Abstract
The recombinant truncated ORF2 (capsid) antigen derived from the Meng strain of swine hepatitis E virus (HEV) differs from that of the Sar-55 strain of human HEV by approximately 5% at the amino acid level. Serial serum samples from two chimpanzees and six rhesus monkeys experimentally infected with HEV were tested with one enzyme immunoassay (EIA) based on the Sar-55 antigen and with a second EIA based on the Meng antigen. We obtained 98% agreement (κ = 0.952) by direct comparison. The virtually identical results obtained with these antigens in detecting seroconversion following infection with HEV suggests that they were reacting with antibodies that detect the same or very similar epitopes of HEV. We then tested human and swine serum samples for anti-HEV in EIAs that utilized one or the other of the two ORF2 antigens and showed that these results were also virtually identical. The specimens tested included swine sera from the United States, Canada, China, Korea, and Thailand and sera from veterinarians, U.S. and non-U.S. volunteer blood donors, and U.S. and non-U.S. animal handlers. We tested 792 swine sera and obtained 93% agreement (κ = 0.839). We similarly tested 882 human sera and obtained 99% agreement (κ = 0.938). Moreover, we found virtually no difference in the levels of prevalence of anti-HEV as measured by the two tests, again suggesting that the antigens derived from human and swine HEV contain the same immunodominant epitopes.
Hepatitis E, which was previously described as epidemic waterborne hepatitis (2, 5), is an acute, self-limiting viral disease. The etiological agent is a single-stranded positive-sense RNA virus that is not enveloped (30). The viral RNA is approximately 7.2 kb in size and contains three partially overlapping open reading frames (ORFs) (30). ORF1 encodes nonstructural proteins, while ORF2 encodes the capsid protein (35) and ORF3 encodes a cytoskeleton-associated phosphoprotein (42). The virus was originally placed within the family Caliciviridae but is presently unclassified (4).
Hepatitis E is an important public health problem in developing countries and a common cause of epidemics. The virus is transmitted primarily via the fecal-oral (20, 21, 30), and large epidemics of hepatitis E have been linked to contaminated water and poor public health conditions (2, 20, 40). In general, onset of symptoms occurs about 28 to 36 days postinfection (3, 36).
Hepatitis E virus is similar to hepatitis A virus with regard to transmission, virulence, and epidemiology, although some reports attribute higher morbidity and mortality to hepatitis E in developing countries (5, 20, 22). Thus, hepatitis E in most individuals is a mild disease, except in pregnant women (particularly in the third trimester), for whom mortality rates as high as 20% have been reported (3, 6, 16).
Hepatitis E is endemic in parts of Asia and northern Africa, and one epidemic was reported in Mexico (7, 10, 12, 17, 22, 41, 43). Hepatitis E has been recognized with increasing frequency in industrialized countries and other areas where infection by hepatitis E virus (HEV) was not thought to be endemic (10, 13, 33). It has been suggested that HEV disease might be zoonotic both in regions of endemicity and in regions where HEV disease is nonendemic (8, 9, 18, 27, 29). Rats and domestic animals such as sheep, cattle, and pigs all appear to be reservoirs of the virus, which might explain the presence of anti-HEV in people with no obvious source of contact with human strains of the virus (11, 18).
Presently, four genotypes of HEV which infect mammals are recognized. The virus infecting swine in the United States was the first animal strain of HEV to be characterized, and it defined a new genotype, genotype 3, which differs from genotypes 1, 2, and 4 by 25.6 to 26.3%, 25.3 to 25.5%, and 23.7 to 24.7%, respectively, at the nucleotide level (39). Subsequently, other genotype 3 HEV strains were recovered from humans in the United States (10, 33) and elsewhere (34). Recently, Haqshenas and colleagues sequenced the ORF2 gene of a newly discovered avian HEV and found that it shared only approximately 47 to 51% homology with genotype 1 strains at the nucleotide level (15). This virus has not been further characterized or classified.
In industrialized countries, the number of HEV infections is probably underestimated (23). There is a genuine need for sensitive, specific, reproducible, and standardized assays to detect markers of HEV infection. Ghabrah et al. suggested that ORF2-based enzyme immunoassays (EIAs) performed better than ORF3-based EIAs (14). Mast and colleagues compared 12 tests for the detection of anti-HEV (23). The results demonstrated both the great disparity in levels of sensitivity and specificity among the different tests as well as the superior sensitivity and specificity of EIAs that used recombinant ORF2-derived capture antigens compared to those of other tests. Cloning of the genotype 3 swine HEV genome by Meng et al. and the subsequent expression of the recombinant ORF2 protein made it possible to develop an EIA for swine antibodies to genotype 3 HEV which was similar to an anti-HEV assay based on a genotype 1 strain used for detection of antibodies in humans (28, 38).
The goal of this study was to evaluate and compare a pair of EIAs for the detection of antibodies to HEV in human and swine sera. Though we tested only swine, nonhuman primate, and human sera, these results likely apply to other species since it is reported that the ORF2 epitopes are broadly reactive across species and strains (1, 19, 24). The assays we describe here are virtually the same but for the capture antigen each employs, namely, a truncated portion of the ORF2 gene product from a strain of swine HEV and a truncated portion from a strain of human HEV. The human strain is the genotype 1 Pakistani Sar-55 strain (7), and the swine strain is the genotype 3 U.S. Meng strain (28).
MATERIALS AND METHODS
Serum samples.
Serial weekly serum samples from two chimpanzees and six rhesus monkeys experimentally infected with HEV were tested with both assays. The chimpanzees were infected with the Pakistani human strain (Sar-55), representing genotype 1, and the rhesus monkeys were infected with the Mexican human strain of HEV, representing genotype 2; the Meng swine strain, representing genotype 3 (26, 27); a U.S. human strain, representing genotype 3 (33); or a Chinese human strain, representing genotype 4 (39).
Another sample set consisted of 792 pig sera (360 samples from the United States, 152 from Canada, 30 from China, 190 from Korea, and 60 from Thailand) and 882 human sera (230 samples from U.S. volunteer blood donors, 603 from U.S. pig handlers, 18 from Thai animal handlers, and 31 from blood bank volunteers in China) (25, 29). Overall, specimens were obtained in areas where HEV genotypes 1, 3, and possibly 4 predominate (34). All samples were unlinked from the identity of their donors, and their use had been previously approved by the Institutional Review Board of Virginia Polytechnic Institute and State University.
Antigen preparation and purification.
The putative HEV capsid protein (ORF2) was expressed from a recombinant baculovirus in insect cells (Sf9) (32, 38). The 72-kDa full-length product was processed in the cells to yield a 63-kDa peptide, a 56-kDa peptide, and a 53-kDa peptide. The 56-kDa antigen was used in the EIA and was purified by anion exchange and gel filtration chromatography (32). The 56-kDa products of the human and swine strains contained amino acids 112 to 607 (496 amino acids) and 112 to 602 (491 amino acids), respectively. Sequence similarity at the amino acid level for these antigens was 95.1%.
EIA for the detection of anti-HEV immunoglobulin G (IgG) in swine and humans.
We used a modification of the EIA described by Tsarev et al. (38). Polystyrene microwell plates (catalog no. 76-381-04; ICN, Costa Mesa, Calif.) were incubated with ORF2 antigen diluted in a carbonate-bicarbonate (pH 9.6) buffer for 18 h at room temperature. The antigen concentration was 0.05 μg/well for the human strain and 0.03 μg/well for the swine strain. The optimal concentrations of capture antigen were established by block titration using an anti-HEV-positive chimpanzee convalescent-phase serum and an anti-HEV-positive swine hyperimmune serum. The wells were washed twice in an automated plate washer with a commercially available wash solution (Kirkegaard & Perry, Gaithersburg, Md.) containing 0.02% Tween 20 in 0.002 M imidazole-buffered saline. The wells were blocked with bovine serum albumin-gelatin for 1 h at 37°C prior to freezing at −20°C in plastic bags. Immediately before use, the blocking buffer was removed and the plates were washed twice with wash buffer as described above.
Ten microliters of each test and control sample was diluted at a ratio of 1:10. The sample was further diluted at a ratio of 1:10 into an antigen-coated test plate (final test dilution, 1:100) and incubated for 30 min at 37°C. Wells were washed five times, and 100 μl of horseradish peroxidase-labeled anti-IgG (Kirkegaard & Perry, Gaithersburg, Md.) was added to each well. The horseradish peroxidase-labeled secondary antibodies were species-specific anti-IgG (heavy and light chain) and were used at a concentration of 1.0 μg/ml. Following a 30-min incubation at 37°C, unbound conjugate was removed by washing five times as described above. 2,2′-azino-di-[3-ethylbenzthiazoline sulfate (6)] (ABTS) substrate was added for color development, and absorbance (405 nm) was read after 30 min.
The cutoff for the swine antigen EIA was established for each test from internal controls and throughout this study ranged between 0.300 and 0.383, with a median of 0.330 (28). The positive cutoff for the human Sar-55 antigen EIA was similarly established (38) and ranged between 0.300 and 0.342 in this study. Previously tested negative blood bank samples, dilution buffer, and preinoculation swine sera served as negative controls.
Statistical analysis.
Calculations for determination of concordance and prevalence were carried out by using the Windows version of S-Plus software as an add-on to Microsoft Excel.
RESULTS
Development of anti-HEV in nonhuman primates as measured by both assays following infection.
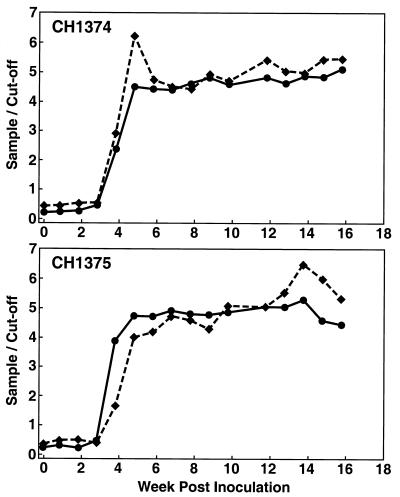
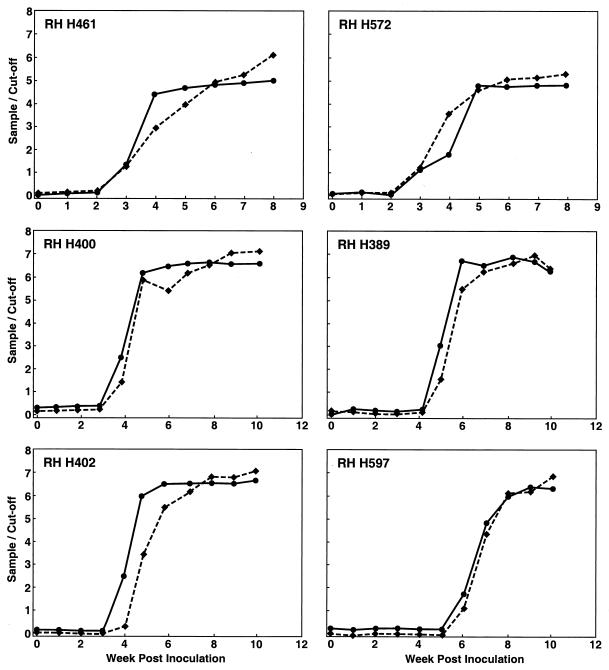
Serial samples from two chimpanzees experimentally infected with the Sar-55 (genotype 1) HEV strain (Fig. 1) and six rhesus monkeys experimentally infected with the Mex-14 (genotype 2) strain, the Meng swine (genotype 3) strain, the US-2 (genotype 3) strain, or a Chinese (genotype 4) HEV strain (Fig. 2) were tested with both EIAs. Very similar values were obtained regardless of whether the capture antigen in the EIA was from the genotype 1 or genotype 3 strain. Agreement of these two sets of data was 98% (κ = 0.952). In all cases, seroconversion was detected at the appropriate time and the patterns of antibody positivity were as expected for a normal infection, thus validating each assay.
FIG. 1.
Anti-HEV IgG responses of two chimpanzees (CH1374 and CH1375) experimentally infected with the Pakistani (genotype 1) strain Sar-55. Anti-HEV was measured by EIAs with capsid antigen generated from the Sar-55 (solid line) and Meng (dashed line) HEV strains. The values are expressed as sample-over-cutoff ratios, and 1.00 is the positive baseline value.
FIG. 2.
Anti-HEV IgG responses of six rhesus monkeys (RH H389, RH H400, RH H402, RH H461, RH H572, and RH H597) experimentally infected with the Mexican (genotype 2) strain Mex-14 (RH H461 and RH H572), swine (genotype 3) strain Meng (RH H400), U.S. (genotype 3) strain US2 (RH H389), and Taiwan (genotype 4) strain 1380 (RH H402 and RH H597) of HEV. Anti-HEV was measured and symbols are defined as described in the legend to Fig. 1.
Seroprevalence of anti-HEV in human serum or plasma samples as determined by both assays.
Human sera or plasma from areas of HEV endemicity and nonendemicity were tested with both EIAs. The overall prevalence of anti-HEV in the human samples was virtually the same regardless of the capture antigen. Prevalence was 13% when evaluated with the human capture antigen versus 12% when evaluated with the swine capture antigen (Table 1). Furthermore, the prevalence values for each of the subgroups were practically equal.
TABLE 1.
Anti-HEV prevalence in human sera as determined by genotype 1 or genotype 3 antigen capture EIAs
| Sample source | No. tested | No. (%) positive for antibody reactive with indicated antigen |
|
|---|---|---|---|
| Sar-55 (genotype 1) | Meng (genotype 3) | ||
| Non-U.S. pig handlers | 18 | 12 (67) | 12 (67) |
| U.S. pig handlers | 603 | 63 (10) | 58 (10) |
| Non-U.S. blood donors | 31 | 5 (16) | 5 (16) |
| U.S. blood bank volunteers | 230 | 31 (13) | 35 (15) |
| Total | 882 | 111 (13) | 110 (12) |
There was a 99% concordance (κ = 0.938) when data from human sera or plasma tested with the human and swine ORF2-coated capture plates were compared (Table 2). Comparisons between data obtained from the two EIAs for non-U.S. pig handlers and blood donors each showed 100% agreement, and comparisons of results for U.S. volunteer blood donors and pig handlers yielded concordance values of 97% (κ = 0.894) and 99% (κ = 0.936), respectively. Therefore, the two antigens yielded virtually identical results in tests of anti-HEV in human sera.
TABLE 2.
Contingency table comparing results of tests on human serum or plasma with the Sar-55 ORF2 and the Meng ORF2 capture antigensa
| Meng ORF2 test result | Result (no.) of Sar-55 ORF2-test |
||
|---|---|---|---|
| Negative | Positive | Total | |
| Negative | 765 | 7 | 772 |
| Positive | 6 | 104 | 110 |
| Total | 771 | 111 | 882 |
Concordance = 99%, as calculated by dividing the sum of concordant values by the sum total. κ value = 0.938.
Seroprevalence of HEV in swine as determined by both assays.
Anti-HEV prevalence in swine sera was also measured by the two EIAs. Once again, the results with the two capture antigens agreed. The EIAs based on genotype 1 and genotype 3 antigens yielded 37% and 35% prevalence, respectively (Table 3).
TABLE 3.
Anti-HEV prevalence in swine sera as determined by genotype 1 or genotype 3 antigen capture EIAs
| Sample source | No. tested | No. (%) positive for antibody reactive with indicated antigen |
|
|---|---|---|---|
| Sar-55 (genotype 1) | Meng (genotype 3) | ||
| United States | 360 | 66 (18) | 69 (19) |
| Canada | 152 | 95 (63) | 86 (57) |
| China | 30 | 5 (17) | 3 (10) |
| Korea | 190 | 97 (51) | 89 (47) |
| Thailand | 60 | 29 (48) | 34 (57) |
| Total | 792 | 292 (37) | 281 (35) |
As seen in Table 4, comparison of test results for swine sera yielded a concordance value of 93% (κ = 0.839). Independently, the subgroups that made up the swine serum set yielded concordance values of 96% (κ = 0.882) for the United States, 86% (κ = 0.714) for Canada, 91% (κ = 0.811) for Korea, 92% (κ = 0.834) for Thailand, and 93% (κ = 0.714) for China. These data demonstrate the comparable ability of each of the capture antigens to identify anti-HEV in swine serum.
TABLE 4.
Contingency table comparing results of tests on swine serum with the Sar-55 ORF2 and the Meng ORF2 capture antigensa
| Meng ORF2 test result | Result (no.) of Sar-55 ORF2-test |
||
|---|---|---|---|
| Negative | Positive | Total | |
| Negative | 476 | 35 | 511 |
| Positive | 24 | 257 | 281 |
| Total | 500 | 292 | 792 |
Concordance = 93%. κ value = 0.839.
DISCUSSION
The almost identical seroconversion profiles obtained with the two EIAs when used to test sera from the experimentally infected nonhuman primates indicated that (i) each assay could reliably discriminate between the absence and presence of anti-HEV and (ii) each antigen reacted with similar levels of sensitivity with antibodies to different genotypes (genotypes 1, 2, 3, and 4). Since the capture antigens were from genotype 1 and genotype 3 strains, the data suggested that both EIAs were effective for assaying antibodies against viruses representing all four of the recognized genotypes.
EIAs are practical, low-cost diagnostic tools to assess markers of infection that lend themselves naturally to seroepidemiological studies. EIAs are particularly useful methods for tracking HEV infections. Several other methods for documentation of HEV infection are available in the form of Western blot assays, PCR protocols, and immune electron microscopy (31, 37), but each of these methods is more cumbersome or less sensitive than EIAs.
EIAs are very sensitive; the best assays are specific, but questions continue to be asked about the high prevalence of antibodies to HEV that are detected by some of the EIAs and whether one antigen is equally able to detect antibodies against different HEVs. Mast et al. convincingly demonstrated that recombinant ORF2-derived capture antigen EIAs could detect anti-HEV in human and chimpanzee sera, and Meng et al. showed the same for swine sera (23, 28).
To address these questions in more depth, we prepared an ORF2 antigen from swine HEV and compared its ability to detect antibody with that of our standard antigen derived from a human strain. We then tested the ability of these EIAs to detect seroconversion following experimental infection of primates with HEVs representing all four genotypes and strains isolated from humans and swine.
The results of the EIAs showed that the antigens were interchangeable with respect to their ability to detect anti-HEV. Therefore, each of these antigens must share important epitopes with the others and it is likely we need only one diagnostic antigen and, by inference, probably only one vaccine to neutralize effectively any genotype of HEV.
Acknowledgments
We thank S. Dea of Centre de Microbiologie et Biotechnologie, INRS-Institut Armand Frappier, Universite du Quebec, Laval, Quebec, Canada, D. Yoo of the Ontario Veterinary College, University of Guelph, Ontario, Canada, and Y. S. Lyoo of the College of Veterinary Medicine, KonKuk University, Seoul, Korea, for swine serum. We thank T. Sirinarumtr and K. Urairong of the Faculty of Veterinary Medicine, Kasetstart University, Kamphaengsaen, Thailand, and D. Wang of the National Control Institute of Veterinary Bioproducts and Pharmaceuticals, Beijing, China, for swine and pig handler samples. Finally, we are grateful to B. Wiseman of the Department of Biomedical Sciences and Pathobiology, College of Veterinary Medicine, Virginia Polytechnic Institute and State University, Blacksburg, Virginia, for swine veterinarian serum samples.
REFERENCES
- 1.Anderson, D. A., F. Li, M. Riddell, T. Howard, H. F. Seow, J. Torresi, G. Perry, D. Sumarsidi, S. M. Shrestha, and I. L. Shrestha. 1999. ELISA for IgG-class antibody to hepatitis E virus based on a highly conserved, conformational epitope expressed in Escherichia coli. J. Virol. Methods 81:131-142. [DOI] [PubMed] [Google Scholar]
- 2.Arankalle, V. A., M. S. Chadha, S. A. Tsarev, S. U. Emerson, A. R. Risbud, K. Banerjee, and R. H. Purcell. 1994. Seroepidemiology of water-borne hepatitis in India and evidence for a third enterically-transmitted hepatitis agent. Proc. Natl. Acad. Sci. USA 91:3428-3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balayan, M. S., A. G. Andjaparidze, S. S. Savinskaya, E. S. Ketiladze, D. M. Braginsky, A. P. Savinov, and V. F. Poleschuk. 1983. Evidence for a virus in non-A, non-B hepatitis transmitted via the fecal-oral route. Intervirology 20:23-31. [DOI] [PubMed] [Google Scholar]
- 4.Berke, T., and D. O. Matson. 2000. Reclassification of the Caliciviridae into distinct genera and exclusion of hepatitis E virus from the family on the basis of comparative phylogenetic analysis. Arch. Virol. 145:1421-1436. [DOI] [PubMed] [Google Scholar]
- 5.Bradley, D., K. Krawczynski, E. H. Cook, K. A. McCaustland, B. H. Robertson, M. A. Kane, J. Spelbring, C. D. Humphrey, I. Weisfuse, A. Andjaparidze, M. Balayan, H. Stetler, and O. Velazquez. 1988. Enterically transmitted non-A, non-B hepatitis: etiology of disease and laboratory studies in non-human primates, p. 138-147. In A. J. Zuckerman (ed.), Viral hepatitis and liver disease. Alan R. Liss, Inc., New York, N.Y.
- 6.Bradley, D. W. 1990. Enterically-transmitted non-A, non-B hepatitis. Br. Med. Bull. 46:442-461. [DOI] [PubMed] [Google Scholar]
- 7.Bryan, J. P., S. A. Tsarev, M. Iqbal, J. Ticehurst, S. Emerson, A. Ahmed, J. Duncan, A. R. Rafiqui, I. A. Malik, R. H. Purcell, and L. J. Legters. 1994. Epidemic hepatitis E in Pakistan: patterns of serologic response and evidence that antibody to hepatitis E virus protects against disease. J. Infect. Dis. 170: 517-521. [DOI] [PubMed] [Google Scholar]
- 8.Clayson, E. T., B. L. Innis, K. S. Myint, S. Narupiti, D. W. Vaughn, S. Giri, P. Ranabhat, and M. P. Shrestha. 1995. Detection of hepatitis E virus infections among domestic swine in the Kathmandu Valley of Nepal. Am. J. Trop. Med. Hyg. 53:228-232. [DOI] [PubMed] [Google Scholar]
- 9.Emerson, S. U., and R. H. Purcell. 2001. Recombinant vaccines for hepatitis E. Trends Mol. Med. 7:462-466. [DOI] [PubMed] [Google Scholar]
- 10.Erker, J. C., S. M. Desai, G. G. Schlauder, G. J. Dawson, and I. K. Mushahwar. 1999. A hepatitis E virus variant from the United States: molecular characterization and transmission in cynomolgus macaques. J. Gen. Virol. 80:681-690. [DOI] [PubMed] [Google Scholar]
- 11.Favorov, M. O., M. Y. Kosoy, S. A. Tsarev, J. E. Childs, and H. S. Margolis. 2000. Prevalence of antibody to hepatitis E virus among rodents in the United States. J. Infect. Dis. 181: 449-455. [DOI] [PubMed] [Google Scholar]
- 12.Fix, A. D., M. Abdel-Hamid, R. H. Purcell, M. H. Shehata, F. Abdel-Aziz, N. Mikhail, H. el Sebai, M. Nafeh, M. Habib, R. R. Arthur, S. U. Emerson, and G. T. Strickland. 2000. Prevalence of antibodies to hepatitis E in two rural Egyptian communities. Am. J. Trop. Med. Hyg. 62:519-523. [DOI] [PubMed] [Google Scholar]
- 13.Garkavenko, O., A. Obriadina, J. Meng, D. A. Anderson, H. J. Benard, B. A. Schroeder, Y. E. Khudyakov, H. A. Fields, and M. C. Croxson. 2001. Detection and characterisation of swine hepatitis E virus in New Zealand. J. Med. Virol. 65:525-529. [PubMed] [Google Scholar]
- 14.Ghabrah, T. M., S. Tsarev, P. O. Yarbough, S. U. Emerson, G. T. Strickland, and R. H. Purcell. 1998. Comparison of tests for antibody to hepatitis E virus. J. Med. Virol. 55:134-137. [PubMed] [Google Scholar]
- 15.Haqshenas, G., H. L. Shivaprasad, P. R. Woolcock, D. H. Read, and X. J. Meng. 2001. Genetic identification and characterization of a novel virus related to human hepatitis E virus from chickens with hepatitis-splenomegaly syndrome in the United States. J. Gen. Virol. 82:2449-2462. [DOI] [PubMed] [Google Scholar]
- 16.Hussaini, S. H., S. J. Skidmore, P. Richardson, L. M. Sherratt, B. T. Cooper, and J. G. O'Grady. 1997. Severe hepatitis E infection during pregnancy. J. Viral Hepat. 4:51-54. [DOI] [PubMed] [Google Scholar]
- 17.Hyams, K. C., M. A. Purdy, M. Kaur, M. C. McCarthy, M. A. Hussain, A. el-Tigani, K. Krawczynski, D. W. Bradley, and M. Carl. 1992. Acute sporadic hepatitis E in Sudanese children: analysis based on a new western blot assay. J. Infect. Dis. 165: 1001-1005. [DOI] [PubMed] [Google Scholar]
- 18.Kabrane-Lazizi, Y., J. B. Fine, J. Elm, G. E. Glass, H. Higa, A. Diwan, C. J. Gibbs, Jr., X. J. Meng, S. U. Emerson, and R. H. Purcell. 1999. Evidence for widespread infection of wild rats with hepatitis E virus in the United States. Am. J. Trop. Med. Hyg. 61:331-335. [DOI] [PubMed] [Google Scholar]
- 19.Khudyakov, Y. E., E. N. Lopareva, D. L. Jue, T. K. Crews, S. P. Thyagarajan, and H. A. Fields. 1999. Antigenic domains of the open reading frame 2-encoded protein of hepatitis E virus. J. Clin. Microbiol. 37:2863-2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khuroo, M. S. 1980. Study of an epidemic of non-A, non-B hepatitis. Possibility of another human hepatitis virus distinct from post-transfusion non-A, non-B type. Am. J. Med. 68:818-824. [DOI] [PubMed] [Google Scholar]
- 21.Krawczynski, K., and D. W. Bradley. 1989. Enterically transmitted non-A, non-B hepatitis: identification of virus-associated antigen in experimentally infected cynomolgus macaques. J. Infect. Dis. 159: 1042-1049. [DOI] [PubMed] [Google Scholar]
- 22.Mast, E. E., and M. J. Alter. 1993. Epidemiology of viral hepatitis: an overview. Semin. Virol. 4:273-283. [Google Scholar]
- 23.Mast, E. E., M. J. Alter, P. V. Holland, R. H. Purcell, et al. 1998. Evaluation of assays for antibody to hepatitis E virus by a serum panel. Hepatology 27:857-861. [DOI] [PubMed] [Google Scholar]
- 24.Meng, J., X. Dai, J. C. Chang, E. Lopareva, J. Pillot, H. A. Fields, and Y. E. Khudyakov. 2001. Identification and characterization of the neutralization epitope(s) of the hepatitis E virus. Virology 288:203-211. [DOI] [PubMed] [Google Scholar]
- 25.Meng, X. J., S. Dea, R. E. Engle, R. Friendship, Y. S. Lyoo, T. Sirinarumitr, K. Urairong, D. Wang, D. Wong, D. Yoo, Y. Zhang, R. H. Purcell, and S. U. Emerson. 1999. Prevalence of antibodies to the hepatitis E virus in pigs from countries where hepatitis E is common or is rare in the human population. J. Med. Virol. 59:297-302. [PubMed] [Google Scholar]
- 26.Meng, X. J., P. G. Halbur, J. S. Haynes, T. S. Tsareva, J. D. Bruna, R. L. Royer, R. H. Purcell, and S. U. Emerson. 1998. Experimental infection of pigs with the newly identified swine hepatitis E virus (swine HEV), but not with human strains of HEV. Arch. Virol. 143:1405-1415. [DOI] [PubMed] [Google Scholar]
- 27.Meng, X. J., P. G. Halbur, M. S. Shapiro, S. Govindarajan, J. D. Bruna, I. K. Mushahwar, R. H. Purcell, and S. U. Emerson. 1998. Genetic and experimental evidence for cross-species infection by swine hepatitis E virus. J. Virol. 72:9714-9721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meng, X. J., R. H. Purcell, P. G. Halbur, J. R. Lehman, D. M. Webb, T. S. Tsareva, J. S. Haynes, B. J. Thacker, and S. U. Emerson. 1997. A novel virus in swine is closely related to the human hepatitis E virus. Proc. Natl. Acad. Sci. USA 94:9860-9865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meng, X. J., B. Wiseman, F. Elvinger, D. K. Guenette, T. E. Toth, R. E. Engle, S. U. Emerson, and R. H. Purcell. 2002. Prevalence of antibodies to hepatitis E virus in veterinarians working with swine and in normal blood donors in the United States and other countries. J. Clin. Microbiol. 40:117-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Purcell, R. H., and S. U. Emerson. 2001. Hepatitis E virus, p. 3051-3061. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed., vol. 2. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 31.Purdy, M. A., K. A. McCaustland, K. Krawczynski, A. Tam, M. J. Beach, N. C. Tassopoulos, G. R. Reyes, and D. W. Bradley. 1992. Expression of a hepatitis E virus (HEV)-trpE fusion protein containing epitopes recognized by antibodies in sera from human cases and experimentally infected primates. Arch. Virol. 123:335-349. [DOI] [PubMed] [Google Scholar]
- 32.Robinson, R. A., W. H. Burgess, S. U. Emerson, R. S. Leibowitz, S. A. Sosnovtseva, S. Tsarev, and R. H. Purcell. 1998. Structural characterization of recombinant hepatitis E virus ORF2 proteins in baculovirus-infected insect cells. Protein Expr. Purif. 12:75-84. [DOI] [PubMed] [Google Scholar]
- 33.Schlauder, G. G., G. J. Dawson, J. C. Erker, P. Y. Kwo, M. F. Knigge, D. L. Smalley, J. E. Rosenblatt, S. M. Desai, and I. K. Mushahwar. 1998. The sequence and phylogenetic analysis of a novel hepatitis E virus isolated from a patient with acute hepatitis reported in the United States. J. Gen. Virol. 79:447-456. [DOI] [PubMed] [Google Scholar]
- 34.Schlauder, G. G., and I. K. Mushahwar. 2001. Genetic heterogeneity of hepatitis E virus. J. Med. Virol. 65:282-292. [DOI] [PubMed] [Google Scholar]
- 35.Tam, A. W., M. M. Smith, M. E. Guerra, C. C. Huang, D. W. Bradley, K. E. Fry, and G. R. Reyes. 1991. Hepatitis E virus (HEV): molecular cloning and sequencing of the full-length viral genome. Virology 185:120-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tsarev, S. A., S. U. Emerson, T. S. Tsareva, P. O. Yarbough, M. Lewis, S. Govindarajan, G. R. Reyes, M. Shapiro, and R. H. Purcell. 1993. Variation in course of hepatitis E in experimentally infected cynomolgus monkeys. J. Infect. Dis. 167: 1302-1306. [DOI] [PubMed] [Google Scholar]
- 37.Tsarev, S. A., T. S. Tsareva, S. U. Emerson, S. Govindarajan, M. Shapiro, J. L. Gerin, and R. H. Purcell. 1994. Successful passive and active immunization of cynomolgus monkeys against hepatitis E. Proc. Natl. Acad. Sci. USA 91:10198-10202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsarev, S. A., T. S. Tsareva, S. U. Emerson, A. Z. Kapikian, J. Ticehurst, W. London, and R. H. Purcell. 1993. ELISA for antibody to hepatitis E virus (HEV) based on complete open-reading frame-2 protein expressed in insect cells: identification of HEV infection in primates. J. Infect. Dis. 168:369-378. [DOI] [PubMed] [Google Scholar]
- 39.Wang, Y., H. Zhang, R. Ling, H. Li, and T. J. Harrison. 2000. The complete sequence of hepatitis E virus genotype 4 reveals an alternative strategy for translation of open reading frames 2 and 3. J. Gen. Virol. 81:1675-1686. [DOI] [PubMed] [Google Scholar]
- 40.Wong, D. C., R. H. Purcell, M. A. Sreenivasan, S. R. Prasad, and K. M. Pavri. 1980. Epidemic and endemic hepatitis in India: evidence for a non-A, non-B hepatitis virus aetiology. Lancet ii:876-879. [DOI] [PubMed]
- 41.Yarbough, P. O., A. W. Tam, K. E. Fry, K. Krawczynski, K. A. McCaustland, D. W. Bradley, and G. R. Reyes. 1991. Hepatitis E virus: identification of type-common epitopes. J. Virol. 65:5790-5797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zafrullah, M., M. H. Ozdener, S. K. Panda, and S. Jameel. 1997. The ORF3 protein of hepatitis E virus is a phosphoprotein that associates with the cytoskeleton. J. Virol. 71:9045-9053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zanetti, A. R., G. J. Dawson, et al. 1994. Hepatitis type E in Italy: a seroepidemiological survey. J. Med. Virol. 42:318-320. [DOI] [PubMed] [Google Scholar]