Abstract
Ovine herpesvirus 2 (OvHV-2), a member of the viral subfamily Gammaherpesvirinae, shares numerous similarities with human herpesvirus 8 (HHV-8). Both viruses are apathogenic in their healthy original host, may cause lymphoprolipherative diseases, cannot routinely be propagated in cell culture, and may be sexually transmitted. However, the pathways of sexual transmission of these viruses, as well as the underlying pathogenetic dynamics, are not well understood. Organs from naturally OvHV-2-infected, as well as OvHV-2-free, sheep were quantitatively analyzed for OvHV-2 by the DNA amplification techniques. The dynamics of OvHV-2 multiplication and excretion were monitored after experimental infections and, most importantly, subsequent to vasectomy. The OvHV-2 DNA load in various tissues and internal organs was not merely reflecting the viral DNA load in the bloodstream, which suggested compartmentalization of OvHV-2. Moreover, OvHV-2 DNA was detected at several portals for virus shedding, i.e., the respiratory, alimentary, and urogenital tracts. Transient OvHV-2 excretion was detected in ejaculates of experimentally infected rams. Upon vasectomy, OvHV-2 DNA reappeared in the ejaculatory plasma, but the titers did not decline after reaching a peak. Spiking and fractionation experiments revealed an inhibitory activity, associated with the spermatozoa, which was able to suppress detection of viral DNA but which was no longer present in samples from vasectomized animals. Therefore, epidemiological studies on viruses that may be transmitted by the ejaculatory pathway and for whose tracing nucleic acid amplification methods are used, i.e., OvHV-2, HHV-8, and the human immunodeficiency virus, should include vasectomized males.
The hallmarks of gamma herpesviruses, i.e., ovine herpesvirus 2 (OvHV-2; the causative agent of malignant catarrhal fever) and the tumorigenic human herpesvirus 8 (HHV-8), include restricted host range and causing host-specific, proliferative, immunopathological diseases (13, 15, 19). HHV-8 and OvHV-2 share at least three important features:
(i) Normally, they are not associated with disease in the healthy original host. However, in the immunologically ill adapted host, they are causative agents of lymphoproliferative diseases. (ii) Natural isolates cannot routinely be propagated in cell culture (7, 18). Therefore, efficient tracing of these viruses relies on DNA amplification techniques. (iii) Several lines of evidence suggest that they may be sexually transmitted (2, 3, 14, 16). However, the reported prevalences of HHV-8 in prostate and semen range from zero to >90% (reviewed in reference 9). To date, hardly any information concerning the pathogenesis and shedding of OvHV-2 has been presented.
Good animal models for studying HHV-8 infections are not available (4). However, specific-OvHV-2-free sheep may be employed to gain significant information on infection, pathogenesis, and transmission of gammaherpesviruses.
To gain insight into the pathogenetic basis for gammaherpesvirus excretion, organs from naturally OvHV-2-infected, as well as specific-OvHV-2-free, sheep were collected and quantitatively analyzed for OvHV-2 DNA. Since HHV-8 is difficult to detect in normal semen, whereas oligozoospermia and clear rather than milky appearance of semen specimens contribute to more reliable detection of HHV-8 (16), experimentally OvHV-2 infected rams were vasectomized and the course of ejaculatory OvHV-2 shedding was monitored and compared to other possible routes of virus shedding. The results showed that OvHV-2 is compartmentalized in its natural host, thereby opening several possible pathways for virus shedding. Moreover, analysis of ejaculatory samples from naturally infected and vasectomized rams clearly shed new light into the conundrum of sexual transmission of gammaherpesviruses, such as OvHV-2 and HHV-8, and of viruses in general.
MATERIALS AND METHODS
Animals.
Eight sheep, six rams (animals 1 and 4 through 8), and two ewes (animals 2 and 3) were selected from a specific-OvHV-2-free flock (7, 14) and introduced into flocks with known OvHV-2 circulation. The ewes and ram 7 were only available for the initial infection studies. Rams 1 and 4 were vasectomized at week 52 after introduction into the positive flock and euthanized for tissue collection at week 72. Rams 5, 6, and 8 had been introduced to the positive flocks 30 weeks later than rams 1 and 4. They were vasectomized at week 22 postintroduction and euthanized at week 42. Tissue samples were collected as listed in Table 1 and stored at −20°C.
TABLE 1.
Ranking of the OvHV-2 load in organs and tissues of infected sheepa
| Rank | Organ or tissue | OvHV-2 load valueb |
||||
|---|---|---|---|---|---|---|
| Ranking value | Max | Mean | Min | Median | ||
| 1 | Small intestinec | 26 | 61,607 | 17,717 | 3,727 | 4,229 |
| 2 | LN retropharyngealisd | 29 | 19,125 | 8,952 | 2,897 | 9,111 |
| 3 | Abomasum | 34 | 54,979 | 16,566 | 2,310 | 8,645 |
| 4 | LN lumbalisd | 35 | 149,835 | 33,640 | 2,305 | 3,857 |
| 5 | Lung | 38 | 31,824 | 11,355 | 1,645 | 3,265 |
| 6 | Vesicular gland | 41 | 39,683 | 11,517 | 2,878 | 4,312 |
| 7 | LN mandibularisd | 41 | 15,796 | 7,543 | 2,603 | 4,159 |
| 8 | Trigeminal ganglion | 43 | 12,992 | 7,104 | 2,353 | 5,667 |
| 9 | Epididymis | 49 | 1,381,214 | 280,573 | 709 | 3,395 |
| 10 | Prostate gland | 53 | 14,981 | 5,416 | 1,455 | 2,557 |
| 11 | Spleen | 54 | 6,317 | 4,046 | 2,098 | 4,435 |
| 12 | Heart | 62 | 12,450 | 4,509 | 907 | 2,548 |
| 13 | Testis | 66 | 28,055 | 7,026 | 706 | 1,902 |
| 14 | Urinary bladder | 67 | 8,074 | 3,339 | 504 | 3,476 |
| 15 | Tonsilla palatina | 68 | 25,743 | 8,031 | 560 | 2,660 |
| 16 | Ampulla of the deferent duct | 72 | 22,707 | 7,556 | 484 | 1,384 |
| 17 | Rhombencephalon | 84 | 27,723 | 6,360 | 0 | 1,455 |
| 18 | Liver | 87 | 2,583 | 1,531 | 198 | 1,985 |
| 19 | Aorta | 88 | 13,191 | 3,892 | 148 | 290 |
| 20 | Spinal cord | 89 | 2,361 | 1,663 | 0 | 2,040 |
| 21 | Kidney | 97 | 5,974 | 1,665 | 42 | 890 |
| 22 | Cornea | 100 | 3,200 | 1,278 | 0 | 887 |
| 23 | Esophagus | 108 | 3,139 | 1,070 | 43 | 382 |
| 24 | Pituitary gland | 109 | 1,618 | 875 | 35 | 974 |
| 25 | Adrenal gland | 109 | 2,019 | 940 | 64 | 763 |
| 26 | Cerebrum | 114 | 1,880 | 813 | 0 | 491 |
| 27 | Skeletal musculature | 117 | 2,424 | 874 | 0 | 451 |
The number of OvHV-2 DNA copies per 6 × 108 copies of cellular DNA was determined for each organ or tissue from each animal.
Ranking value, the OvHV-2 DNA load in each tissue was ranked for each individual tested. The sum of the individual rankings gave the ranking value. Max, Mean, Min, and Median numbers refer the load of OvHV-2 DNA copies per 6 × 108 copies of cellular DNA. Max, the maximal value detected among individual organs and tissues is shown; Mean, the mean value calculated from individual values; Min, the minimal value found among the individual organs and tissues; Median, the median value of OvHV-2 DNA load per organ and tissue.
Including Peyer's patches.
LN, lymph node.
Sampling.
Nasal, ocular, and oral swabs, as well as fecal specimens (each collected three times per week) and EDTA blood samples (weekly), were taken at intervals and processed as follows.
Freshly taken swabs were placed in a tube containing 5 ml of phosphate-buffered saline (PBS), agitated vigorously, squeezed out, and discarded. Afterward, the fluid was filtered through a double layer of sterile gauze before being centrifuged at 4°C for 10 min at 217 × g. Each pellet was resuspended in 500 μl of PBS.
Then, 40 ml of lysis buffer (0.15 M NH4Cl, 10 mM CHKO3, 0.1 mM EDTA; pH 7.2) was added to 10 ml of EDTA-treated blood samples, followed by incubation for 5 min at room temperature to lyse the erythrocytes and centrifugation at 4°C for 10 min at 868 × g. The buffy coat cells were resuspended and washed in 50 ml of PBS before being centrifuged (4°C, 10 min, 868 × g) to gain the pellet consisting of buffy coat cells. All samples, including untreated fecal specimens, were stored at −20°C until further use.
Ejaculatory samples.
To get ejaculatory samples at weekly intervals, the rams were trained to mount a restrained ewe treated with estrogen, and the samples were collected by artificial vagina as described elsewhere (5, 8).
The fresh ejaculate was diluted 1:2 with PBS and cooled at 4°C for 1 h to reduce the motility of the spermatozoa. The sample was then separated into three fractions as described previously (21). The supernatant, containing the ejaculatory plasma, was obtained after centrifugation of 30 to 300 μl (depending on the total volume of the ejaculate) of the diluted ejaculates at 13,000 rpm for 30 s in an Eppendorf microcentrifuge. The plasma was supplemented with 2 volumes of buffer 1 (0.15 M NaCl, 0.75% sodium-N-lauroylsarcosine, 1.5 mg of freshly added proteinase K/ml) and digested at 60°C for 1 h.
The pellet was resuspended in 1 volume of PBS and lysed in 2 volumes of buffer 1. The mixture was incubated at 60°C for 1 h before being resedimented (13,000 rpm, 30 s) in a microcentrifuge. The supernatant from this process constituted the intermediate fraction, containing the nonsperm cells. The spermatozoa were present in the second pellet, which was designated as the sedimentable fraction. For lysis of the sedimentable fraction, the pellet was resuspended in 1 volume of PBS and 2 volumes of buffer 2 (buffer 1 supplemented with 40 mM 1,4-dithiothreitol (Boehringer, Mannheim, Germany) and incubated at 60°C for 1 h. To obtain DNA from unfractionated ejaculates, aliquots of the samples were immediately lysed in buffer 2.
Urine samples.
Urine samples (15 to 35 ml) were obtained from seven OvHV-2 positive (five ewes and two rams) and four negative sheep (two of each sex). Sterile urine was obtained by puncturing the urinary bladder. Alternatively, spontaneous urine was collected. After removal of an aliquot of total urine, the samples were fractionated by centrifugation (868 × g, 4°C, 10 min). The supernatant was designated as the soluble fraction. The pellet was resuspended in 30 ml of PBS and washed by repeated centrifugation and resuspension in PBS. Finally, the sedimentable fraction of the urine was resuspended in 200 μl of PBS. The processed samples were stored at 4°C until further use.
DNA extraction.
Care was taken to select protocols for DNA extraction, which resulted in only minimal inhibition of PCR amplifications. With the exception of total urine and urine supernatant, the DNA of all of the different samples was extracted by a QIAamp DNA Mini kit (Qiagen, Basel, Switzerland). Carrier DNA (50 μg of salmon sperm DNA/ml; Life Technologies AG, Basel, Switzerland) was added to the elution buffer (AE) to elute the DNA of the semen samples (22). As suggested by the manufacturer, the QIAamp Viral RNA Mini kit containing a special buffer (AVL) was used to extract the DNA of total urine and the supernatant.
Fluorogenic PCRs and absolute and relative quantitations.
The fluorogenic PCRs specific for OvHV-2 and genomic sheep DNA, respectively, as well as absolute and relative quantitations, were performed essentially as described previously (7). Briefly, 10 μl of eluted DNA was used as starting material. The amount of amplifiable viral DNA in the sample was compared to the amount of cellular DNA, which was determined in a parallel PCR. To minimize potential inhibitory activities inherent to specific tissues, several dilutions of DNA template were evaluated (data not shown). To allow comparison of the results obtained with different animals and their tissue samples, the results were normalized to the arbitrary value of 6 × 108 copies of cellular DNA. Alternatively, the result was expressed as a function of the volume of the sample.
Vasectomy and determination of the spermatozoa counts.
Vasectomy was done according to the cranial midscrotal approach (12). The spermatozoa were counted in an improved Neubauer hemocytometer counting chamber according to the method of the World Health Organization (1).
RESULTS
Quantitative distribution of OvHV-2 in organs and tissues.
The presence and the quantity of infectious virus at the site of excretion are essential prerequisites for virus transmission. For a comparative view of the content of OvHV-2 DNA in 27 different organ and tissue samples of naturally infected sheep (7), we determined the amount of viral DNA per location as a function of cellular DNA (Table 1).
Although previous results had shown that OvHV-2 DNA was persistently present in white blood cells (7), these results suggested that in its natural host OvHV-2 is compartmentalized rather than distributed freely over the entire organism. Remarkably, both heavily vascularized (e.g., the liver) and nearly blood-free tissues (e.g., the cornea) were positive for viral DNA. The surprisingly high titers of OvHV-2 DNA in the vesicular gland (rank 6 of 27), the epididymis (rank 9), and the pars disseminata of the prostate gland (rank 10) indicated that the virus may use the genital tract as its portal to sexual transmission. However, the presence of high titers of viral DNA in the abomasum (rank 3) and the small intestine (rank 1), on the one hand, and in the tonsils (rank 15), the retropharyngeal lymph nodes (rank 2), and the lung (rank 5), on the other, argued for a possible respiratory or fecal-oral transmission. Finally, with the urine bladder (rank 14) containing considerable amounts of OvHV-2 DNA, the possibility of a urinary pathway of OvHV-2 transmission also had to be considered.
Chronological appearance of OvHV-2 at different sites after natural infection.
To analyze infection and virus excretion, we introduced specific OvHV-2-negative sheep into flocks with known history of OvHV-2 circulation (7, 14). In analogy to the alcelaphine herpesvirus 1, we expected that OvHV-2 would first replicate in the upper respiratory tract (17). However, the first positive signal was always found in buffy coat cells, which had previously been shown to represent a reservoir of OvHV-2 DNA in infected sheep (7, 14). Nasal swabs turned positive only 7 to 9 days later. After another 7 days, we occasionally found OvHV-2 DNA in ocular swabs. The maximal amounts of virus detected in either the nasal or the ocular swabs were at least 1 order of magnitude below the maximal amounts found in the buffy coat cell fraction. Notably, we were unable to detect viral DNA in either oral swabs, fecal samples, or urinary specimens. In contrast, studies with other herpesviruses had indicated that they may well be excreted through the urinary pathway (6).
OvHV-2 DNA was detected in the urogenital tract of all five sacrificed OvHV-2 positive rams, i.e., in the kidneys, the urinary bladder, the testis, the epididymis, the vesicular gland, the ampulla of the deferent duct, and the pars disseminata of the prostate gland (Table 2). In general, the mean values of OvHV-2 DNA concentrations in the tissues of the genital tract exceeded those of the values measured in the kidney or the urinary bladder. These results suggested that OvHV-2 is excreted via the urogenital tract. Semen may be most important for male-to-female transmission. The amount of virus in urine is low. Nevertheless, as numerous animals are constantly exposed, urine may be important for transmission among males, to sexually inactive members of the flock, and even to other animal species.
TABLE 2.
Comparison of the OvHV-2 load in the organs of the urogenital tract
| Organ | Ov HV-2 loada |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Sheep |
Mean | Median | Max | Min | |||||
| 1 | 4 | 5 | 6 | 8 | |||||
| Epididymis | 709 | 16,593 | 1,381,214 | 3,395 | 952 | 280,573 | 3,395 | 1,381,214 | 709 |
| Vesicular gland | 4,312 | 3,382 | 7,329 | 39,683 | 2,878 | 11,517 | 4,312 | 39,683 | 2,878 |
| Ampulla of the deferent duct | 484 | 534 | 12,670 | 22,707 | 1,384 | 7,556 | 1,384 | 22,707 | 484 |
| Testis | 706 | 1,902 | 28,055 | 3,264 | 1,204 | 7,026 | 1,902 | 28,055 | 706 |
| Prostate gland | 1,455 | 1,747 | 14,981 | 2,557 | 6,342 | 5,416 | 2,557 | 14,981 | 1,455 |
| Urinary bladder | 504 | 8,074 | 3,476 | 848 | 3,791 | 3,339 | 3,476 | 8,074 | 504 |
| Kidney | 42 | 1,036 | 890 | 382 | 5,974 | 1,665 | 890 | 5,974 | 42 |
| Urine | - | - | - | - | - | - | - | - | - |
The numbers indicate the load of OvHV-2 DNA copies per 6 × 108 copies of cellular DNA. Individual values, as well as maximum (Max), minimum (Min), median, and mean values are shown.
-, Negative, i.e., no OvHV-2 DNA was detected.
Similar to virus shedding in the respiratory tract, semen was not positive until after viral DNA had been detected in the buffy coat cells. However, from day 2 until day 21 after the buffy coat cells had turned PCR positive, we transiently detected OvHV-2 DNA in the semen.
Dynamics of OvHV-2 excretion in semen.
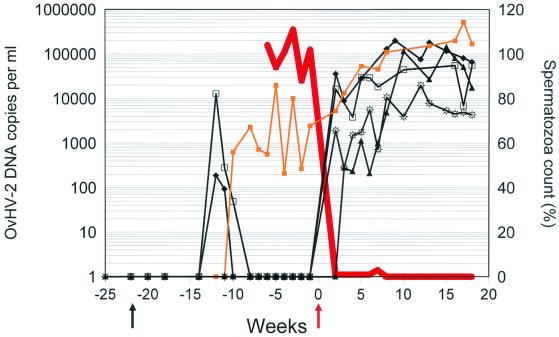
One ram (animal 5) continuously excreted OvHV-2 DNA in the semen. Since its ejaculatory virus titers fluctuated independently of the virus load in buffy coat cells (data not shown), we hypothesized that virus was shed actively by the seminal route. To explore this possibility further, we subjected OvHV-2-positive rams to surgical vasectomy. Within 2 or 3 weeks after vasectomy, coincident with the drop in spermatozoa counts, OvHV-2 DNA reappeared in the ejaculatory plasma samples from rams 1, 4, 6, and 8 (Fig. 1). Remarkably, this was clearly not a consequence of higher lymphocyte counts or inflammatory cells in the ejaculate.
FIG. 1.
Emergence of OvHV-2 DNA in ejaculates of rams prior and after vasectomy. The time in weeks is indicated on the x axis. Vasectomy was done in week zero (red arrow). The y1 axis (left) indicates the concentration of OvHV-2 in the ejaculates of originally specific OvHV-2-free rams, whose individual values are indicated by symbols. Rams 1 (sunburst symbols) and 4 (solid triangles) had been introduced into the sheep flock with natural circulation of OvHV-2 at week −52 prior to vasectomy. Rams 5 (gold squares), 6 (diamonds), 8 (open squares) were introduced into the flock at week −22 (black arrow). The y2 axis (right) indicates the relative spermatozoa counts. The average value of all rams is indicated by a red line.
Surprisingly, titers did not decline after reaching a peak. On the contrary, they continued to fluctuate in a range that was equivalent or even higher than that occurring during the acute phase of primary infection. Similarly, we observed a persisting increase of the viral DNA load by up to 2 orders of magnitude in the ejaculatory plasma samples of ram 5. Thus, it appeared as if vasectomy had not induced reactivation of OvHV-2 from a latent state. Rather, these results suggested that the absence of spermatozoa facilitated the detection of viral DNA in the ejaculatory plasma. Indeed, this notion is in agreement with the observation that a previously unrecognized variable in the analyses of semen specimens is oligozoospermia and reduced sperm production (16). Consequently, HHV-8 is difficult to detect in normal semen, whereas oligozoospermia and a clear rather than a milky appearance of semen specimens are factors that contribute to more reliable detection of HHV-8 (16).
Thus, these results and considerations raised concerns about the reliability of detection of OvHV-2 DNA in urogenital samples due to the possible presence of inhibitors of detection. Therefore, we centrifuged both ejaculatory and urine samples from OvHV-2-positive animals to gain and analyze soluble and sedimentable (urinary samples) or soluble (ejaculatory plasma), intermediate, and sedimentable (spermatozoa-associated) fractions.
With one exception, we did not find OvHV-2 DNA in either the soluble or the sedimentable fractions of urine from OvHV-2-positive animals, although amplifiable cellular DNA was readily detected in both fractions. Similarly, unfractionated ejaculatory samples of persistently infected animals always reacted negatively with regard to viral DNA. However, upon fractionation by sedimentation, we were able to detect OvHV-2 DNA in the supernatant of centrifuged ejaculates from positive rams and, with sporadic exceptions, at a reduced titer also in the intermediate fraction. In contrast, positive signals attributable to the cellular fraction were not detected in the sedimented spermatozoa. However, on rare occasions a weak signal was discernible and attributed to contaminating supernatants.
Spiking experiments made clear that the addition of as little as 10 molecules of plasmid-cloned OvHV-2 DNA to either total urine, fractionated urinary sediment, or supernatant could be equally well detected. Thus, PCR detection of OvHV-2 DNA in urinary specimens was not inhibited. In contrast, upon spiking fractionated ejaculates from OvHV-2 negative rams, we observed an inhibitory effect in association with the sedimentable fraction containing the spermatozoa. In two of three experiments, the spiking of the spermatozoa fraction with 10 or 100 molecules of plasmid-cloned OvHV-2 DNA led to negative results and, in a third experiment, to a strongly reduced signal. However, a signal within the expected range was detected under the same conditions, when the spiking DNA was added to either undiluted ejaculatory plasma and intermediate fractions or to prediluted (1:10 and 1:100) fractions containing the spermatozoa.
To finally test whether OvHV-2 DNA in positive samples was associated with the spermatozoa or not, we retested the fractions containing sedimented spermatozoa at 10- and 100-fold dilutions. However, even under those conditions, the spermatozoa fractions remained negative.
These results indicated that components of the sedimentable ejaculatory fluids (i.e., the spermatozoa) but not of urine were able to suppress the sensitivity of DNA detection in urogenital samples by approximately 2 orders of magnitude. Nevertheless, OvHV-2 DNA was not associated with spermatozoa but rather with the intermediate and supernatant fractions of the ejaculatory fluids.
DISCUSSION
We provide evidence that the gammaherpesvirus OvHV-2 is compartmentalized in its natural host, which opens the pathways for a variety of routes of virus shedding. The relevance of the ejaculatory shedding emerged only after we tested naturally infected animals before and after surgical vasectomy. Similar to OvHV-2, HHV-8 cannot be serially propagated in cell culture, and animal models are not available (13, 18). Obviously, transmission studies with HHV-8 cannot ethically be performed in the original host, whereas they can be done with OvHV-2 in sheep. The possibility of sexual transmission has been suggested for both of the two viruses (2, 14, 16). However, it is well known that detection of viruses in semen is difficult because semen may be toxic for cell cultures, whereas PCR-based detection methods may be inhibited by seminal components (reviewed in reference 13). It is equally well known that virus transmission in nature may be successful, even though in vitro tests are unable to detect the virus under scrutiny (10).
An inhibitory activity, able to suppress detection of viral DNA, associated with the spermatozoa, was no longer present in samples from vasectomized animals. Thus, the key issues that arise from the results presented here are relevant not only for the understanding of the pathogenesis and transmission of OvHV-2 but also for HHV-8 and probably any other virus that may be transmitted by the ejaculatory pathway. Indeed, controversial reports on the quantity of HHV-8 in human semen (2, 3, 11, 16) speak for the existence of such spermatozoa-associated inhibitors in humans. Even the human immunodeficiency virus is cultured more often from seminal cells than from seminal plasma, although the frequency of viral RNA detection does not decrease after vasectomy (9, 20). Future studies on sexual transmission of viruses should therefore focus on results obtained with vasectomized males.
However, the occasional presence of OvHV-2 DNA in nasal and other bodily secretions of infected animals speaks also for potential alternative routes of OvHV-2 transmission, which may be equally important. For example, the respiratory route, which is typical for the transmission of alcelaphine herpesvirus 1 among wildebeest (17), may be essential also for OvHV-2. Therefore, it must not be concluded from these data that seminal shedding should represent the single only relevant pathway for transmission of OvHV-2 or of any of the viruses mentioned above.
Acknowledgments
We thank Sarun Keo for excellent technical assistance; Christoph Lischer for vasectomy of the rams; and Julius Burri, Paul Mastnak, Hanspeter Müller, and Hanspeter Rutschi for taking care of the animals. Finally, we acknowledge the critical comments of David Nadal, Ueli Hübscher, and Phil Pellet.
This work was supported by the Swiss Veterinary Services, the Robert and Dorothea Wyler donation, and the Kanton of Zurich.
REFERENCES
- 1.Anonymous. 1992. WHO laboratory manual for the examination of human semen and sperm-cervical mucus interaction, p. 11-13. Cambridge University Press, Cambridge, England.
- 2.Beral, V., T. A. Peterman, R. L. Berkelman, and H. W. Jaffe. 1990. Kaposi's sarcoma among persons with AIDS: a sexually transmitted infection? Lancet 335:123-128. [DOI] [PubMed] [Google Scholar]
- 3.Couturier, E., R. A. Ancelle-Park, I. de Vincenzi, A. M. Downs, and J. B. Brunet. 1990. Kaposi sarcoma as a sexually transmitted disease. Lancet 335:1105. [DOI] [PubMed] [Google Scholar]
- 4.Dittmer, D., C. Stoddart, R. Renne, V. Linquist-Stepps, M. E. Moreno, C. Bare, J. M. McCune, and D. Ganem. 1999. Experimental transmission of Kaposi's sarcoma-associated herpesvirus (KSHV/HHV-8) to SCID-hu Thy/live mice. J. Exp. Med. 190:1857-1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Evans, G., and W. M. C. Maxwell (ed.). 1987. Salamon's artificial insemination of sheep and goats, p. 85-91. Butterworths Pty., Ltd., Sidney, Australia.
- 6.Gautheret-Dejean, A., J. T. Aubin, L. Poirel, J. M. Huraux, J. C. Nicolas, W. Rozenbaum, and H. Agut. 1997. Detection of human Betaherpesvirinae in saliva and urine from immunocompromised and immunocompetent subjects. J. Clin. Microbiol. 35:1600-1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hüssy, D., N. Stäuber, C. M. Leutenegger, S. Rieder, and M. Ackermann. 2001. Quantitative fluorogenic PCR assay for measuring ovine herpesvirus 2 replication in sheep. Clin. Diagn. Lab. Immunol. 8:123-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Janett, F., D. Hüssy, C. Lischer, M. Hässig, and R. Thun. 2001. Semen characteristics after vasectomy in the ram. Theriogenology 56:485-491. [DOI] [PubMed] [Google Scholar]
- 9.Krieger, J. N., A. Nirapathpongporn, M. Chaiyaporn, G. Peterson, I. Nikolaeva, R. Akridge, S. O. Ross, and R. W. Coombs. 1998. Vasectomy and human immunodeficiency virus type 1 in semen. J. Urol. 159:820-825. [PubMed] [Google Scholar]
- 10.Kupferschmied, H. U., U. Kihm, P. Bachmann, H. K. Müller, and M. Ackermann. 1986. Transmission of IBR/IPV virus in bovine semen: a case report. Theriogenology 25:439-443. [DOI] [PubMed] [Google Scholar]
- 11.Lin, J.-C., S.-C. Lin, E.-C. Mar, P. E. Pellet, F. R. Stamey, J. A. Stewart, and T. J. Spira. 1995. Is Kaposi's sarcoma-associated herpesvirus detectable in semen of HIV-infected homosexual men. Lancet 346:1601-1602. (Retraction, Lancet 351: 1365, 1998.) [DOI] [PubMed] [Google Scholar]
- 12.Lofstedt, R. M. 1982. Vasectomy in ruminants: a cranial midscrotal approach. J. Vet. Med. Assoc. 181:373-375. [PubMed] [Google Scholar]
- 13.Moore, P. S., and Y. Chang. 2001. Kaposi's sarcoma-associated herpesvirus, p. 2803-2833. In D. M. Knipe and P. M. Howley (ed.), Fields virology, vol. II, 4th ed. Lippincott/Williams & Wilkins, Philadelphia, Pa.
- 14.Müller-Doblies, U. U., J. Egli, H. Li, U. Braun, and M. Ackermann. 2001. Bösartiges Katarrhalfieber in der Schweiz. 1. Teil: Epidemiologie. Schweiz. Arch. Tierheilkd. 143:173-183. [PubMed] [Google Scholar]
- 15.Müller-Doblies, U. U., H. Li, B. Hauser, H. Adler, and M. Ackermann. 1998. Field validation of laboratory tests for clinical diagnosis of sheep-associated malignant catarrhal fever. J. Clin. Microbiol. 36:2970-2972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pellett, P. E., T. J. Spira, O. Bagasra, C. Boshoff, L. Corey, P. Rimessi, C. Sosa, C. Wood, and J. A. Stewart. 1999. Multicenter comparison of PCR assay for detection of human herpesvirus 8 DNA in semen. J. Clin. Microbiol. 37:1298-1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Plowright, W. 1990. Malignant catarrhal fever virus, p. 123-150. In Z. Dinter and B. Morein (ed.), Virus infections in ruminants, vol. 3. Elsevier Science Publishers, Amsterdam, The Netherlands.
- 18.Renne, R., D. Blackbourn, D. Whitby, J. Levy, and D. Ganem. 1998. Limited transmission of Kaposi's sarcoma-associated herpesvirus in cultured cells. J. Virol. 72:5182-5188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roizman, B., and P. E. Pellett. 2001. The family herpesviridae: a brief introduction, p. 2381-2397. In D. M. Knipe and P. M. Howley (ed.), Fields virology, vol. II, 4th ed. Lippincott/Williams & Wilkins, Philadelphia, Pa.
- 20.Tachet, A., E. Dulioust, D. Salmon, M. De Almeida, S. Rivalland, L. Finkielsztejn, I. Heard, P. Jouannet, D. Sicard, and C. Rouzioux. 1999. Detection and quantification of HIV-1 in semen: identification of a subpopulation of men at high potential risk of viral sexual transmission. AIDS 13:823-831. [DOI] [PubMed] [Google Scholar]
- 21.Van Engelenburg, F. A. C., R. K. Maes, J. T. van Oirschot, and F. A. M. Rijsewijk. 1993. Development of a rapid and sensitive polymerase chain reaction assay for detection of bovine herpesvirus 1 in bovine semen. J. Clin. Microbiol. 31:3129-3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vögtlin, A., C. Fraefel, S. Albini, C. M. Leutenegger, E. Schraner, B. Spiess, H. Lutz, and M. Ackermann. 2002. Quantification of feline herpesvirus 1 DNA in ocular fluid samples of clinically diseased cats by real-time TaqMan PCR. J. Clin. Microbiol. 40:519-523. [DOI] [PMC free article] [PubMed] [Google Scholar]