Abstract
There are about 350 million chronic hepatitis B virus (HBV) carriers worldwide. A proactive approach to the management of this disease is likely to reduce the morbidity and mortality caused by HBV. This study aimed to evaluate the diagnostic performance of a novel tool for discriminating between infected and noninfected subjects, the hepatitis B sAg/eAg test (Binax Inc., Portland, Maine). The test is designed to rapidly and accurately detect both the HBV surface antigen (HBsAg) and the HBV e antigen (HBeAg). A cohort of 942 subjects was tested. The serum clinical sensitivity of the hepatitis B sAg/eAg test was 99.75 and 96.37% for HBsAg and HBeAg, respectively. Serum clinical specificity was 99.32% for HBsAg and 98.99% for HBeAg. Analytical sensitivity was satisfactory for the purposes of population screening. Visual evaluation showed that the test signals were stable for at least 3 h after the recommended evaluation time. No interference or cross-reactivity was observed with known interfering substances and virologic markers. These results indicate that the hepatitis B sAg/eAg test is well suited to the accurate detection of HBV carriers. In addition to the good clinical specificity and sensitivity of this test, its stability and user-friendly design mean that a correct performance, even under field conditions, is highly likely. Consequently, the hepatitis B sAg/eAg test has the potential to identify subjects who require HBV vaccination (HBsAg− and HBeAg−) and HBV-infected individuals who might benefit most from antiviral therapy (HBsAg+ and HBeAg+).
Until recently, the management of hepatitis B disease has mostly been reactive, yet a proactive approach is more likely to reduce the morbidity and mortality caused by this viral infection. An approach involving prevention or early treatment could be used in the management of hepatitis B, an infection for which both effective vaccines and treatment options exist. More than 116 countries have included hepatitis B vaccination as part of their routine infant or adolescent immunization programs and, with the support of the Global Alliance for Vaccines and Immunization, mass immunization will soon be recommended in the great majority of the remaining countries (6, 7). However, there are currently about 350 million people worldwide who are chronically infected with hepatitis B virus (HBV) (3, 8), 15 to 40% of whom will develop serious sequelae during their lifetime (4). Ideally, these chronic carriers should be identified and medical interventions implemented to reduce the risk of premature death. Furthermore, measures should be introduced to prevent further spread of the virus to the unprotected population.
A diagnostic procedure allowing discrimination between an infected and noninfected subject within minutes, using just a few droplets of blood, would facilitate and add to the success of a proactive approach towards HBV disease management. An assay that enables the simultaneous detection of HBV surface antigen (HBsAg) and HBV e antigen (HBeAg) would allow a distinction to be made between subjects who might benefit from antiviral treatment (HBsAg+ and HBeAg+), those who are less prone to respond to antiviral therapy (HBsAg+ and HBeAg−), and those in need of vaccination (HBsAg− and HBeAg−). The cost, logistic complexity, and loss of compliance associated with additional laboratory testing will determine whether HBsAg− HBeAg− subjects should be tested for antibodies specific to HBV, prior to vaccination.
In this study, we report the evaluation of a rapid diagnostic test for the simultaneous detection of HBsAg and HBeAg. This investigation has been conducted with a view to possibly implementing the test within a proactive approach to the management of hepatitis B. Although assessment of this test has been performed in a clinical microbiology laboratory, its prospective use under field conditions has been a major consideration.
MATERIALS AND METHODS
Clinical samples.
The hepatitis B sAg/eAg test (Binax Inc., Portland, Maine) is designed for use with whole blood samples obtained by finger or heel puncture. However, as it was impractical for large numbers of HBV-infected subjects to provide fresh whole blood drawn via finger puncture, serum that had been stored at −80°C for no longer than 24 months was analyzed.
A total of 942 samples were examined for the presence of HBsAg and HBeAg. All sera derived from 403 patients with biopsy-proven chronic HBV infection tested positive for HBsAg (using AxSym HBsAg V2; Abbott Laboratories) and HBV DNA (using HBV Amplicor; Roche Molecular Diagnostics). HBeAg was detected in 303 of these 403 serum samples by using AxSym HBe 2.0 (Abbott Laboratories). HBsAg- and HBeAg-free sera (as determined using the AxSym HBsAg and HBe tests) were acquired from 295 healthy volunteers who had participated in a clinical vaccine evaluation trial. Whole blood samples that were negative for HBsAg and anti-HBV core antigen (HBc; as determined using Enzygnost HBsAg and Enzygnost anti-HBc monoclonal; Dade Behring, Marburg, Germany) were obtained as anticoagulated EDTA specimens from 244 healthy, voluntary blood donors through the Blood Transfusion Centre of the Belgian Red Cross (Ghent Branch).
Hemolytic and icteric serum samples, as well as those positive for serologic markers of Epstein-Barr virus, cytomegalovirus, human immunodeficiency virus, and hepatitis C virus infection, were acquired from the Laboratory for Clinical Pathology of the Ghent University Hospital. Sera positive for rheumatoid factor were provided by the Department of Rheumatology, Ghent University Hospital.
The hepatitis B sAg/eAg test was used during this study to detect HBsAg and HBeAg in whole blood, serum, or plasma, according to the manufacturer's guidelines. The only deviation from the recommended protocol was introduced to avoid a volume bias: when using serum as the test sample, 60 μl was used instead of the 100-μl volume recommended for whole blood. Whole blood samples were applied in the prescribed 100-μl volume. The assays were performed as follows: (i) the assay card was opened and positioned on a flat area; (ii) using a precision pipette, 100 μl of anticoagulated whole blood or 60 μl of serum was added to the top of the pink and white pad on the assay card; (iii) it took about 30 s to 1 min for the sample to move from the white into the pink area and wet it; (iv) the adhesive liner was then removed and discarded; (v) subsequently, the assay card was closed and the timing was started; (vi) 10 min later, the result was read through the viewing window.
Reference methods.
Microparticle enzyme immunoassays for HBsAg and HBeAg were performed according to the manufacturer's instructions with the AxSym HBsAg V2 and AxSym HBe 2.0 systems, respectively. The accuracy and stability of these tests were continuously monitored by the internal quality assessment program of this study.
Reference materials.
The international standard for HBsAg (subtype ad, code 80/549; National Institute for Biological Standards and Control [NIBSC]) was purchased from the NIBSC (Hertfordshire, United Kingdom). The HBeAg reference antigen (HBe-Referenzantigen 82) was purchased from the Paul-Ehrlich Institute (PEI), Bundesamt für Sera und Impfstoffe, Langen, Germany.
Evaluation methodology.
The methods used to evaluate the analytical and diagnostic properties of the hepatitis B sAg/eAg test were mainly based on the National Committee for Clinical Laboratory Standards EP12-P guidelines (5). In brief, the analytical sensitivity was determined by performing a series of 20 repeat tests. The lowest concentration that scored positive in 19 of 20 repeats (95%) was deemed the limit of analytical sensitivity. The linear dilutions were prepared from an in-house standard that was calibrated against the international reference material. Clinical sensitivity, clinical specificity, the predictive value of a positive (PPV) or negative (NPV) test result, and efficiency were assessed by comparing the results obtained using the hepatitis B sAg/eAg test with the true clinical diagnosis for HBsAg status and the AxSym HBe 2.0 (Abbott Laboratories) result for HBeAg. Robustness and cross-reactivity were assessed by analyzing samples containing known interfering substances or that were seropositive for one or more virologic markers. All these samples were analyzed using both the hepatitis B sAg/eAg and AxSym HBe 2.0 systems. To evaluate the stability of the test signal, six assays (three negative and three positive) were analyzed and visually scored from 5 min to 3 h past the recommended 10-min evaluation time.
RESULTS
Analytical sensitivity of the hepatitis B sAg/eAg test for HBsAg and HBeAg.
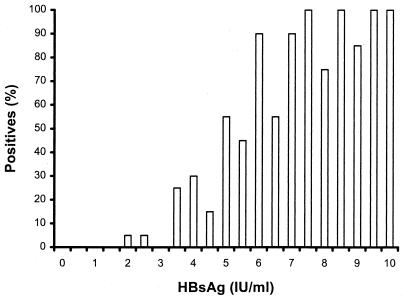
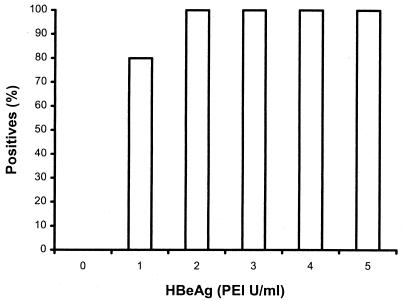
The lower detection limit of the assay for HBsAg was determined by measuring a series of control serum samples containing known concentrations of HBsAg ranging from 0 to 10 IU/ml, at increments of 0.5 IU/ml. Each sample was measured 20 times, and the lowest concentration at which 19 of the 20 measurements scored positive was considered the detection limit. As shown in Fig. 1, this result was achieved at a concentration of 9.5 IU/ml. Similarly, a series of samples containing 0 to 5 PEI standard units (PEI U) of HBeAg/ml, with increments of 1 PEI U/ml, was prepared and analyzed. The detection limit for HBeAg was 2 PEI U/ml (Fig. 2).
FIG. 1.
Percentage of HBsAg-positive subjects detected using the hepatitis sAg/eAg test versus the HBsAg standard provided by NIBSC.
FIG. 2.
Percentage of HBeAg-positive subjects detected using the hepatitis sAg/eAg test versus the PEI HBeAg standard.
Diagnostic performance of the hepatitis B sAg/eAg test.
A qualitative evaluation of the diagnostic performance of the test, with respect to detection of HBsAg, was based on the analysis of 942 samples (Table 1). The clinical sensitivity for serum was 99.75%, and the overall specificity was 99.63% (99.32% for serum and 100.00% for whole blood). The qualitative evaluation of HBeAg detection using the hepatitis B sAg/eAg test on samples whose HBeAg status had been evaluated using the AxSym HBe 2.0 method showed a clinical sensitivity of 96.37% for serum and an overall specificity of 99.37% (98.99% for serum and 100.00% for whole blood) (Table 2).
TABLE 1.
Diagnostic performance of the hepatitis B sAg/eAg system for HBsAga
| Sample | Test outcome | Clinical diagnosis |
Total subjects | |
|---|---|---|---|---|
| Chronic HBV carriers | Healthy control subjects | |||
| Serum (S) | HBsAg+ | 402 | 2 | 404 |
| HBsAg− | 1 | 293 | 294 | |
| Total | 403 | 295 | 698 | |
| Whole blood (WB) | HBsAg+ | 0 | 0 | |
| HBsAg− | 244 | 244 | ||
| Total | 244 | 244 | ||
| Total (S+WB) | HBsAg+ | 402 | 2 | 404 |
| HBsAg− | 1 | 537 | 538 | |
| Total | 403 | 539 | 942 | |
Clinical sensitivity for serum, 99.75%; clinical specificity for serum, 99.32%; clinical specificity for whole blood, 100.00%; clinical specificity for all samples, 99.63%.
TABLE 2.
Diagnostic performance of the hepatitis B sAg/eAg system for HBeAga
| Sample | Test outcome | HBeAg status of subjects by AxSymHBe 2.0 |
Total subjects | |
|---|---|---|---|---|
| HBeAg+ | HBeAg− | |||
| Serum (S) | HBeAg+ | 292 | 4 | 296 |
| HBeAg− | 11 | 391 | 402 | |
| Total | 303 | 395 | 698 | |
| Whole blood (WB) | HBeAg+ | 0 | 0 | |
| HBeAg− | 244 | 244 | ||
| Total | 244 | 244 | ||
| Total (S+WB) | HBeAg+ | 292 | 4 | 296 |
| HBeAg− | 11 | 635 | 646 | |
| Total | 303 | 639 | 942 | |
Clinical sensitivity for serum, 96.37%; clinical specificity for serum, 98.99%; clinical specificity for whole blood, 100.00%; clinical specificity for all samples, 99.37%.
Three tests showing positive results and three with negative results were monitored for extended periods of time and were found to be stable for at least 3 h after the recommended 10-min evaluation time. This extended incubation did not further increase the sensitivity of the test and did not lead to negative signals. Although it is preferable that the manufacturer's guidelines be followed, it is diagnostically advantageous that a slight deviation from the recommended evaluation time does not invalidate the results.
Robustness and cross-reactivity.
A series of samples which had been proven to be negative for HBV markers using the AxSym system were examined for possible interference by hemolysis (n = 5), bilirubin (n = 5), rheumatoid factor (n = 4), Epstein-Barr virus immunoglobulin G (IgG) (n = 9), cytomegalovirus IgG (n = 12), human immunodeficiency virus antibodies (n = 4), and hepatitis C virus antibodies (n = 5). No interference was observed.
DISCUSSION
The analytical and diagnostic qualities of the hepatitis B sAg/eAg system for the combined detection of HBsAg and HBeAg were evaluated.
The analytical sensitivity for HBsAg was 9.5 IU/ml. The sensitivity detailed by the manufacturer in the hepatitis B sAg/eAg test package insert is 5 ng/ml, which is equivalent to approximately 5 IU/ml. Most enzyme immunoassays available on the market are more sensitive, detecting levels of 0.2 to 0.7 ng/ml (2). However, these systems require various degrees of sophisticated equipment and, furthermore, do not provide results within 10 min at the point of care. The extreme sensitivity of enzyme immunoassays is essential for safe screening of blood and blood derivatives but is of lesser importance in the context of population screening, where the prime goal is to discriminate HBV carriers from noninfected subjects. It is notable that HBsAg generally circulates at concentrations of 50 to 300 μg/ml in chronic carriers (1) and that values below 20 IU/ml (approximately 20 ng/ml) are rarely observed. Data reported in this study indirectly confirm this statement, as the diagnostic sensitivity of the hepatitis B sAg/eAg test for HBsAg in a cohort of 403 chronic hepatitis B patients was 99.75% (402 of 403 subjects). Further results showed that the clinical specificity of the hepatitis B sAg/eAg test, evaluated in a cohort of 539 HBV-free subjects (295 serum, 244 whole blood) was 99.63% (537 of 539 subjects).
Within the total cohort evaluated (n = 942), the PPV for HBsAg using the hepatitis B sAg/eAg test was 99.50%, while the NPV was 99.81% and the overall efficiency of the test was 99.68%. The distribution of HBV-positive and HBV-negative subjects in our study population (403 HBV positives, 539 HBV negatives) is not a reflection of the real-life situation, in which the prevalence of HBV is not 42.78%. If the test were applied in a setting where the prevalence of chronic HBV infection was 5%, the PPV would be 93.42% and the NPV would be 99.99%.
The analytical sensitivity observed for HBeAg was 2 PEI U/ml. In the qualitative evaluation, the hepatitis B sAg/eAg test was used on samples of known HBeAg status, as determined using the AxSym HBe 2.0 system. This produced a clinical sensitivity of 96.37% (292 of 303 samples) and an overall clinical specificity of 99.37% (98.99%, or 391 of 395 with serum samples; 100.00%, or 244 of 244 with whole blood). Within the total cohort evaluated (n = 942), the PPV for HBeAg using the hepatitis B sAg/eAg test was 98.65%, the NPV was 98.30%, and the overall efficiency of the test was 98.41%. If the test was used in a setting where the prevalence of HBeAg-positive chronic hepatitis B patients was 5%, the PPV would be 88.95% and the NPV would be 99.81%.
These results indicate that the hepatitis B sAg/eAg test is extremely well suited to detecting HBV carriers, even in populations in areas with intermediate endemicity. Its user-friendly design and its stability mean that a correct performance is highly likely, even under conditions where facilities and resources may be limited. This test has the potential to identify subjects who require vaccination and HBV-infected subjects who may benefit most from antiviral treatment (HBsAg+, HBeAg+). Thus, the hepatitis B sAg/eAg test is a useful and appropriate instrument in the proactive management of HBV disease.
Acknowledgments
L. Van Renterghem (Virology Laboratory, University Hospital, Ghent, Belgium), B. Vandekerckhove (Blood Transfusion Centre, Ghent, Belgium), and F. De Keyzer (Department of Rheumatology, University Hospital, Ghent) are gratefully acknowledged for providing the serum and whole blood samples used in this survey. We further thank S. Couvent and F. Clinckspoor for their assistance in reading the tests.
REFERENCES
- 1.Ganem, D., and R. J. Schneider. 2001. Hepadnaviridae and their replication, p. 2703-2737. In D. M. Knipe, P. M. Howley, R. M. Chanook, T. P. Monath, B. Roizman, and S. E. Straus (ed.), Fields virology, 4th ed. Lippincott Williams and Wilkins, Philadelphia, Pa.
- 2.Hollinger, F. B., and T. J. Liang. 2001. Hepatitis B virus, p. 2971-3036. In D. M. Knipe, P. M. Howley, R. M. Chanook, T. P. Monath, B. Roizman, and S. E. Straus (ed.), Fields virology, 4th ed. Lippincott Williams and Wilkins, Philadelphia, Pa.
- 3.Lee, W. 1997. Hepatitis B virus infection. N. Engl. J. Med. 337:1733-1745. [DOI] [PubMed] [Google Scholar]
- 4.McMahon, B. J. 1997. Hepatocellular carcinoma and viral hepatitis, p. 315-330. In R. A. Wilson (ed.), Viral hepatitis. Marcel Dekker, New York, N.Y.
- 5.National Committee for Clinical Laboratory Standards. 2000. User protocol for evaluation of qualitative test performance; proposed guideline EP12-P, vol. 20, no. 15. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 6.Van Damme, P. 2001. Hepatitis B: vaccination programmes in Europe—an update. Vaccine 19:2375-2379. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization. 2001. Introduction of hepatitis B vaccine into childhood immunization services—management guidelines, including information for health workers and parents. WHO, Geneva, Switzerland.
- 8.World Health Organization. 2001. Expanded programme on immunization: hepatitis B vaccine—making global progress. Update, October 1996. WHO, Geneva, Switzerland.