Abstract
Campylobacteriosis is a zoonotic disease in which birds have been suggested to play an important role as a reservoir. We investigated the prevalence of Campylobacter jejuni subsp. jejuni in black-headed gulls (Larus ridibundus) in southern Sweden with the aim of examining the nature of C. jejuni infection in this bird species. Birds were sampled in four sampling series each year during 1999 (n = 419) and 2000 (n = 365). Longitudinally sampled C. jejuni isolates from individual gulls were subjected to macrorestriction profiling (MRP) by pulsed-field gel electrophoresis to investigate the genotypical stability during the natural course of infection. Furthermore, a subset (n = 76) of black-headed gull isolates was compared to isolates from broiler chickens (n = 38) and humans (n = 56) originating from the same geographic area. We found a pronounced seasonal variation in C. jejuni carriage, with the highest rates found in late autumn. MRP similarities were higher between isolates of human and broiler chicken origin, than between those of wild bird origin and either of the other two hosts. However, identical MRPs were found in two gull isolates and one human isolate after digestion with two restriction enzymes, strongly indicating that they may have been colonized by the same clone of C. jejuni. The MRPs most prevalent in gull isolates did not occur among isolates from humans and broiler chickens, suggesting the existence of a subpopulation of C. jejuni adapted to species-specific colonization or environmental survival.
Campylobacter jejuni, the main human pathogen of the Campylobacter taxon, is regarded as the most common cause of food-borne bacterial enteritis in humans in many parts of the industrialized world (31). The main source of infection for sporadic cases of human campylobacteriosis is considered to be consumption of or contact with undercooked chicken or cross-contamination from raw chicken products to food that is consumed without further heating (14, 30). Through case control studies, other sources of infection (e.g., unpasteurized milk and contaminated drinking water) (16) have also been identified, but there are still many uncertainties about the epidemiology of Campylobacter infections (21).
In Sweden, strict hygienic procedures undertaken by producers and industry have resulted in a reduction of Campylobacter infections in broiler chickens from approximately 40 to 60% to <10% during the last 10 years (J. Lindblad, personal communication). Despite this decrease, the annual number of domestically acquired Campylobacter infections in humans has remained nearly unchanged over the last decade (1, 20). In most countries, human Campylobacter infections show a seasonal variation, with a peak during the warmer period of the year (13, 22)—in Sweden typically in late July through August (20). In broiler chickens, there is also a seasonal variation in rates of infection, but the peak occurs slightly later than in humans (4), the reverse of what would be expected if the main cause of the peak in human cases was the temporary increase in number of infected broiler chicken flocks.
It is known that many avian and mammalian species carry Campylobacter, and there seems to be a particularly strong association between Campylobacter and birds, both wild and domesticated (19, 35). Wild birds therefore could be possible reservoirs or vectors for human pathogenic C. jejuni, either directly or via poultry. Outbreaks in humans in the United Kingdom and Norway support this idea, with hundreds of people becoming ill after consumption of milk and drinking water contaminated by birds (27, 33). Nevertheless, relatively few studies have examined whether isolates of C. jejuni found among wild birds are comparable to those found in humans. In some of these investigations, serotypes commonly found among patients were shown to occur as well among wild birds (28, 35). However, a recent study showed significant differences in serotype distributions among isolates from wildlife, broiler chickens, and humans (26). Although the sample size for wild birds (n = 15) was not as large as that for mammals, the results nevertheless suggested that both wild mammals and birds play only a minor role in the epidemiology of human C. jejuni infections.
In the present study, we investigated the prevalence and seasonal variation of C. jejuni in black-headed gulls (Larus ridibundus), an omnivorous species that is among the most likely wild bird candidates for transmission of zoonotic agents, at a single locality over a 2-year period. We also genetically compared the gull isolates to those from humans and broiler chickens collected during the corresponding time period. We found a marked seasonal prevalence variation in the gulls. The genotypic comparison revealed that two isolates from gulls were indistinguishable from a human isolate. The predominate genotypes among gull isolates, however, were not found in any of the human or broiler chicken isolates, indicating the presence of C. jejuni adapted to colonization of black-headed gulls or to environmental survival.
MATERIALS AND METHODS
Sampling and culturing from black-headed gulls.
A total of 419 individual fecal samples were obtained from gulls in 1999, and 367 samples were obtained in 2000 (Table 1). The birds, all apparently healthy with no signs of diarrhea, were caught at a pond in a public park (Pildammsparken) in the central part of the city Malmö, in southern Sweden. Sampling was performed yearly in four sampling periods, with each sampling corresponding to one of the four seasons: winter (January and February), spring (March and April), summer (July and August), and autumn (October and November). The sampling was performed by insertion of a sterile swab into the cloaca. For previously ringed birds, the ring number was noted, while unringed individual birds were ringed, and each fecal sample was marked with the corresponding number. Determination of the ages of birds was performed according to the method of Baker (2). Each swab was placed in Amies charcoal transport medium (Transwab; BioDisc, Solna, Sweden), transported to the laboratory by regular mail, and cultured within 24 to 72 h after sampling. The fecal samples were plated onto Campylobacter selective medium, consisting of 42.5 g of Columbia blood agar base (II) per liter (Acumedia Manufacturers, Baltimore, Md.), 5% defibrinated horse blood, 10 mg of vancomycin per liter, 2,500 IU of polymyxin B per liter, and 5 mg of trimethoprim per liter, and incubated at 42°C under microaerobic (85% N2, 10% CO2, 5% O2) conditions. The plates were checked for growth of Campylobacter-like colonies after 24 and 48 h of incubation. From each plate with growth of suspected Campylobacter, one colony was isolated and further investigated. Isolates showing a Campylobacter-like morphology on blood agar plates (containing 42.5 g of Columbia blood agar base [II] per liter plus 5% defibrinated horse blood), gram-negative seagull-like cell morphology under light microscopy and positive reactions in catalase and oxidase tests were considered putative Campylobacter spp., and those positive according to the hippurate-hydrolysis test (17) were considered putative C. jejuni.
TABLE 1.
Numbers of individual birds in each sampling period and prevalence of C. jejuni colonization
| Group | Result for sampling period: |
|||||||
|---|---|---|---|---|---|---|---|---|
| January-February |
March-April |
July-August |
October-November |
|||||
| Total no. | % with C. jejuni | Total no. | % with C. jejuni | Total no. | % with C. jejuni | Total no. | % with C. jejuni | |
| 1999 | ||||||||
| Juveniles | 21 | 4.8 | 33 | 33.3 | 70 | 34.3 | 80 | 41.2 |
| Adults | 36 | 8.3 | 142 | 17.6 | 37 | 29.7 | ||
| All ages | 57 | 7.0 | 175 | 20.6 | 117 | 37.6 | ||
| 2000 | ||||||||
| Juveniles | 17 | 23.5 | 31 | 38.7 | 86 | 32.6 | 99 | 59.6 |
| Adults | 20 | 20.0 | 114 | 17.5 | ||||
| All ages | 37 | 21.6 | 145 | 22.1 | ||||
Resampling and longitudinal investigation.
Occasionally, previously sampled birds were recaptured. Because this offered a unique opportunity to investigate the natural course of Campylobacter colonization of wild birds, a selective survey was initiated during the course of the main investigation. During the latter part of year 2000, ornithologists in Malmö were given the ring numbers of previously Campylobacter-positive gulls and asked to resample such birds when possible. These selected samplings were not included in the prevalence data. Furthermore, from January to March 2001, an additional 119 gulls were sampled with the aim of receiving additional samples from previously caught birds. When applicable, multiple colonies were isolated from the selective plates at resampling. All C. jejuni isolates from individuals with two or more culture-positive samples were subjected to pulsed-field gel electrophoresis (PFGE) to determine their macrorestriction profiles (MRPs).
Multiple isolation.
For 11 of the fecal samples, 4 to 10 colonies were obtained from the selective plates with the aim of investigating whether individual birds could carry C. jejuni isolates with different MRPs.
Broiler chicken and human isolates.
Fifty-two Campylobacter isolates obtained from broiler chickens and 67 isolates originating from humans were included in the study. Broiler chickens originating from flocks raised in Skåne County were sampled during the period from 26 May to 8 October 1999 as part of the Swedish Campylobacter Control Program. The fecal samples were cultured at the Department of Food Hygiene, Swedish University of Agricultural Sciences, Uppsala, according to methods routinely used in the laboratory: 24 to 42 h of incubation in Preston Campylobacter enrichment broth (Oxoid CM67 supplemented with 50 ml of saponin-lysed horse blood, and 2 ampoules of Oxoid supplement SR117 per 1,000 ml) at 42°C, followed by incubation of a volume of the broth on Preston Campylobacter selective agar (37 g of Oxoid agar CM, 50 ml of lysed horse blood, and 2 ampoules of Oxoid supplement SR117 per 1,000 ml) at 42°C for 48 to 72 h. Human isolates, from patients with no history of recent travel outside Sweden, were obtained from 26 May to 5 August 1999 in Skåne County. Patient fecal samples were cultivated at the Microbiological Laboratory in Lund according to routine methods: cultivation on selective plates containing blood agar base (LabM, Bury, Lancashire, United Kingdom) supplemented with 4% citrated horse blood, 10 mg of vancomycin per liter, 2,500 IU of polymyxin B per liter, and 5 mg of trimethoprim per liter in a microaerobic atmosphere (80% N2, 6% O2, 10% CO2, 4% H2). Broiler chicken and human isolates were sent to the Department of Molecular Biology, Umeå, where they were subcultured on blood agar and incubated for 48 h in a microaerobic environment before being frozen in Trypticase soy broth supplemented with 15% glycerol at −80°C for subsequent analysis.
Molecular identification to the species and genus level.
Putative identification to the genus and species level of gull, broiler chicken, and human isolates was later confirmed. All isolates were subjected to a slightly modified version of a previously described C. jejuni- and Campylobacter coli-specific multiplex PCR (32). One microliter of each frozen culture was used as a template. Isolates negative in this PCR were cultivated on blood agar plates for 24 h before boiled lysates or purified chromosomal DNA samples were prepared (Puregene DNA isolation kit; Gentra Systems, Minneapolis, Minn.) to be used as templates. Isolates still negative in the multiplex PCR were subjected to a second PCR method, which is specific for thermotolerant Campylobacter spp. (8). The 23S ribosomal DNA (rDNA) PCR products were subsequently digested with AluI to produce species-specific patterns (8). Subsets of isolates producing atypical digestion patterns were investigated by full-length or partial sequencing of the 16S rRNA gene. The genes were amplified by PCR with chromosomal DNA as the template, and the products were purified and used as templates for DNA sequencing (BigDye Terminator Cycle Sequencing Ready Reaction; Applied Biosystems, Warrington, England). Nucleotide sequence analyses were performed by using the University of Wisconsin Genetics Computer Group GCG sequence analysis software for VAX computers.
MRP and PFGE.
All C. jejuni isolates of human and chicken origin and gull isolates obtained from January to October 1999 were subjected to MRP and PFGE. A modified version of previously described methods (24, 25) was used. Briefly, cultures were incubated for 24 to 48 h on blood agar plates and then suspended to a cell density corresponding to an optical density of 1.4 at 405 nm. Phenylmethylsulfonyl fluoride solution was excluded from the protocol and replaced by an additional washing in Tris-EDTA (TE) buffer. All isolates were subjected to digestion with SmaI, while isolates with identical SmaI MRPs were also subjected to digestion with KpnI. Each gel contained two or three lanes with a molecular marker (λ ladder; New England Biolabs), and for gels with SmaI-digested blocks, an internal reference strain (99C287) was included. Separation of restriction fragments was performed by electrophoresis with GeneMapper XA (Bio-Rad Laboratories, Sundbyberg, Sweden). Ramping parameters for SmaI digests were 5 s to 10 s for 4 h, 10 s to 40 s for 14 h, and 50 s to 60 s for 4 h. Those for KpnI were 4 s to 20 s for 23 h. The electrophoresis was performed at 13°C. When two fragments of similar sizes were suspected to be superimposed on each other after PFGE of SmaI digests, a second electrophoresis was performed to identify double bands. Ramping parameters further separating fragments between approximately 200 and 350 kb were 18.27 s to 30.82 s for 33.45 h, and those for fragments between approximately 120 and 250 kb were 6.75 s to 21.79 s for 31.37 h. With the two latter programs, electrophoresis was performed at 14°C, and Tris-borate-EDTA (TBE) buffer was replaced after approximately 24 h. Gels were stained in ethidium bromide, destained in water, and photographed under UV light.
For plasmid identification, undigested PFGE blocks were subjected to PFGE with ramping parameters of 0.5 s for 30 min, 8 s for 30 min, 1 s for 3 h, 2 s for 3 h, 4 s for 6 h, and 8 s for 8 h (5).
Computer analysis.
Negative photographs of gels containing SmaI-digested samples were scanned with a ScanJet 6100C (Hewlett Packard) and analyzed with the GelCompare version 4.0 (Applied Maths, Kortrijk, Belgium) software program to produce a dendrogram. Untypeable strains (not digestible with SmaI) were excluded from the computer analysis. In order to avoid a false high homology between broiler chicken isolates, only one isolate was included when multiple isolates with identical MRPs had been obtained from one chicken production unit within a 1-week period. One Campylobacter coli isolate was included as an out-group. A band position tolerance of 1.0% and an increase tolerance of 1.5% were attributed to the analysis. Fragments of all sizes were included.
Statistical methods.
The gulls were divided into two age groups: “juveniles” (less than 1 year old) and “adults” (more than 1 year old). We tested by cross-tabulation for differences in prevalence within and between different age groups for various sampling occasions during each year. The proportions of infected birds to the number of sampled birds for each age class and sample occasion were calculated, the values were approximated to normal, and differences between age groups or sample occasions were tested with a paired test. P values <0.05 were considered significant.
RESULTS
Prevalence.
Campylobacter spp. were isolated in 117 (27.9%) of the 419 black-headed gull samples obtained during 1999 and in 133 (36,2%) of the 367 samples obtained during 2000. Among these, 108 (92.3%) and 127 (95.5%), respectively, were identified as C. jejuni (Table 1), which corresponds well to previous reports on the proportion of C. jejuni over hippurate hydrolysis-negative Campylobacter spp. in black-headed gulls (11, 29). In 1999, seven isolates were identified as Campylobacter lari, and two were identified as C. coli. Among samples from the year 2000, five C. coli isolates and only one C. lari isolate were identified.
Age and seasonal variation.
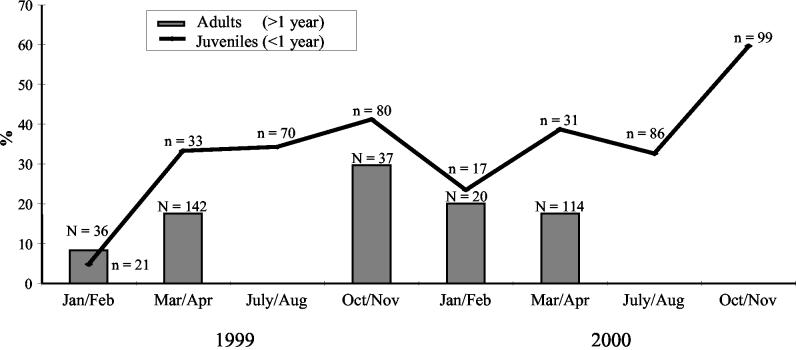
We found no significant differences in the prevalences of C. jejuni between juvenile and adult birds (Table 1): 1999, winter, P = 0.585; spring, P = 0.074; and autumn, P = 0.29; 2000, winter, P = 0.796; and spring, P = 0.361. Within both age groups, however, C. jejuni prevalences differed between sampling occasions. Among juvenile birds, a pronounced seasonal variation was observed (Fig. 1). During both years studied, the colonization rates were lowest in winter, increased during spring and summer, and reached their highest level in late autumn at the October-November sampling. The winter prevalence for juvenile birds was significantly lower than on the following sampling occasions in 1999: winter versus spring, P = 0.002; winter versus summer, P < 0.001; and winter versus autumn, P < 0.001. In 2000, the prevalence of C. jejuni in juvenile birds was significantly lower at winter, spring, and summer samplings compared to autumn sampling: winter versus autumn, P = 0.002; spring versus autumn, P = 0.038; and summer versus autumn, P < 0.001. All other interseasonal comparisons were nonsignificant. A similar seasonal variation was seen during both years studied, even if the prevalence rate was significantly higher at the autumn sampling in 2000 compared to the same period in 1999 (autumn 2000 versus autumn 1999, P = 0.013).
FIG. 1.
Prevalence of C. jejuni at different sampling series. N, total number of samples from adult birds; n, total number of samples from juvenile birds.
Longitudinal data.
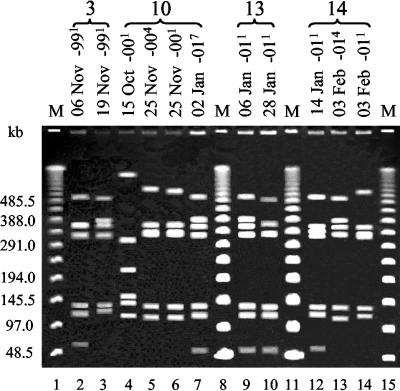
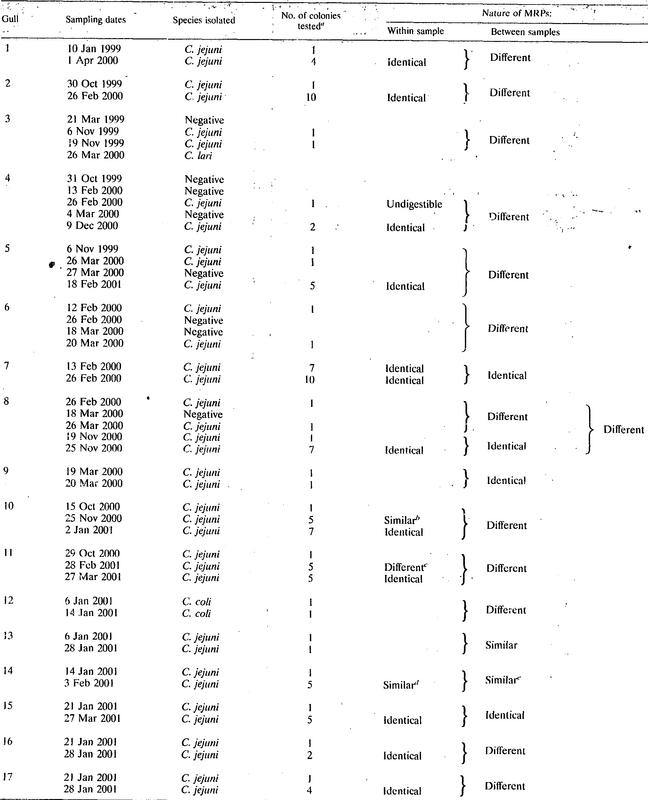
Forty-eight gulls were recovered and resampled on at least one occasion. Thirty-eight birds were sampled twice. Out of these, 15 gulls were culture negative on both occasions, 13 were negative on one occasion and C. jejuni positive on another, and 10 were Campylobacter positive on both occasions. Nine of the latter were positive for C. jejuni on both sampling occasions, while one was positive for C. coli on both occasions. Four birds were sampled three times, four birds were sampled four times, and two birds were sampled five times. Out of these, three birds were culture positive on no occasion or one occasion only, while the remaining birds were Campylobacter positive two or more times. Among all 17 birds that were Campylobacter positive on more than one occasion, only 1 bird was colonized by different Campylobacter species at different samplings. Identical MRPs were found in C. jejuni isolates from two sampling occasions in five birds sampled at 1-, 6-, 13-, 27-, and 65-day intervals, respectively. From the majority of individuals, however, C. jejuni isolates with different MRPs were found at different samplings. In some of these cases, the MRPs found showed a high similarity between samplings (Fig. 2). Details on sampling dates and the results of cultivation and of MRP by PFGE are given in Table 2 for the 17 birds that were Campylobacter positive on at least two occasions.
FIG. 2.
Examples of SmaI MRPs of C. jejuni isolates from resampled black-headed gulls. Sampling dates and bird numbers (above braces) corresponding to numbers in Table 2 are given above the gel. Superscripts indicate number of colonies from each sample with the corresponding MRP. M, molecular mass marker (λ ladder).
TABLE 2.
Resampled black-headed gulls Campylobacter positive on two or more occasions
Number of colonies investigated by PFGE.
MRP from one colony differed by having a slightly shorter largest fragment.
Two colonies gave one MRP, and three gave a different MRP.
Four colonies were identical, and one differed slightly.
All three types were highly similar.
Multiple isolation.
Multiple isolates were characterized from each of 11 gulls. In general, the level of heterogeneity between MRPs of different colonies from a given individual was low. In multiple isolates from nine of the birds tested, only one MRP was found among the colonies from each individual. In isolates from one bird, the MRPs were identical in four out of five colonies, while the MRP of the fifth showed a small difference in the length of the largest fragment. For the last bird, from which 10 colonies were retrieved, the MRP of one clone lacked a fragment of approximately 50 kb. The corresponding fragment, occurring in the MRPs of the remaining colonies, was shown to be a plasmid. It is notable that, at primary isolation, the colony lacking the plasmid was contaminated by a swarming bacterium and therefore had to be subcultured several times before a pure culture could be obtained. Thus, loss of the plasmid could well have occurred in the laboratory as a result of the repeated subculturing.
Species determination.
A total of 369 isolates (250 from gulls, 52 from broiler chickens, and 67 from humans) were investigated by the hippurate hydrolysis test and molecular typing by C. jejuni- and C. coli-specific multiplex PCR, by production of species-specific restriction enzyme digestion patterns of 23S rDNA PCR products, or by sequencing of 16S rDNA PCR products. The results were concordant in 368 cases. One broiler chicken isolate was repeatedly negative in the hippurate hydrolysis test, but repeatedly positive for C. jejuni in the multiplex PCR, and therefore regarded as hippurate negative C. jejuni. A total of 56 isolates were negative in the multiplex PCR and therefore were referred to the 23S PCR method. Out of these, seven isolates (all from gulls) were shown to be C. lari, and consequently the negative results of the multiplex PCR were as expected for these isolates. Another 38 isolates (1 from broiler chickens, 4 from humans, and 33 from gulls) produced typical C. jejuni restriction patterns after digestion with AluI, and 6 isolates (all from gulls) produced typical C. coli patterns. Four hippurate hydrolysis-positive isolates (two from gulls and two of broiler chicken origin) produced identical atypical patterns after enzymatic digestion of the 23S rDNA PCR products. The largest fragment of the typical C. jejuni pattern was missing, while two smaller fragments appeared, indicating that these isolates might have an additional restriction site for AluI in their 23S rDNA genes. One of these isolates was subjected to full-length sequencing of the 16S rDNA, and the highest homology (99.8%) found in a BLAST search was to the 16S rDNA sequence of C. jejuni NCTC11168 (GenBank accession no. AL139076); thus, these four isolates were considered to be C. jejuni. Furthermore, one hippurate hydrolysis-negative isolate of gull origin produced an atypical digestion pattern, resembling the one of C. lari, but with the smallest fragment slightly larger. The 16S rDNA sequence showed 99.8% homology to a previously published urease-positive thermophilic Campylobacter isolate (GenBank accession no. AB066098), and our isolate was regarded as C. lari. Sixty-three human isolates were identified as C. jejuni, and 4 were identified as C. coli. Among the 52 broiler chicken isolates investigated, 50 were identified as C. jejuni, and 2 were identified as C. coli.
MRP, PFGE, and cluster analysis.
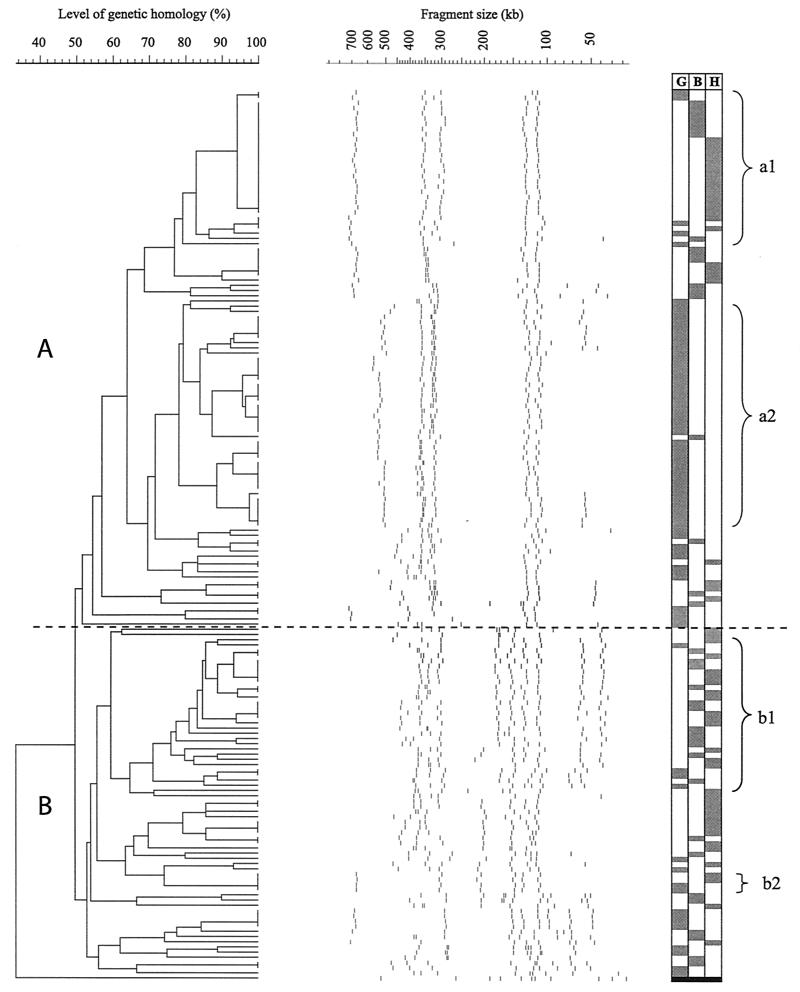
Out of the 192 C. jejuni isolates subjected to MRP and PFGE, a total of 15 isolates (3 from gulls, 7 from humans, and 5 from broiler chickens) were excluded from the computer analysis because their DNA was indigestible with SmaI. Another seven broiler chicken isolates were excluded because their SmaI MRPs were identical to that of at least one other isolate obtained within the same week from the same production unit. Cluster analysis of the remaining 170 C. jejuni isolates (76 from gulls, 38 from broiler chickens, and 56 from humans) resulted in two main clusters at a 50% level of similarity (Fig. 3). Cluster A contained 103 isolates, of which 60 were from gulls, 18 were from broiler chickens, and 25 were from humans. Cluster B contained 67 isolates, of which 16 were from gulls, 20 werefrom broiler chickens, and 31 were from humans. While human and broiler chicken isolates were fairly evenly distributed between the two clusters, with approximately 50% of isolates in each cluster, the majority (78.9%) of the gull isolates were found in cluster A, where 43 of the gull isolates formed a distinctive subcluster (marked “a2” in Fig. 3). Isolates from all sampling periods and from both juvenile and adult birds were represented in this gull subcluster. It is notable that 13 of 15 isolates from the autumn sampling belonged to this group of isolates.
FIG. 3.
Dendrogram of SmaI MRPs of C. jejuni from black-headed gulls (G), broiler chickens (B), and humans (H). Cluster analysis was performed by using GelCompare version 4.0 with the unweighted pair group method with arithmetic mean (UPGMA). Gray boxes behind MRPs indicate host origin of isolates and a black bar at the bottom indicates a C. coli isolate used as the out-group. A dashed line indicates the demarcation between clusters A and B. For definition of a1, a2, b1, and b2, see the text.
With a cutoff level of 90%, 11 gull isolates grouped together with isolates from either of the two other hosts. Two of these gull isolates were isolated in winter, five were isolated in spring, two were isolated in summer, and two were isolated in autumn. The dendrogram showed six gull isolates (four isolated in spring and one each in summer and autumn) with SmaI MRPs identical to those of isolates of either human or chicken origin. Subsequent digestion with KpnI of isolates in these groups of mixed origin produced a variety of MRPs in most groups. However, in one group (marked “b2” in Fig. 3) consisting of two human and two black-headed gull isolates, identical KpnI MRPs were found in two black-headed gull isolates and one human isolate. These two gull isolates were both isolated during the spring sampling.
DISCUSSION
The black-headed gull was chosen for investigation because it is one of the wild birds most likely to be involved in the epidemiology of human-associated enteropathogenic bacteria. It is an omnivorous and opportunistic species that feeds both in natural habitats, such as seashores, lakes, and rivers, as well as in farmlands and urban surroundings (e.g., at sewage outlets, refuse dumps, or recreational areas where food leftovers can be found) (15). In Pildammsparken, these gulls feed to a large extent on bread and, to a lesser extent, on herring brought there by humans—mainly for feeding of other bird species. The gulls are also seen searching for food at other places: for example, around hot dog stands, in dustbins, and on the lawns of the parks and gardens in different parts of the city.
Our results show that in juvenile black-headed gulls in Malmö, like in humans and broiler chickens, there is pronounced seasonal variation in C. jejuni infection rates (Fig. 1). The highest number of infected gulls was found in October and November, and thus the peak differs from that found in humans and broiler chickens, where C. jejuni infection rates peak in July to August and September, respectively. Only juveniles were possible to sample during all of the sampling periods, and therefore we could make only a comprehensive description of the seasonal prevalence variation for young birds. However, prevalence rates in adult birds sampled in 1999 indicate that there exists a corresponding variation in prevalence in this age group as well. The interpretation of the data at hand is complicated by the fact that black-headed gulls, like many other species of wild birds, are migratory. Recoveries of ringed birds show that several different populations utilize the Malmö area (3). There is one stationary population of gulls, in which individuals spend the entire year in the region. Another population breeds in Malmö and spends the winter in northwestern Europe. Still other birds breed further to the northeast (in southeast Sweden and Finland, the Baltic states, and parts of both Belarus and Russia) and either remain in the Malmö area during winter or just stop over on their migration. The observed pattern with a peak in late autumn may therefore have more than one explanation. It could reflect a sampling bias of heavily-C. jejuni-colonized populations of migratory individuals, or it could reflect a seasonal variation in the presence of C. jejuni in the local environment of the sampling area, with gulls becoming infected locally. Because the late-autumn sampling series was performed over 4-week periods each year, the latter explanation is more plausible. Furthermore, results of the resampling of individual birds indicate that there might be a rapid shift in colonizing strains, which also suggests that many birds become infected locally. This in turn indicates that the occurrence of C. jejuni in the environment is particularly high during this time of the year. Interestingly, previous investigations from England have shown a seasonal variation in numbers of Campylobacter organisms in surface waters, with low counts in June to August and maximum counts in winter (18, 23). Even though campylobacters are microaerophilic organisms, and thus sensitive to atmospheric oxygen, reports have shown that Campylobacter spp. have the ability to survive for prolonged periods in cold water, possibly being protected by biofilms (6, 7). This could enhance C. jejuni survival in the environment during cooler periods of the year and thereby increase the exposure of birds to C. jejuni organisms. Another explanation could be that there is less exposure to UV light during autumn and winter, which may be expected to influence Campylobacter survival in the environment (18).
All gulls in this investigation appeared healthy at the time of sampling, and a number of previously C. jejuni-positive individuals were either captured or observed on later occasions. It is therefore most likely that, under normal circumstances, C. jejuni infection is predominantly subclinical in black-headed gulls. Little is known about C. jejuni infections in this bird species, including how long infection persists and whether infection induces protective immunity that would prevent reinfection. In a study on housed herring gulls (Larus argentatus), all but 1 out of 27 previously C. jejuni-positive birds were culture negative after 4 weeks of housing. When 20 of these birds were exposed to the same biotype of C. jejuni 1 year after natural infection, only 1 individual was reinfected (10). However, if infection by C. jejuni would cause the development of a general protective immunity in gulls, we would have expected to find a greater difference in prevalence between age groups, since the likeliness of infection and subsequent development of immunity would increase with time. In this study, however, no significant difference in prevalence between age groups was observed. Also the results of the resampling argue against the development of a general immunity. Many individuals were repeatedly C. jejuni positive, and often a shift in MRPs had occurred between samplings. These findings indicate that immunity to C. jejuni in gulls is either time limited or protective only to certain clones. However, the differences in MRPs from different samplings do not necessarily have to be caused by reinfections. Previous reports on C. jejuni isolated from broiler chicken products and live chickens have shown that C. jejuni may undergo genetic rearrangements (12, 34). In some gulls, comparatively small differences in MRPs were found in isolates from different samplings, which may indicate that genetic instability occurs as well in C. jejuni colonizing gulls. In order to elucidate this possibility, isolates from single birds need to be further investigated.
In the present study, we used PFGE to compare the SmaI MRPs of C. jejuni isolates from black-headed gulls, broiler chickens, and human clinical cases of infection. In this type of investigation, in which a larger number of isolates without known epidemiological relationships are compared, the chance of finding identical isolates is small. Therefore, we chose not only to look for identical MRPs, but also to identify groups of isolates exhibiting similar patterns. Even if there was a high level of heterogeneity among the isolates in this investigation, groups of such basic patterns could be identified, some of them recognizable from other investigations. For example, the basic pattern of subcluster a1 (Fig. 3), consisting of five (on one occasion six) fragments, was previously shown to be prevalent in clinical cases of human campylobacteriosis, as well as in isolates originating from poultry (13). Also, in our investigation, variants of this pattern were prevalent among human and broiler chicken isolates, but the pattern was also represented by a few isolates of gull origin. Another basic pattern reported as common in C. jejuni isolates from humans and poultry is the pattern indicated as b1 in Fig. 3 (9, 26). Again, this was a common theme of both human and chicken isolates in our investigation, and as before, this pattern was represented by a few gull isolates. The levels of identity and similarity between MRPs of isolates from broiler chickens and humans were markedly higher than those between isolates from black-headed gulls and the other two hosts. A few gull isolates with MRPs identical or nearly identical to such from humans or broiler chickens were found, however. The finding of two gull isolates with MRPs identical to that of one human isolate, after digestion with two separate enzymes, suggests that black-headed gulls may have a role in the epidemiology of C. jejuni strains that are pathogenic to humans. It is important to note, however, that the direction of transmission is unknown.
One of the most striking features of the dendrogram, apart from existence of genotypes shared by isolates from gulls and humans or broiler chickens, is the existence of a subcluster almost exclusively made up by isolates from black-headed gulls (a2 in Fig. 3). Even if the computer analysis placed one broiler chicken isolate in this subcluster, the MRP of this isolate differs from the others, because it does not exhibit the characteristic double fragment in the size range of approximately 300 to 320 or 330 to 360 kb that is seen among the gull isolates (e.g., lanes 2, 5, 6, 10, and 14 in Fig. 2). Hence, the basic pattern of subcluster a1 does not occur in any of the human or broiler chicken isolates. Among black-headed gulls, this is the most prevalent basic pattern, and the subcluster contains more than half (56.6%) of the total number of gull isolates. Isolates representing all four seasons were found within the gull subcluster. Interestingly, 87% of all isolates from the autumn sampling belonged to this group, while only approximately 50% of the total number of isolates from winter, spring, and summer samplings were found within the subcluster. Taken together, this could suggest that these isolates represent a pool of C. jejuni adapted to colonization of wild birds in general or black-headed gulls in particular or that these isolates represent a subpopulation adapted to environmental survival. As we have already stated, previous investigations have shown that the levels of environmental C. jejuni are especially high during late autumn, and our results also comply with the findings of a study on Campylobacter carriage in herring gulls in Scotland (35). A high proportion of the herring gull isolates were nonserotypeable, and the authors suggested they were environmentally associated.
Our results indicate that wild birds, such as black-headed gulls, should be taken into consideration when the epidemiology of C. jejuni is discussed, because we found strong indications of strain sharing between wild gulls, broiler chickens, and humans. However, because the majority of isolates found among wild birds differed in genotype from those commonly isolated from humans, we believe that gulls in Malmö may function more as accidental vehicles rather than as a true reservoir of C. jejuni lineages important for clinical disease in humans. It is therefore of great importance to further investigate possible phenotypic and genotypic differences between isolates from different reservoirs and to evaluate the possible existence of environmental pools of C. jejuni.
Acknowledgments
This work was supported by the Health Research Council of Southeast Sweden (2001-02), the Centre for Environmental Research, the Medical Faculty of Umeå University, the Swedish Society of Medicine, and the Swedish Council for Forestry and Agricultural Research.
We gratefully acknowledge the dedicated work of ornithologists K. Bengtsson and L. Blomquist, without whom this project would not have been possible. We also thank E. Holst at the Clinical Microbiological Laboratory in Lund, Sweden, for kindly providing the human clinical isolates included in the study; Paul D. Haemig for valuable comments on the manuscript; and P. Arnqvist for statistical analysis.
REFERENCES
- 1.Anonymous. 2001. Communicable disease in Sweden 2000. The annual report of the Department of Epidemiology. Swedish Institute for Infectious Disease Control, Stockholm, Sweden.
- 2.Baker, K. 1993. Identification guide to the European non-Passerines. BTO guide 24. British Trust for Ornithology, Thetford, United Kingdom.
- 3.Bengtsson, K., and L. Blomquist. 2001. Origin, migration, and site fidelity of Black-headed gulls Larus ridibundus ringed at Malmö. Ornis Svecica 11:59-77. (In Swedish.) [Google Scholar]
- 4.Berndtson, E. 1996. Ph.D. thesis. Swedish University of Agricultural Sciences, Uppsala, Sweden.
- 5.Burman, N. 1994. Ph.D. thesis. Umeå University, Umeå, Sweden.
- 6.Buswell, C. M., Y. M. Herlihy, L. M. Lawrence, J. T. M. McGuiggan, P. D. Marsh, C. W. Keevil, and S. A. Leach. 1998. Extended survival and persistence of Campylobacter spp. in water and aquatic biofilms and their detection by immunofluorescent-antibody and -rRNA staining. Appl. Environ. Microbiol. 64:733-741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ekweozor, C. C., C. E. Nwoguh, and M. R. Barer. 1998. Transient increases in colony counts observed in declining populations of Campylobacter jejuni held at low temperature. FEMS Microbiol. Lett. 158:267-272. [DOI] [PubMed] [Google Scholar]
- 8.Fermér, C., and E. O. Engvall. 1999. Specific PCR identification and differentiation of the thermophilic campylobacters, Campylobacter jejuni, C. coli, C. lari, and C. upsaliensis. J. Clin. Microbiol. 37:3370-3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gibson, J. R., C. Fitzgerald, and R. J. Owen. 1995. Comparison of PFGE, ribotyping and phage-typing in the epidemiological analysis of Campylobacter jejuni serotype HS2 infections. Epidemiol. Infect. 115:215-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glunder, G., U. Neumann, and S. Braune. 1992. Occurrence of Campylobacter spp. in young gulls, duration of Campylobacter infection and reinfection by contact. Zentbl. Vetmed. Reihe B 39:119-122. [DOI] [PubMed] [Google Scholar]
- 11.Glunder, G., U. Neumann, S. Braune, J. Pruter, S. Petersen, and G. Vauk. 1991. Zum Vorkommen von Campylobacter spp. und Salmonella spp. bei Möwen in Norddeutschland. Dtsch. Tierärztl. Wochenschr. 98:152-155. [PubMed] [Google Scholar]
- 12.Hänninen, M.-L., M. Hakkinen, and H. Rautelin. 1999. Stability of related human and chicken Campylobacter jejuni genotypes after passage through chick intestine studied by pulsed-field gel electrophoresis. Appl. Environ. Microbiol. 65:2272-2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hänninen, M.-L., P. Perko-Mäkela, A. Pitkälä, and H. Rautelin. 2000. A three-year study of Campylobacter jejuni genotypes in humans with domestically acquired infections and in chicken samples from the Helsinki area. J. Clin. Microbiol. 38:1998-2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harris, N. V., N. S. Weiss, and C. M. Nolan. 1986. The role of poultry and meats in the etiology of Campylobacter jejuni/coli enteritis. Am. J. Public Health 76:407-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hatch, J. 1996. Threats to public health from gulls (Laridae). Int. J. Environ. Res. 6:5-16. [Google Scholar]
- 16.Hopkins, R. S., R. Olmsted, and G. R. Istre. 1984. Endemic Campylobacter jejuni infection in Colorado: identified risk factors. Am. J. Public Health 74:249-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hwang, M.-N., and G. M. Ederer. 1975. Rapid hippurate hydrolysis method for presumptive identification of group B streptococci. J. Clin. Microbiol. 1:114-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones, K., M. Betaieb, and D. R. Telford. 1990. Thermophilic campylobacters in surface waters around Lancaster, UK: negative correlation with campylobacter infections in the community. J. Appl. Bacteriol. 69:758-764. [DOI] [PubMed] [Google Scholar]
- 19.Kapperud, G., and O. Rosef. 1983. Avian wildlife reservoir of Campylobacter fetus subsp. jejuni, Yersinia spp., and Salmonella spp. in Norway. Appl. Environ. Microbiol. 45:375-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lindbäck, J., and Å. Svensson. 2001. Campylobacter infections in Sweden—a statistical analysis of temporal and spatial distributions of notified sporadic campylobacter infections, p. 1-34. Stockholm University, Stockholm, Sweden.
- 21.Neal, K. R., and R. C. Slack. 1997. Diabetes mellitus, anti-secretory drugs and other risk factors for campylobacter gastro-enteritis in adults: a case-control study. Epidemiol. Infect. 119:307-311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nylen, G., F. Dunstan, S. R. Palmer, Y. Andersson, F. Bager, J. Cowden, G. Feierl, Y. Galloway, G. Kapperud, F. Megraud, K. Molbak, L. R. Petersen, and P. Ruutu. 2002. The seasonal distribution of campylobacter infection in nine European countries and New Zealand. Epidemiol. Infect. 128:383-390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Obiri-Danso, K., and K. Jones. 1999. Distribution and seasonality of microbial indicators and thermophilic campylobacters in two freshwater bathing sites on the River Lune in northwest England. J. Appl. Microbiol. 87:822-832. [DOI] [PubMed] [Google Scholar]
- 24.On, S. L. W., E. M. Nielsen, J. Engberg, and M. Madsen. 1998. Validity of SmaI-defined genotypes of Campylobacter jejuni examined by SalI, KpnI, and BamHI polymorphisms: evidence of identical clones infecting humans, poultry, and cattle. Epidemiol. Infect. 120:231-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.On, S. L. W., and P. Vandamme. 1997. Identification and epidemiological typing of Campylobacter hyointestinalis subspecies by phenotypic and genotypic methods and description of novel subgroups. Syst. Appl. Microbiol. 20:238-247. [Google Scholar]
- 26.Petersen, L., E. M. Nielsen, J. Engberg, S. L. W. On, and H. H. Dietz. 2001. Comparison of genotypes and serotypes of Campylobacter jejuni isolated from Danish wild mammals and birds and from broiler flocks and humans. Appl. Environ. Microbiol. 67:3115-3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Phillips, C. A. 1995. Bird attacks on milk bottles and campylobacter infection. Lancet 346:386. [DOI] [PubMed] [Google Scholar]
- 28.Rosef, O., G. Kapperud, S. Lauwers, and B. Gondrosen. 1985. Serotyping of Campylobacter jejuni, Campylobacter coli, and Campylobacter laridis from domestic and wild animals. Appl. Environ. Microbiol. 49:1507-1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sixl, W., R. Karpiskova, Z. Hubalek, J. Halouzka, M. Mikulaskova, and J. Salava. 1997. Campylobacter spp. and Salmonella spp. in black-headed gulls (Larus ridibundus). Cent. Eur. J. Public Health 5:24-26. [PubMed] [Google Scholar]
- 30.Studahl, A., and Y. Andersson. 2000. Risk factors for indigenous campylobacter infection: a Swedish case-control study. Epidemiol. Infect. 125:269-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tauxe, R. V. 2001. Incidence, trends and sources of campylobacteriosis in developed countries: an overview, p. 42-43. In World Health Organization (ed.), The increasing incidence of human campylobacteriosis. Report and proceedings of a WHO consultation of experts. (WHO/CDS/CSR/APH 2001.7) World Health Organization, Copenhagen, Denmark.
- 32.Vandamme, P., L. J. Van Doorn, S. T. al Rashid, W. G. Quint, J. van der Plas, V. L. Chan, and S. L. W. On. 1997. Campylobacter hyoilei Alderton et al. 1995 and Campylobacter coli Veron and Chatelain 1973 are subjective synonyms. Int. J. Syst. Bacteriol. 47:1055-1060. [DOI] [PubMed] [Google Scholar]
- 33.Varslot, M., J. Resell, and I. G. Fostad. 1996. Water-borne Campylobacter infection—probably caused by pink-footed geese. Two outbreaks in Nord-Trøndelag, Stjørdal in 1994 and Verdal in 1995. Tidsskr. Nor. Laegeforen. 116:3366-3369. [PubMed] [Google Scholar]
- 34.Wassenaar, T. M., B. Geilhausen, and D. G. Newell. 1998. Evidence of genomic instability in Campylobacter jejuni isolated from poultry. Appl. Environ. Microbiol. 64:1816-1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Whelan, C. D., P. Monaghan, R. W. Girdwood, and C. R. Fricker. 1988. The significance of wild birds (Larus sp.) in the epidemiology of Campylobacter infections in humans. Epidemiol. Infect. 101:259-267. [DOI] [PMC free article] [PubMed] [Google Scholar]