Abstract
While dengue virus is thought to replicate in mononuclear phagocytic cells in vivo, attempts to detect it in peripheral blood mononuclear cells (PBMC) by virus isolation or antigen detection have had variable and generally low rates. In this study, we developed a reverse transcription (RT)-real-time PCR assay to quantify positive- and negative-sense RNA of dengue virus type 2 within the cells. The assay includes an RT step using either sense or antisense primer followed by a real-time PCR step using the designed primers and probe, which target a capsid region highly conserved in dengue virus type 2 strains. It can be used to monitor the dynamic change of intracellular dengue virus RNA species during the course of infection. When this assay is employed in quantification of dengue virus RNA species in PBMC from 10 patients infected with dengue virus type 2, both positive- and negative-sense dengue RNA can be detected, indicating that dengue virus is actively replicating in PBMC in vivo. Moreover, the amounts of negative-sense dengue virus RNA in PBMC correlate very well with the viral load of dengue virus in plasma, suggesting that quantification of negative-sense dengue virus RNA in PBMC may provide another indicator of dengue virus replication in vivo. Use of this convenient, sensitive, and accurate method of quantification in clinical samples from patients with different disease severity would further our understanding of the pathogenesis of dengue.
Dengue virus belongs to the genus Flavivirus of the family Flaviviridae. It contains a positive-sense single-stranded RNA genome of approximately 11 kb (9, 22). Flanked by the two nontranslated regions at both ends, the single open reading frame consists of three structural genes at the 5′ one-fourth and seven nonstructural genes at the 3′ three-fourths. Among the 80 or so arthropod-borne flaviviruses, epidemics of infection by the four serotypes of dengue virus (DEN-1, DEN-2, DEN-3, and DEN-4) continue to be a major public health problem in tropical and subtropical areas (4, 9, 14, 25). It has been estimated that approximately 100 million dengue infections occur annually worldwide (9, 11, 25).
The clinical presentations of dengue virus infection range from asymptomatic, or a mild self-limited illness, dengue fever (DF), to a severe and potentially life-threatening disease, dengue hemorrhage fever/dengue shock syndrome (DHF/DSS) (9, 14, 37). Following an incubation period of 3 to 14 days, fever and a variety of symptoms occur, coinciding with the appearance of dengue virus in blood (9, 14). Based on the pathological findings in experimentally infected rhesus monkeys and in humans with fatal infections, dengue virus is thought to replicate in mononuclear phagocytic cells in vivo (1, 2, 9, 10, 11, 14, 23, 26). Several studies have attempted to detect dengue virus in peripheral blood mononuclear cells (PBMC). Scott et al. isolated dengue virus from peripheral blood leukocytes in 76 (22.9%) of 332 patients with acute dengue infection (30). Similarly, Blok et al. isolated dengue virus from PMBC during the acute phase in 13 (18.8%) out of 69 patients (3). Using both virus isolation and fluorescent-antibody staining methods to examine PBMC from 19 patients with acute dengue infection, Waterman et al. reported an isolation rate of 15.8% and antigen detection rate of 5.3% (36). Kittigul et al. developed an enzyme-linked immunosorbent assay (ELISA) to detect dengue virus antigen in PBMC in 53.8% of cases (15). Overall, the detection rates of dengue virus in PBMC through either virus isolation or antigen detection method were variable and generally low (3, 15, 30, 36).
Recently, several reverse transcription-PCR (RT-PCR)-based methods, which are both rapid and sensitive, have been developed for the detection or quantification of dengue virus in plasma (6, 12, 13, 19, 21, 22, 27, 31, 34, 35). In this study, we developed a sensitive and convenient RT-real-time PCR assay to quantify dengue virus RNA species in PBMC from infected patients. Both positive- and negative-sense dengue virus RNA were detected in PBMC from all 10 DEN-2 patients examined, indicating active replication of dengue virus in PBMC in vivo. Moreover, our findings suggest that the amounts of negative-sense dengue virus RNA in PBMC may indicate the level of dengue virus replication in vivo.
MATERIALS AND METHODS
Study participants.
The diagnosis of DF or DHF followed the World Health Organization clinical definition (37). Ten DF patients from the Yuan General Hospital during an outbreak in 2001 in Kaohsiung, a city in southern Taiwan, were included in this study. The day of onset of fever (oral temperature, ≥38°C) is defined as day 1 of illness (d1). Acute-phase blood samples were collected in EDTA-containing tubes between days 1 and 5 of illness. Plasma was prepared within 6 h of collection and stored at −80°C until use (35). PMBC were prepared using the Ficoll-Paque Plus isolation solution (Amersham Pharmacia Biotech, Uppsala, Sweden), washed four times with phosphate-buffered saline (PBS), and then resuspended in RPMI 1640 (Gibco/BRL, Life Technologies) containing 30% fetal calf serum (FCS), penicillin (100 U/ml), streptomycin (100 μg/ml), and 10% dimethyl sulfoxide, and stored in liquid nitrogen until use. The serotypes of the viruses infecting 10 dengue patients were all determined to be DEN-2 by using a previously described RT-PCR assay, which can distinguish the four dengue virus serotypes by the sizes of the products (12, 19).
Virus infection assay.
BHK cells were seeded on a 24-well plate (105 cells/well) in minimal essential medium (MEM) (Gibco/BRL, Life Technologies) plus 10% FCS at 37°C overnight. The DEN-2 virus, 16681 strain, was added to each well at a multiplicity of infection (MOI) of 0.1 in quadruplicate and incubated at 37°C for 2 h. After the wells were washed with PBS four times, MEM plus 2% FCS was added to each well in a total volume of 1 ml. Culture supernatants were collected on days 1, 3, 5, and 7 postinfection. At the same time point, cells from one well were washed four times with PBS, trypsinized, and counted. For infection of PBMC, 3.5 × 105 PBMC from a healthy donor were infected with the DEN-2 16681 strain at MOI of 1.0 and incubated at 37°C for 2 h. After the cells were washed with PBS four times, RPMI 1640 plus 10% FCS was added to a final volume of 2 ml in a six-well plate. One-fourth of the culture was removed on each of day 1, 3, 5, and 7 postinfection. Supernatants were collected after centrifugation at 1,500 rpm on a model A-4-62 rotor (Eppendorf, Hamburg, Germany) for 10 min, and cells were washed four times with PBS and counted.
Plaque assay.
BHK cells were seeded in a 24-well plate in MEM plus 10% FCS at 37°C overnight until confluence. Culture supernatants were serially diluted and added to each well in triplicates. After incubation at 37°C for 2 h, medium containing MEM and 1% methyl cellulose (1:1) (Sigma, St. Louis, Mo.) with 2% FCS was added, and the plates were incubated at 37°C for 7 days. Plaques were counted after fixation with 3.7% formaldehyde, removal of an agarose plug, and staining with 1% crystal violet solution in 20% methanol.
Isolation of viral RNA.
Dengue virus RNA was isolated from aliquots of culture supernatants or plasma, using the QIAamp viral RNA mini kit (Qiagen, Hilden, Germany) to a final volume of 50 μl (35). Plasma samples obtained from two hepatitis C virus (HCV) carriers and two dengue-naive healthy donors were subjected to RNA isolation. RNA was also isolated from stock viruses of the four dengue serotypes, the Hawaii (DEN-1), New Guinea (DEN-2), H-87 (DEN-3), and H-241 (DEN-4) strains, as well as from three Japanese encephalitis virus strains as described previously (35). Total RNA was isolated from a known number of dengue virus-infected cells or PBMCs from dengue patients and dengue-naive healthy donors by using the RNeasy mini kit to a final volume of 50 μl (Qiagen). A commercial immunoglobulin M and immunoglobulin G capture ELISA (PanBio Dengue Duo, Brisbane, Australia) was used to check sera of healthy donors to identify the dengue-naive donors (32).
Primer design.
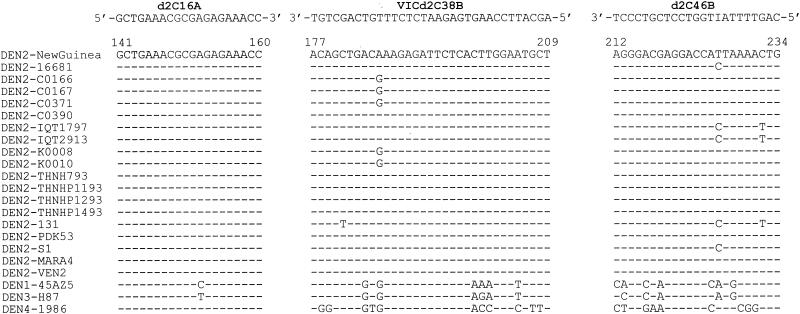
Through an analysis of all dengue virus sequences available in GenBank, a region in the capsid gene that is highly conserved in DEN-2 but not in other serotypes or other flaviviruses was identified. A primer pair, d2C16A and d2C46B, and a flurogenic probe, VICd2C38B, in this region were thus designed (Fig. 1).
FIG. 1.
Alignment of the designed primers and probe with DEN-2 sequences available in GenBank and representative DEN-1, DEN-3, and DEN-4 sequences. The genome positions according to the DEN-2 Jamaica strain (5) are shown at the top. Dashes indicate identity.
Generation of the construct and RNA.
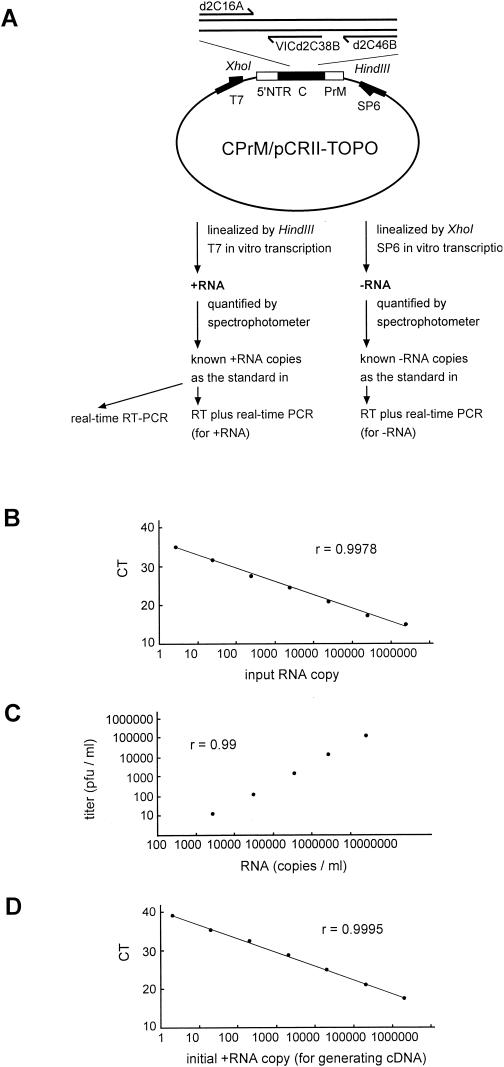
CPrM/pCRII-TOPO contains the entire capsid gene and the N-terminal 54 amino acids of the precursor membrane (PrM) region of the DEN-2 strain PL046 in the vector pCRII-TOPO (Invitrogen, San Diego, Calif.) (35) (see Fig. 2A). The in vitro- transcribed positive- sense RNA generated by T7 transcription (Promega, Madison, Wis.) of the HindIII-linealized CPrM/pCRII-TOPO was purified by phenol-chloroform extraction and quantified by spectrophotometry as described previously (35). The copy number of the positive-sense RNA was calculated based on the concentration measured and its molecular weight. The in vitro-transcribed negative-sense RNA was generated by SP6 transcription of the XhoI-linealized CPrM/pCRII-TOPO and quantified by spectrophotometry (see Fig. 2A). The copy number of negative-sense RNA was thus calculated.
FIG.2.
(A) Schematic diagram of the construct, CPrM/pCRII-TOPO, and the protocol used in generating the positive-sense RNA (+RNA) and negative-sense RNA (−RNA) as standards for the real-time RT-PCR and the RT-real-time PCR assays. The relative positions of the primers and probe are shown. (B) Relationship of known input RNA copies to the threshold cycle (CT) in the real-time RT-PCR assay. (C) Relationship between the RNA copy number determined by the real-time RT-PCR assay (copies per milliliter) and the virus titer (PFU per milliliter). RNA templates derived from serial 10-fold dilutions of the DEN-2 New Guinea virus were subjected to the real-time RT-PCR assay. (D) Relationship between the initial positive-sense RNA copies used in generating cDNA and the threshold cycle (CT) in the real-time PCR assay. r is the correlation coefficient.
Real-time RT-PCR and RT-real-time PCR assays.
Real-time RT-PCR or RT-real-time PCR was performed in a separate room from that used for RNA isolation, and precautions for PCR were followed to avoid contamination (18). A real-time RT-PCR assay was developed for quantification of dengue virus RNA in culture supernatants or plasma. An aliquot (2.5 μl) of purified RNA and known amounts of positive-sense RNA (2.5, 25, 250, 2,500, 25,000, 250,000, and 2,500,000 copies) were subjected to real-time RT-PCR using the designed primers (d2C16A and d2C46B) and probe (VICd2C38B), and the TaqMan one-step RT-PCR master mix reagent kit (PE Biosystems, Foster City, Calif.). The amplification conditions were 48°C for 30 min and 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min, as recommended by the manufacturer. The ABI Prism 7700 sequence detector was used to analyze the emitted fluorescence during amplification. A positive result is defined by the cycle number (CT value) required to reach the threshold, which is 10 times the standard deviation of the mean baseline emission calculated for PCR cycles 3 to 15. Since 2.5 μl of the 50-μl RNA eluates, which were derived from 140 μl of culture supernatant or plasma, was used in each reaction, the number of dengue virus RNA copies per reaction was divided by 7 μl (140 μl × 2.5 μl/50 μl) and multiplied by 1,000 to determine the number of RNA copies per ml of supernatant or plasma. The sensitivity of the assay is 2.5 copies of RNA per reaction, corresponding to 357 copies per ml of plasma or supernatant.
An RT-real-time PCR assay was developed to quantify intracellular positive-sense or negative-sense dengue RNA by using different primers in the RT step. For quantification of positive-sense dengue RNA, an aliquot (2 μl) of total RNA isolated from dengue virus-infected or uninfected cells and known amounts of positive-sense RNA (2, 20, 200, 2,000, 20,000, 200,000, and 2,000,000 copies) were subjected to RT using a previously described antisense primer, C69B (35), and a cDNA synthesis kit (Life Technologies, Rockville, Md.). For quantification of negative-sense dengue virus RNA, an aliquot (2 μl) of total RNA and known amounts of negative-sense RNA (2 to 2,000,000 copies) were subjected to RT using a sense primer C14A (35). An aliquot (2 μl) of the cDNA was then subjected to real-time PCR using the designed primers and probe, the TaqMan universal PCR master mix reagent kit (PE Biosystems), and the ABI prism 7700 sequence detector. The amplification conditions were 50°C for 2 min and 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. The amounts of intracellular positive- and negative-sense dengue virus RNA were determined by the copy number of positive- and negative-sense RNA, respectively, divided by the number of cells.
Experimental design.
The real-time RT-PCR assay was first established to quantify DEN-2 stock virus and DEN-2 viruses produced in supernatants during the course of infection. The RT-real-time PCR assay was then employed to quantify the positive- and negative-sense dengue virus RNA in DEN-2 virus-infected cells in vitro (BHK cells and PBMC) as well as in PBMC from patients with dengue infection.
RESULTS
Establishment of a real-time RT-PCR assay.
A closer examination of dengue virus sequences available in GenBank revealed a small region in the capsid gene that was highly conserved in isolates of DEN-2 viruses but not in other serotypes or other flaviviruses. To establish a real-time RT-PCR assay for DEN-2 virus, a primer pair, d2C16A and d2C46B, and a fluorescent probe, VICd2C38B, in this region were designed (Fig. 1).
The feasibility of the real-time RT-PCR assay was examined first. Known amounts of the in vitro-transcribed positive-sense RNA, were subjected to real-time RT-PCR using the designed primers and probe and the Taqman one-step RT-PCR protocol. As shown in Fig. 2B, a linear curve was obtained as the amount of input positive-sense RNA increased from 2.5 copies to 2,500,000 copies per reaction. To evaluate this assay in quantification of DEN-2 virus, dengue virus RNA isolated from a DEN-2 virus, the New Guinea strain, as well as from other serotypes including DEN-1 (Hawaii), DEN-3 (H-87), and DEN-4 (H-241), was subjected to the analysis. A positive signal was detected in the reaction containing the RNA templates derived from DEN-2 virus but not in those derived from other serotypes (Fig. 2C and data not shown). Only a background signal was detected in the reactions containing no RNA template or RNA templates derived from other flaviviruses prevalent in Taiwan, including Japanese encephalitis virus and HCV, and from the plasma of two healthy individuals (data not shown). These results were consistent with our primer design and indicated that the real-time RT-PCR assay can quantify DEN-2 virus but not other serotypes or the two flaviviruses tested. Serial 10-fold dilutions of the DEN-2 New Guinea virus were subjected to viral RNA isolation, and the viral RNA was subjected to the real-time RT-PCR assay. A linear relationship was found between the determined RNA copy number per milliliter of supernatant and the virus titer (PFU per milliliter), indicating the accuracy of this assay (correlation coefficient, r = 0.99) (Fig. 2C).
Quantification of DEN-2 virus by the real-time RT-PCR assay during the course of infection is shown in Fig. 3. While only background signals were detected in the supernatants of the mock-infected cells, the amounts of dengue virus RNA in the supernatants of infected cells increased and peaked on day 3. Quantification of the culture supernatants by the plaque assay revealed a similar growth kinetic curve (Fig. 3). These results demonstrate the applicability of this assay in monitoring viral replication during the course of infection.
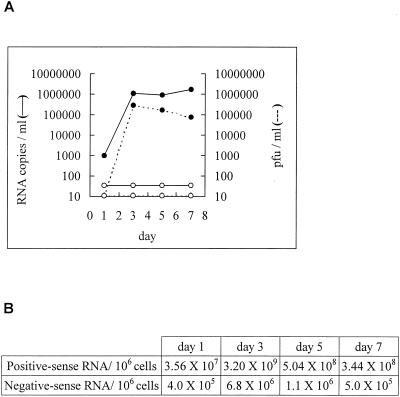
FIG. 3.
Replication kinetics of DEN-2 virus in BHK cells. Quantification of dengue virus in the culture supernatants (A) and dengue virus RNA species within the cells (B) is shown. BHK cells were infected with DEN-2 virus (16681 strain) at MOI of 0.1, and culture supernatants collected at different time points were subjected to a plaque assay and real-time RT-PCR assay. Solid circles: DEN-2 virus infections; open circles: mock infections. Cells were counted and subjected to the RT-real-time PCR assay. Amounts of positive- and negative-sense RNA per million cells are shown.
Quantification of intracellular dengue virus RNA species by an RT-real-time PCR assay.
As shown in Fig. 2D, a linear standard curve was obtained as the input cDNA derived from 2 copies of positive-sense RNA increased to that derived from 2,000,000 copies. DEN-2 virus-infected BHK cells were collected, and the amount of intracellular positive-sense dengue RNA was determined by the copy number of positive-sense RNA divided by the number of cells. As shown in Fig. 3B, the amount of positive-sense dengue virus RNA in the infected BHK cells increased from 35.6 copies per cell on day 1 to 3,200 copies per cells on day 3, in parallel with the growth kinetic curve. Only background signals were detected in the reaction containing cDNA derived from mock-infected cells or from negative-sense RNA as the control (data not shown).
In the quantification of negative-sense dengue virus RNA, a linear standard curve was also obtained as the input cDNA derived from negative-sense RNA increased (data not shown). In agreement with a previous report, the amount of intracellular negative-sense dengue virus RNA, which was determined by the copy number of negative-sense RNA divided by the number of cells, was smaller than that of the positive-sense dengue RNA (7). As shown in Fig. 3B, intracellular negative-sense dengue virus RNA increased from 4 × 105 copies per million cells on day 1 to 6.8 × 106 copies per million cells on day 3, consistent with the increase in the amount of positive-sense dengue virus RNA and the growth kinetic curve. Only background signals were detected in the reaction containing cDNA derived from mock-infected cells or from positive-strand RNA as the control (data not shown).
Quantification of dengue virus RNA species in dengue virus-infected PBMC.
The RT-real-time PCR assay was also employed in quantification of dengue virus RNA species within primary cells infected by DEN-2 virus in vitro. The amount of intracellular positive-sense dengue RNA peaked on day 1 (261 copies per cell) and decreased gradually (data not shown). Similarly, the intracellular negative-sense dengue virus RNA peaked on day 1 (1.45 × 106 copies per million cells) and then decreased. Quantification of dengue virus in the supernatants revealed a similar trend, except that the growth kinetic curve peaks on day 3, lagging 2 days behind the intracellular dengue RNA species (data not shown).
Quantification of dengue virus RNA species in PBMC from dengue patients.
As shown in Table 1, positive- and negative-sense dengue virus RNAs were detected in PBMC from all 10 dengue patients but not in PBMC from dengue-naive healthy donors (data not shown). In agreement with the experiments of in vitro infection of PBMC, the amounts of intracellular positive-sense dengue RNA, ranging from 5,400 to 1.1 × 106 copies per 106 cells, were larger than those for the negative-sense RNA (ranging from 40 to 11000 copies per million cells). Examination of the amounts of intracellular dengue virus RNA species and plasma viral load revealed a good correlation between the amount of the negative-sense dengue RNA in PBMC and the level of dengue virus RNA in plasma (correlation coefficient, r = 0.96), suggesting that intracellular negative-sense dengue virus RNA in PBMC may indicate the extent of dengue virus replication in vivo.
TABLE 1.
Quantification of plasma and intracellular dengue virus RNA in dengue patients
| Patient ID | Sampling daya | Amt of plasma RNAb (copies/ml) | Amt of positive-sense RNAc (copies/106 PBMC) | Amt of negative-sense RNAc (copies/106 PBMC) |
|---|---|---|---|---|
| P10 | d2 | 3.93 × 105 | 3.85 × 104 | 1.00 × 103 |
| P2 | d2 | 3.72 × 106 | 5.51 × 104 | 2.40 × 103 |
| P3 | d1 | 7.28 × 105 | 4.71 × 104 | 1.90 × 103 |
| P4 | d1 | 3.17 × 105 | 1.45 × 105 | 1.50 × 103 |
| P5 | d2 | 7.72 × 106 | 1.10 × 106 | 1.10 × 104 |
| P6 | d2 | 4.09 × 105 | 6.59 × 104 | 3.00 × 103 |
| P7 | d2 | 3.85 × 106 | 8.91 × 104 | 3.20 × 103 |
| P8 | d4 | 8.95 × 104 | 1.31 × 104 | 4.00 × 102 |
| P9 | d4 | 9.15 × 103 | 1.00 × 104 | 1.00 × 102 |
| P10 | d5 | 3.08 × 103 | 5.40 × 103 | 4.0 × 101 |
The day of onset of fever is defined as day 1 (d1) of illness.
The amount of RNA is determined by the real-time RT-PCR assay.
The amount of RNA is determined by the RT-real-time PCR assay.
DISCUSSION
Although dengue virus replicates in a variety of cells in vitro, including cells of the myeloid, lymphoid, epithelial, endothelial, and fibroblastic lineages, the cell types supporting dengue virus replication in vivo are thought to be the cells of a mononuclear phagocyte lineage based on the pathological findings in monkeys and in fatal human cases (1, 2, 7, 9, 10, 11, 14, 17, 23, 26). Attempts to detect dengue virus in PBMC as a simple way of examining dengue virus replication in vivo have revealed variable and low rates of detection (3, 15, 30, 36). Using the sensitive RT-real-time PCR assay established in this study, we were able to detect and quantify both positive- and negative-sense dengue virus RNA within the PBMC from all 10 DEN-2 patients during the acute stage of infection. These findings demonstrate that dengue virus is actively replicating in PBMC. Moreover, a linear relationship was found between the amount of negative-sense dengue RNA in PBMC and viral load of dengue virus in plasma, suggesting that the amount of negative-sense dengue virus RNA in PBMC can be an indicator of the level of dengue virus replication in vivo.
The RT-real-time PCR assay, in combination with the real-time RT-PCR assay, which quantifies cell-free virus in the supernatants, was very useful in monitoring the dynamic changes of intracellular dengue virus RNA species and dengue virus released outside the cells during the course of infection. The replication kinetics observed correlate well with those based on the traditional plaque assay (Fig. 3). Of note was the observation that the RNA copy number in the supernatants was larger than the titer of the virus (PFU per milliliter) determined by plaque assay, with a ratio on the order of 1 to 2 log units. This was similar to what has been reported previously (13, 31, 35). The discrepancy could be due to the presence of genetically defective viruses resulting from the error-prone viral RNA polymerase, sensitivity of the flavivirus envelope to changes in the pH of the medium, or the instability of other viral components. Higher ratios of genome copy number to infectious unit have been observed in other viruses, such as human immunodeficiency virus type 1, where the ratios range from 104 to 107 (28).
The sensitivity of the RT-real-time PCR assay is 2 copies of positive- or negative-stranded per reaction. Consistent with our original primer design, the specificity of this assay for DEN-2 virus was revealed by real-time RT-PCR assay, which used the same primers and probe and can detect templates derived from DEN-2 virus but not those from other serotypes. Due to the limitation in the length of product (optimal: less than 150 bp) in the real-time PCR assay, we were not able to design a primer pair covering a region that is smaller than 150 bp and highly conserved by all four dengue virus serotypes. Using a similar approach, it is possible to develop different RT-real-time PCR assays for quantification of intracellular dengue virus RNA species for other serotypes. Compared to the previously reported asymmetric competitive RT-PCR assay (7), which requires the construction of both positive- and negative-sense competitor plasmids and RNA as well as replicate reactions, our RT-real-time PCR assay is simpler and more convenient and has a wider range of detection (Fig. 2D).
It has been reported recently that infection of different cells by dengue virus is modulated by cell types and viral strains (7). When the same DEN-2 strain (16681) was used, it was found that the levels of positive-sense and negative-sense dengue RNA in BHK cells detected in our study were slightly lower than those reported in HepG2 cells, another epithelial cell line (7). This could be due to the lower MOI (0.1) in our study compared to the MOI of 3 in that study. The levels and kinetics of intracellular positive-sense and negative-sense dengue virus RNA in the PBMC infection study were different from those in BHK cells or HepG2 cells (Fig. 3B and data not shown) (7). Different cell types and different MOI (1.0 in our PBMC infection assay) may account for the difference. We also compared the levels of plasma dengue virus load and viral RNA species in PBMC derived from dengue patients with the levels of dengue virus in culture supernatants and virus RNA species in PBMC that were infected in vitro. While the levels of dengue virus RNA in plasma on days 1 and 2 of infection (ranging from 7.72 × 106/ml to 3.17 × 105/ml for patients P1 to P7) were similar to the peak level in culture supernatants, the levels of intracellular dengue virus RNA species in PBMC from dengue patients were about 1 to 2 log units lower than those from in vitro infection (Table 1 and data not shown). These findings suggest that only a small fraction of circulating PBMC in dengue patients were infected by dengue virus. Mononuclear cells from tissues, such as macrophages, histiocytes, dendritic cells, skin Langerhans' cells, or Kupffer cells in liver, probably contributed to a large proportion of plasma dengue virus RNA (9, 14, 38). In this regard, it should be noted that infection of different cell types in vivo could vary by viral strain. Therefore, whether the correlation between the viral load of dengue virus in plasma and negative-sense dengue RNA in PBMC also holds true for other DEN-2 strains remains to be investigated in the future.
Accurate quantification of plasma viral loads has been successfully utilized in studies of several viral infectious diseases, such as human immunodeficiency virus type 1 and HCV, to assess the clinical status and response to therapy (20, 24). Several sensitive RT-PCR and virus isolation methods designed for quantification of dengue virus in plasma have shown recently that higher viral loads of dengue virus correlate with increased disease severity (27, 33). Our RT-real-time PCR assay that can quantify dengue virus RNA species within the cells would complement these assays and provide another important parameter. Applications of this assay in monitoring the sequential changes of dengue virus RNA species within PBMC or other clinical specimens from patients with different disease severity, in combination with measurements of the viral load of dengue virus in plasma and several other immune activation markers during the course of infection (8, 9, 14, 29), would shed new light on the pathogenesis of dengue viral infection.
Acknowledgments
We thank Yi-Ling Lin at the Institute of Biomedical Sciences, Academia Sinica, Taipei, Taiwan, for kindly providing plasmid CPrM/pCR3.1 for generating CPrM/pCRII-TOPO, and we thank Dai-Yu Chao, Hui-Ting Wang, and Su-Ru Lin for technical assistance. We also thank D. J. Gubler for the DEN-1 Hawaii strain and DEN-2 New Guinea strain, and we thank the Department of Health, Center for Disease Control, Taiwan, for the DEN-4 H-241 strain.
This work was supported in part by the National Science Council (NSC91-2320-B-002-191) and by the National Health Research Institute (NHRI-CN-CL8903P), Taiwan, Republic of China.
REFERENCES
- 1.Aung-Khin, M., K. Ma-Ma, Z. Thant, and M. Tin-U. 1975. Changes in the tissues of the immune system in dengue hemorrhagic fever. J. Trop. Med. Hyg. 47:256-261. [PubMed] [Google Scholar]
- 2.Bhamarapravati, H., P Tuchinda, and V. Boonyapaknavik. 1967. Pathology of Thailand hemorrhagic fever: a study of 100 autopsy cases. Ann. Trop. Med. Parasitol. 61:500-510. [DOI] [PubMed] [Google Scholar]
- 3.Blok, J., B. H. Kay, R. A. Hall, and B. M. Gorman. 1988. Isolation and characterization of dengue virus serotype 1 from an epidemic in northern Queensland, Australia. Arch Virol. 100:213-220. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control. 1994. Dengue surveillance—United States 1986-1992. Morb. Mortal. Wkly. Rep. 43(SS-2):7-19. [Google Scholar]
- 5.Deubel, V., R. M. Kinney, and D. W. Trent. 1986. Nucleotide sequence and deduced amino sequence of the structural proteins of dengue type 2 virus, jamaica genotype. Virology 155:365-377. [DOI] [PubMed] [Google Scholar]
- 6.Deubel, V. 1997. The contribution of molecular techniques to the diagnosis of dengue infection, p. 335-366. In D. J. Gubler and G. Kuno (ed.), Dengue and dengue hemorrhagic fever. CAB International, London, United Kingdom.
- 7.Diamond, M. S., D. Edgil, T. G. Roberts, B. Lu, and E. Harris. 2000. Infection of human cells by dengue virus is modulated by different cell types and viral strains. J. Virol. 74:7814-7823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Green, S., D. W. Vaughn, S. Kalayanarooj, S. Nimmannitya, S. Suntayakorn, A. Nisalak, R. Lew, B. L. Innis, I. Kurane, A. L. Rothman, and F. A. Ennis. 1999. Early immune activation in acute dengue illness is related to development of plasma leakage and disease severity. J. Infect. Dis. 179:755-762. [DOI] [PubMed] [Google Scholar]
- 9.Gubler, D. J. 1998. Dengue and dengue hemorrhagic fever. Clin. Microbiol. Rev. 11:480-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Halstead, S. B., and E. J. O'Rourke. 1977. Dengue viruses and mononuclear phagocytes. II. Identity of blood and tissue leukocytes supporting in vitro infection. J. Exp. Med. 146:218-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Halstead, S. B. 1988. Pathogenesis of dengue: challenges to molecular biology. Science 239:476-481. [DOI] [PubMed] [Google Scholar]
- 12.Harris, E., G. Roberts, L. Smith, J. Selle, L. D. Kramer, S. Valle, E. Sandoval, and A. Balmaseda. 1998. Typing of dengue viruses in clinical specimens and mosquitoes by singe-tube multiplex reverse transcriptase PCR. J. Clin. Microbiol. 36:2634-2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Houng, H. S. H., D. Hritz, and N. Kanesa-thasan. 2000. Quantitative detection of dengue 2 virus using fluorogenic RT-PCR based on 3′-noncoding sequence. J. Virol. Methods 86:1-11. [DOI] [PubMed] [Google Scholar]
- 14.Innis, B. L. 1995. Dengue and dengue hemorrhagic fever, p. 103-146. In J. S. Porterfield (ed.), Exotic viral infections—1995. Chapman & Hall, London, United Kingdom.
- 15.Kittigul, L., N. Meethien, D. Sujirarat, C. Kittigul, and S. Vasanavat. 1997. Comparison of dengue virus antigens in sera and peripheral blood mononuclear cells from dengue infected patients. Asian Pac. J. Allergy Immunol. 15:187-191. [PubMed] [Google Scholar]
- 16.Kuno, G., G. J. J. Chang, R. Tsuchiya, N. Karabatsos, and C. B. Cropp. 1998. Phylogeny of the genus Flavivirus. J. Virol. 72:73-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kurane I., U. Kontny, J. Janus, and F. A. Ennis. 1990. Dengue-2 virus infection of human mononuclear cell lines and establishment of persistent infections. Arch. Virol. 110:91-101. [DOI] [PubMed] [Google Scholar]
- 18.Kwok, S., and R. Higuchi. 1989. Avoiding false positive with PCR. Nature 339:237-238. [DOI] [PubMed] [Google Scholar]
- 19.Lanciotti, R. S., C. H. Calisher, D. J. Gubler, G-J. Chang, and A. V. Vorndam. 1992. Rapid detection and typing of dengue viruses from clinical samples by using reverse transcriptase-polymerase chain reaction. J. Clin. Microbiol. 30:545-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lau, J. Y. N., G. L. Davis, J. Kniffen, K. P. Qian, M. S. Urdea, C. S. Chan, M. Mizokami, P. D. Neuwald, and J. C. Wilber. 1993. Significance of serum hepatitis C virus RNA levels in chronic hepatitis C. Lancet 341:1501-1504. [DOI] [PubMed] [Google Scholar]
- 21.Laue, T., P. Emmerich, and H. Schmitz. 1999. Detection of dengue virus RNA in patients after primary or secondary dengue infection by using the TaqMan automated amplification system. J. Clin. Microbiol. 37:2543-2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lindenbach, B. D., and C. M. Rice. 2001. Flaviviridae: the viruses and their replication, p. 991-1041. In D. M. Knipe, P. M. Howley, D. E. Griffin, et al. (ed.), Fields virology, 4th ed. Lippincott William & Wilkins, Philadelphia, Pa.
- 23.Marchette, N. J., S. B. Halstead, W. A. Falkler, Jr., and D. Nash. 1973. Studies on the pathogenesis of dengue infection in monkeys. III. Sequential distribution of virus in primary and heterologous infections. J. Infect. Dis. 128:23-30. [DOI] [PubMed] [Google Scholar]
- 24.Mellors, J. W., C. R. Rinaldo, P. Gupta, R. M. White, J. A. Todd, and L. A. Kinsley. 1996. Prognosis in HIV-1 infection predicted by the quantity of virus in plasma. Science 272:1167-1170. [DOI] [PubMed] [Google Scholar]
- 25.Monath, T. P. 1994. Dengue, the risk to developed and developing countries. Proc. Natl. Acad. Sci. USA 91:2395-2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morens, D. M., N. J. Marchette, M. C. Chu, and S. B. Halstead. 1991. Growth of dengue type 2 virus isolates in human peripheral blood leukocytes correlates with severe and mild dengue disease. Am. J. Trop. Med. Hyg. 45:644-651. [DOI] [PubMed] [Google Scholar]
- 27.Murgue, B., C. Roche, E. Chungue, and X. Deparis. 2000. Prospective study of the duration of magnitude of viremia in children hospitalized during the 1996-1997 dengue-2 outbreak in French Polynesia. J. Med. Virol. 60:432-438. [DOI] [PubMed] [Google Scholar]
- 28.Piatak, M., M. S. Saag, L. C. Yang, S. J. Clark, J. C. Kappes, K. C. Luk, B. H. Hahn, G. M. Shaw, and J. D. Lifson. 1993. High levels of HIV-1 in plasma during all stages of infection determined by competitive PCR. Science 259:1749-1754. [DOI] [PubMed] [Google Scholar]
- 29.Rothman, A. L., and F. A. Ennis. 1999. Immunopathogenesis of dengue hemorrhagic fever. Virology 257:1-6. [DOI] [PubMed] [Google Scholar]
- 30.Scott, R. M., A. Nisalak, U. Cheamudon, S. Seridhoranakul, and S. Nimmannitya. 1980. Isolation of dengue viruses from peripheral blood leukocytes of patients with hemorrhagic fever. J. Infect. Dis. 141:1-6. [DOI] [PubMed] [Google Scholar]
- 31.Sudiro, T. M., H. Ishiko, S. Green, D. W. Vaughn, A. Nisalak, S. Kalayanarooj, A. L. Rothman, B. Raengsakulrach, J. Janus, I. Kurane, and F. A. Ennis. 1997. Rapid diagnosis of dengue viremia by reverse transcriptase-polymerase chain reaction using 3′-noncoding region universal primers. Am. J. Trop. Med. Hyg. 56:424-429. [DOI] [PubMed] [Google Scholar]
- 32.Vaughn, D. W., A. Nisalak, T. Solomon, S. Kalayanarooj, N. M. Dung, R. Kneen, A. Cuzzubbo, and P. L. Devine. 1999. Rapid serologic diagnosis of dengue virus infection using a commercial capture ELISA that distinguishes primary and secondary infections. Am. J. Trop. Med. Hyg. 60:693-698. [DOI] [PubMed] [Google Scholar]
- 33.Vaughn, D. W., S. Green, S. Kalayanarooj, B. L. Innis, S. Nimmannitya, S. Suntayakorn, T. P. Endy, B. Raengsakulrach, A. L. Rothman, F. A. Ennis, and A. Nisalak. 2000. Dengue viremia titer, antibody response pattern, and virus serotype correlate with disease severity. J. Infect. Dis. 181:2-9. [DOI] [PubMed] [Google Scholar]
- 34.Vorndam, V., and G. Kuno. 1997. Laboratory diagnosis of dengue virus infections, p. 313-334. In D. J. Gubler and G. Kuno (ed.), Dengue and dengue hemorrhagic fever. CAB International, London, United Kingdom.
- 35.Wang, W. K., C. N. Lee, C. L. Kao, Y. L. Lin, and C. C. King. 2000. Quantitative competitive reverse transcription-PCR for quantification of dengue virus RNA. J. Clin. Microbiol. 38:3306-3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Waterman, S. H., G. Kuno, D. J. Gubler, and G. E. Sather. 1985. Low rate of antigen detection and dengue virus isolation from the peripheral blood leukocytes of dengue fever patients. Am. J. Trop. Med. Hyg. 34:380-384. [DOI] [PubMed] [Google Scholar]
- 37.World Health Organization. 1997. Dengue hemorrhagic fever: diagnosis, treatment and control, 2nd ed. World Health Organization, Geneva, Switzerland.
- 38.Wu, S.-J. L., G. Grouard-Vogel, W. Sun, J. R. Mascola, E. Brachtel, R. Putvatana, M. K. Louder, L. Filgueira, M. A. Marovich, H. K. Wong, A. Blauvelt, G. S. Murphy, M. L. Robb, B. L. Innis, D. L. Birx, C. G. Hayes, and S. S. Frankel. 2000. Human skin Langerhans cells are targets of dengue virus infection. Nat. Med. 6:816-820. [DOI] [PubMed] [Google Scholar]