Abstract
We evaluated a new immunoblot assay (Helicoblot 2.1) for Helicobacter pylori infection and CagA and VacA status by using serum samples from 222 patients. The test accurately detected H. pylori infection and VacA status, but improvements in the interpretation criteria are required before it can be recommended for determination of CagA status.
There is considerable interest in determining Helicobacter pylori infection outcomes in relation to the putative H. pylori virulence factors CagA and VacA. Molecular methods can identify cagA and vacA genotypes of H. pylori; however, these methods require cultured strains or gastric biopsy specimens and thus are not suitable for routine clinical use or most epidemiologic studies. Serology-based detection methods have been used to assess CagA and VacA status on the basis of the presence of anti-CagA or anti-VacA antibodies, but their accuracy has been questioned (2, 5, 7, 8). We evaluated the efficacy of a new immunoblot assay (Helicoblot [HB] 2.1; Genelabs Diagnostics, Singapore) for the diagnosis of H. pylori infection and determination of the CagA and VacA status of H. pylori.
We studied serum samples from 222 patients (120 patients from Japan and 102 from the United States). The Japanese patients were 30 H. pylori-negative patients and 90 H. pylori-infected patients, including 10 infected with CagA-negative strains. The U.S. patients consisted of 25 H. pylori-negative and 77 H. pylori-infected patients, including 20 infected with CagA-negative strains and 57 infected with CagA-positive strains. Informed consent was obtained from all patients, and the hospital ethics committees approved the protocols by which the specimens were obtained.
The “gold standard” definition of H. pylori infection included histology, culture, and serology (HM-CAP; Enteric Product Inc., Westbury, N.Y.). All three tests were required to be positive for H. pylori-positive patients and negative for H. pylori-negative patients. CagA status was based on the results of an immunoblot assay of H. pylori cultured from each patient and used a polyclonal antibody prepared by immunizing a mouse with recombinant CagA (Acambis Inc., Cambridge, Mass.) as previously described (9). VacA status was evaluated by detecting in vitro vacuolating cytotoxin activity as previously described (10). For determination of relative vacuolating cytotoxin activity, concentrated (approximately 20-fold) culture supernatants were serially diluted (from 1:1 to 1:128 in 2-fold increments) in Vero cell culture medium. The relative vacuolating cytotoxin activity was expressed as the maximum dilution score yielding more than 50% Vero cell vacuolation (1 [1:1] to 8 [1:128]). OipA status was based on the oipA gene switch status as previously described (11, 12).
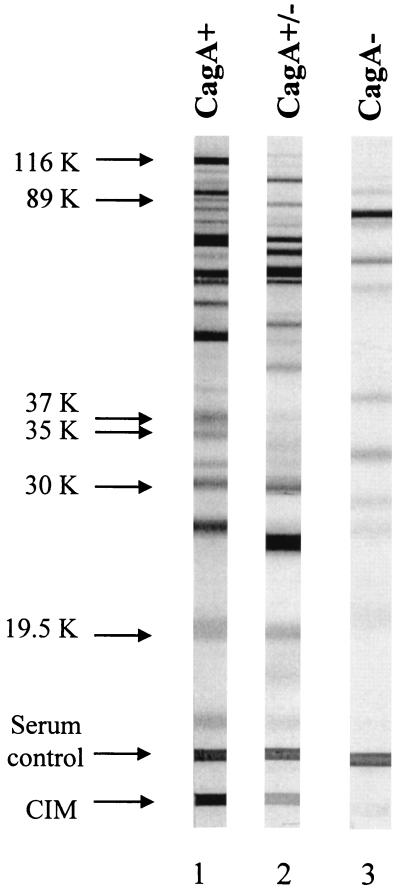
Serum samples were analyzed by two immunoblot assays (HB 2.0 and HB 2.1; Genelabs Diagnostics) in accordance with the manufacturer's instructions. Both immunoblots used whole-cell H. pylori antigens but from different H. pylori isolates. HB 2.0 contained antigens with molecular weights of 19,500, 26,500, 30,000, 35,000, 89,000 (VacA), and 116,000 (CagA) derived from H. pylori strain NCTC 11916. HB 2.1 contained antigens with molecular weights of 19,500, 30,000, 35,000, 37,000, 89,000 (VacA), and 116,000 (CagA) from H. pylori strain ATCC 49503. For HB 2.0, a positive result was defined as the presence of a single band at an Mr of 116,000, 89,000, or 35,000 or any two bands from among the 30,000-, 26,500-, and 19,500-Mr antigens. Patient serum samples that showed no reactivity or in which the pattern of bands did not meet these criteria were reported as negative. HB 2.1 contained an additional antigen line, designated the current infection marker (CIM) (Fig. 1). The CIM had been originally identified by screening of immunogenic proteins of H. pylori and was synthesized by recombinant technology (4). The CIM was located at the bottom of the strip as an independent band; the location did not correspond to the molecular mass.
FIG. 1.
Examples of CagA band immunoblot patterns obtained by using serum samples with HB 2.1. Lanes: 1, CagA-positive case (very prominent higher-molecular-weight band of the doublet bands around an Mr of 116,000); 2, cases of CagA inconsistency between our criteria and the manufacturer's criteria (the doublet bands are faint, and the higher-molecular-weight band is not prominent compared with the lower-molecular-weight band); 3, CagA-negative case (no prominent band around an Mr of 116,000). The serum control band serves as a check for serum and reagent addition in the assay.
The HB 2.1 criteria for H. pylori seropositivity were (i) a positive result for the 116,000-Mr band and one or more of the 89,000-, 37,000-, 35,000-, 30,000-, and 19,500-Mr bands together or along with the presence of the CIM; (ii) the presence of the 89,000-, 37,000-, or 35,000-Mr band; and (iii) the presence of both the 30,000- and 19,500-Mr bands. Because interobserver variation in the interpretation of these immunoblots had been previously reported (1), all of the strips were sent to the manufacturer for interpretation by an investigator who remained blinded to the sample origin. All strips were also interpreted by one of us (Y.Y.) in accordance with the instruction manual.
With the gold standard and the manufacturer's criteria, the overall sensitivity and specificity for H. pylori status were 99 and 98% for HB 2.1 and 95 and 89% for HB 2.0, respectively (Table 1). The CIM as a single marker of H. pylori infection was not superior to the immunoblot considered without it (HB 2.1). The CIM yielded sensitivity and specificity values of >90%, irrespective of the region of origin of the sample, consistent with a report that a rapid test incorporating the CIM had sensitivity and specificity values of 94 and 90%, respectively, when tested on 148 patients, 78 of whom were H. pylori infected (3).
TABLE 1.
Accuracy of immunoblot assays for H. pylori status
| Test | Samples | Infection status | Gold standard |
Kappa | % Sensitivity | % Specificity | % PPVa | % NPVb | |
|---|---|---|---|---|---|---|---|---|---|
| H. pylori+ | H. pylori− | ||||||||
| HB 2.0 | Total | H. pylori+ | 159 | 6 | 0.780 | 95 | 89 | 96 | 86 |
| H. pylori− | 8 | 49 | |||||||
| Japan | H. pylori+ | 87 | 3 | 0.822 | 97 | 90 | 97 | 90 | |
| H. pylori− | 3 | 27 | |||||||
| United States | H. pylori+ | 72 | 3 | 0.734 | 94 | 88 | 96 | 81 | |
| H. pylori− | 5 | 22 | |||||||
| HB 2.1 | Total | H. pylori+ | 165 | 1 | 0.929 | 99 | 98 | 99 | 96 |
| H. pylori− | 2 | 54 | |||||||
| Japan | H. pylori+ | 89 | 1 | 0.892 | 99 | 97 | 99 | 97 | |
| H. pylori− | 1 | 29 | |||||||
| United States | H. pylori+ | 76 | 0 | 0.974 | 99 | 100 | 100 | 99 | |
| H. pylori− | 1 | 25 | |||||||
| CIM | Total | H. pylori+ | 155 | 2 | 0.841 | 93 | 96 | 99 | 82 |
| H. pylori− | 12 | 53 | |||||||
| Japan | H. pylori+ | 83 | 0 | 0.856 | 92 | 100 | 100 | 81 | |
| H. pylori− | 7 | 30 | |||||||
| United States | H. pylori+ | 72 | 2 | 0.822 | 94 | 92 | 97 | 82 | |
| H. pylori− | 5 | 23 | |||||||
PPV, positive predictive value.
NPV, negative predictive value.
With the presence of CagA protein from the clinical isolates as the gold standard, the overall sensitivity and specificity of HB 2.0 for CagA were both 90%, with the interpretations by the local investigator and the manufacturer being entirely consistent. With HB 2.1, there were doublet bands near an Mr of 116,000 (higher- and lower-molecular-weight bands) (Fig. 1). The higher-molecular-weight band correlated with the presence of CagA, and CagA status was considered positive by the manufacturer only if the higher-molecular-weight band was prominent (Matthew Mak, Genelabs Diagnostics, personal communication). As this is a judgment call requiring experience, we scored serum samples as CagA positive if the higher-molecular-weight band was present, irrespective of its intensity. With these criteria, the sensitivity was 99% but the specificity was only 53%, which differed markedly from the readings at the manufacturer, where the sensitivity and specificity were 99 and 90%, respectively. Importantly, of the 19 CagA-negative isolates studied, 3 (15.7%) were incorrectly categorized by the HB 2.1 immunoblot. If the CagA band was considered alone, there were 9 (16%) false-positive CagA cases among the 55 H. pylori-negative cases. Use of the CagA positivity criteria requiring both the presence of CagA and that of one or more of the H. pylori proteins improved the specificity of HB 2.1 for H. pylori status to 98% (54 of 55). Figueiredo et al. (1) also reported the sensitivity and specificity of HB 2.1 for CagA status in a Dutch population to be only 78.6 and 54.2%, respectively. Bands were often present in the molecular mass range of CagA (e.g., 16%) among CagA-negative cases, and these may represent cross-reaction with antigens of other bacteria with similar molecular weights.
With vacuolating cytotoxin activity as the gold standard, the sensitivity of HB 2.1 for the 89,000-Mr VacA antigen was 93% and the specificity was 88% (Table 2). There were no inconsistencies between the analysis done by us and that done by the manufacturer. We also examined the relationship between the concentration of in vitro vacuolating cytotoxin activities and the intensity of the VacA band. The intensity of the VacA band was scored compared with that of the VacA band with the H. pylori-positive control samples provided by the kits (scores: 1, the intensity of the band was faint; 2, the intensity of the band was clear but weaker than that of the control band; 3, the intensity of the band was similar to or stronger than that of the control band). Spearman's correlation coefficient (r) was 0.86 within VacA band-positive cases. Overall, detection and measurement of the activity of VacA by HB 2.1 was simple and accurate, obviating endoscopy with biopsy and in vitro assays of vacuolating cytotoxic activity.
TABLE 2.
Accuracy of serology for each putative virulence factor of H. pylori
| Test | Samples | Antigen status | Gold standard |
Kappa | % Sensitivity | % Specificity | % PPVa | % NPVb | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CagA+ | CagA− | Vacuolating toxin+ | Vacuolating toxin− | oipA on | oipA off | ||||||||
| HB 2.0 | Total | CagA+ | 123 | 3 | 0.26 | 90 | 90 | 98 | 66 | ||||
| CagA− | 14 | 27 | |||||||||||
| Japan | CagA+ | 70 | 2 | 0.5 | 88 | 80 | 97 | 44 | |||||
| CagA− | 10 | 8 | |||||||||||
| United States | CagA+ | 53 | 1 | 0.84 | 93 | 95 | 98 | 83 | |||||
| CagA− | 4 | 19 | |||||||||||
| Total | VacA+ | 70 | 5 | 0.47 | 60 | 90 | 93 | 49 | |||||
| VacA− | 47 | 45 | |||||||||||
| Japan | VacA+ | 23 | 0 | 0.21 | 34 | 100 | 92 | 41 | |||||
| VacA− | 44 | 23 | |||||||||||
| United States | VacA+ | 47 | 5 | 0.77 | 94 | 82 | 90 | 79 | |||||
| VacA− | 3 | 22 | |||||||||||
| Total | OipA+ | 93 | 20 | 0.25 | 68 | 33 | 82 | 19 | |||||
| OipA− | 44 | 10 | |||||||||||
| Japan | OipA+ | 68 | 6 | 0.2 | 85 | 40 | 92 | 25 | |||||
| OipA− | 12 | 4 | |||||||||||
| United States | OipA+ | 25 | 14 | 0.2 | 67 | 55 | 81 | 37 | |||||
| OipA− | 32 | 6 | |||||||||||
| HB 2.1 | Total | CagA+ | 135 | 3 | 0.54/0.69c | 99/99 | 53/90 | 91/98 | 89/93 | ||||
| CagA− | 2 | 16 | |||||||||||
| CagA±d | 11 | ||||||||||||
| Japan | CagA+ | 80 | 1 | 0.88/0.94 | 100/100 | 80/90 | 99/99 | 100/100 | |||||
| CagA− | 0 | 8 | |||||||||||
| CagA± | 0 | 1 | |||||||||||
| United States | CagA+ | 55 | 2 | 0.44/0.87 | 96/96 | 40/90 | 82/96 | 80/90 | |||||
| CagA− | 2 | 8 | |||||||||||
| CagA± | 10 | ||||||||||||
| Total | VacA+ | 109 | 6 | 0.63 | 93 | 88 | 95 | 85 | |||||
| VacA− | 8 | 44 | |||||||||||
| Japan | VacA+ | 64 | 2 | 0.83 | 96 | 91 | 90 | 84 | |||||
| VacA− | 3 | 21 | |||||||||||
| United States | VacA+ | 45 | 4 | 0.75 | 90 | 85 | 94 | 83 | |||||
| VacA− | 5 | 23 | |||||||||||
| Total | OipA+ | 69 | 20 | 0.12 | 50 | 33 | 78 | 13 | |||||
| OipA− | 68 | 10 | |||||||||||
| Japan | OipA+ | 44 | 6 | 0.07 | 55 | 40 | 88 | 10 | |||||
| OipA− | 36 | 4 | |||||||||||
| United States | OipA+ | 25 | 14 | 0.2 | 44 | 30 | 64 | 16 | |||||
| OipA− | 32 | 6 | |||||||||||
PPV, positive predictive value.
NPV, negative predictive value.
Values when we used our local criteria/when we used the manufacturer's criteria.
±, inconsistent bands; we regarded this as CagA positive by our criteria and CagA negative by the manufacturer's criteria.
Recently, the 34,000-Mr outer inflammatory protein OipA has been described as a new putative virulence factor (11, 12). We were unable to confirm the recent speculation that the 35,000-Mr antigen seen with the HB immunoblot might represent OipA (6). With oipA gene “on” status as the gold standard for the presence of OipA, the overall sensitivity of the 35,000-Mr antigen was 68% with HB 2.0 and 50% with HB 2.1, with low specificity (33%) (Table 2). These data are consistent with the notion that the 35,000-Mr antigen does not represent OipA or that OipA is not immunogenic.
Acknowledgments
This work was supported in part by the Office of Research and Development, Medical Research Service, Department of Veterans Affairs, and by Public Health Service grants DK53659 and DK56338, which fund the Texas Gulf Coast Digestive Diseases Center.
We acknowledge Matthew Mak and Theresa Chow (Genelabs Diagnostics) for fruitful discussion. Genelabs Diagnostics provided serological test kits used in this study.
REFERENCES
- 1.Figueiredo, C., W. Quint, N. Nouhan, H. van den Munckhof, P. Herbrink, J. Scherpenisse, W. de Boer, P. Schneeberger, G. Perez-Perez, M. Blaser, and L. J. van Doorn. 2001. Assessment of Helicobacter pylori vacA and cagA genotypes and host serological response. J. Clin. Microbiol. 39:1339-1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Graham, D. Y., R. M. Genta, D. P. Graham, and J. E. Crabtree. 1996. Serum CagA antibodies in asymptomatic subjects and patients with peptic ulcer: lack of correlation of IgG antibody in patients with peptic ulcer or asymptomatic Helicobacter pylori gastritis. J. Clin. Pathol. 49:829-832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hung, C. T., W. K. Leung, F. K. L. Chan, and J. Y. Sung. 2002. Comparison of two new rapid serology tests for diagnosis of Helicobacter pylori infection in Chinese patients. Digest. Liver Dis. 34:111-115. [DOI] [PubMed] [Google Scholar]
- 4.Leung, W. K., F. K. L. Chan, M. S. Falk, R. Suen, and J. J. Sung. 1998. Comparison of two rapid whole-blood tests for Helicobacter pylori infection in Chinese patients. J. Clin. Microbiol. 30:1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mitchell, H. M., S. L. Hazell, Y. Y. Li, and P. J. Hu. 1996. Serological response to specific Helicobacter pylori antigens: antibody against CagA antigen is not predictive of gastric cancer in a developing country. Am. J. Gastroenterol. 91:1785-1788. [PubMed] [Google Scholar]
- 6.Treiber, G. G., M. Schwabe, and P. Malfertheiner. 2001. H. pylori toxicity factors: the clinical significance of oip-A in comparison to cag-A. Gut 49(Suppl. A16):11.
- 7.Yamaoka, Y., T. Kodama, D. Y. Graham, and K. Kashima. 1998. Comparison of four serological tests to determine the CagA or VacA status of Helicobacter pylori strains. J. Clin. Microbiol. 36:11:3433-3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamaoka, Y., and D. Y. Graham. 1999. CagA status and gastric cancer unreliable serological tests produce unreliable data. Gastroenterology 117:745. [DOI] [PubMed] [Google Scholar]
- 9.Yamaoka, Y., T. Kodama, K. Kashima, D. Y. Graham, and A. R. Sepulveda. 1998. Variants of the 3′ region of the cagA gene in Helicobacter pylori isolates from different H. pylori-associated diseases. J. Clin. Microbiol. 36:2258-2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamaoka, Y., T. Kodama, M. Kita, J. Imanishi, K. Kashima, and D. Y. Graham. 1998. Relationship of vacA genotypes of Helicobacter pylori to cagA status, cytotoxin production, and clinical outcome. Helicobacter 4:241-253. [DOI] [PubMed] [Google Scholar]
- 11.Yamaoka. Y., D. H. Kwon, and D. Y. Graham. 2000. A Mr 34,000 proinflammatory outer membrane protein (oipA) of Helicobacter pylori. Proc. Natl. Acad. Sci. USA 97:7533-7538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamaoka, Y., S. Kikuchi, H. M. El-Zimaity., O. Gutierrez., M. S. Osato., and D. Y. Graham. 2002. Importance of Helicobacter pylori oipA in clinical presentation, gastric inflammation, and mucosal interleukin 8 production. Gastroenterology 123:414-424. [DOI] [PubMed] [Google Scholar]