Abstract
The National Centre for Streptococcus (NCS) (Canada) determined the group A streptococcal (GAS) M types of 4,760 Canadian isolates submitted between 1993 and 1999 by classic serotyping. The 10 most frequently identified M types were M1 (26.4%), M12 (9.8%), M28 (8.9%), M3 (6.8%), M4 (6.2%), M11 (4.8%), M89 (3.1%), M6 (3.0%), M2 (2.6%), and M77 (1.9%). Nontypeable isolates accounted for 15.4% of the collection. The province of Ontario submitted 51.1% of the isolates, followed by Quebec (21.2%) and Alberta (13.9%). Together, these three provinces constituted 71.3% of the Canadian population in 1996. The numbers of M types M1, M12, M28, and M3 occurred most frequently in subjects whose ages were <1 to 15 years and 25 to 45 years, as well as in the elderly (60 to 90 years). Further analysis found that the four most frequently identified M types from blood, brain, and cerebrospinal fluid were M1 (28.2%), M28 (9.2%), M12 (9.1%), and M3 (8.2%), with 13.4% of isolates being nontypeable. The four isolates from throats most frequently identified were M1 (19.5%), M12 (15.3%), M3 (8.6%), and M28 (5%) with 19.4% of isolates being nontypeable. The sic gene of a subset of M1 strains (9.5% of the M1 collection) was sequenced. Of 36 sic types identified, the four most common were sic1.01 (22.8%), sic1.02 (14.9%), sic1.135 (10.5%), and sic1.178 (9.6%). Together these four sic types further characterized nearly 60% of the M1 strains sequenced. In summary, from the years 1993 to 1999, the NCS detected 54 M types, of which 10 different M types constituted 73.5% of the collection. M1 was the most common GAS M type circulating in the Canadian population, responsible for more than a quarter of the isolates typed. The most common throat isolates differed in M-type and proportion from those of invasive isolates. Sequencing the sic gene further characterized the most common M-type serotype 1 in a fashion that may be useful for epidemiologic investigations.
Group A streptococci (GAS) are associated with a wide spectrum of diseases, ranging from mild pharyngitis to life-threatening streptococcal toxic shock and necrotizing fasciitis (4, 7, 16, 17, 19, 23). Early efforts at understanding the spread of this organism in relation to the diseases it causes led to the development of the currently used serological typing system based on the surface-exposed GAS M protein (20, 21). The M protein is a long coiled coil protein projecting from the surface of the streptococcal cell wall. Amino acid variation in the amino-terminal portion of the protein serves as the basis for the determination of the M type (12). Currently there are 86 distinct GAS M types officially recognized worldwide. These M types are numbered sequentially, M1 through M93; however, this sequence includes four M types from non-group A streptococci and three M types that were later found to be duplicates of previously described M types (www.cdc.gov/ncidod/biotech/strep/strepindex) (10).
In addition to serological typing, investigators have reported emm gene sequencing (the emm gene codes for the M protein) as a method of typing GAS. Incomplete international collections of M antisera limit the ability to serologically characterize all of the new emm types, and these are therefore currently referred to as emm types rather than M types. Recent reports list emm94 to emm124 as the latest extensions of the Lancefield classification (9, 11).
Of the serologically classified M types, the most frequent M type currently seen in cases of invasive GAS disease is M1 (8, 10, 15, 23). Other M types frequently associated with invasive disease are M3, M28, M12, and M4 (8, 10). Since M1 is associated with the majority of disease, it is important to subdivide this M type to provide epidemiologic information on circulating M1 strains within a community. Hoe et al. have shown that the M1 type can be characterized based on variation seen within the sic (streptococcal inhibitor of complement) gene (13). The sic gene encodes a protein that inhibits cytolytic function of the complement cascade in vitro and is involved in adherence to human epithelial cells (14). This gene exhibits a high level of polymorphism, permitting differentiation of strains within the M1 serotype and thereby providing a tool for GAS epidemiology (13). sic typing is specific for the M1 type only.
We report the results of GAS M typing and sic gene variation in M1 strains from Canadian GAS isolates submitted to the National Centre for Streptococcus (NCS), Canada, from 1993 to 1999.
MATERIALS AND METHODS
Source of GAS isolates.
The NCS, located within the Provincial Laboratory of Public Health for Alberta in Edmonton, Alberta, Canada, is mandated to provide national reference services for streptococci. This includes serologic typing of GAS. A total of 4,760 isolates of GAS were submitted to the NCS from within Canada, during the years 1993 to 1999. The isolates were categorized as (i) blood, brain, and cerebrospinal fluid (CSF); (ii) miscellaneous sites (including soft tissue, knee, hand, abscess, joint, eye, ear, hip, sputum, pleural, vagina, pustule, finger, and penis, etc.); and (iii) throat.
Serologic typing and opacity factor determination.
M and T typing and opacity factor determination were performed on all isolates using standardized methods (18). In addition, anti-opacity factor (AOF) typing was performed on all isolates that were opacity factor positive (18). All antisera were prepared in-house. The following reagents were used: M typing antisera 1, 2, 3, 4, 5, 6, 12, 13, 14, 15, 17, 18, 19, 22, 23, 24, 26, 27, 29, 30, 31, 32, 33, 39, 41, 43, 44, 46, 47, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 68, 71, 72, 73, 74, 75, 76, 79, 80, 81, 83 (PT2110), 87 (PT2841), 89 (PT4245), and 90 (PT4931) (total, 61) and AOF typing antisera 2, 4, 9, 11, 22, 25, 27, 28, 44, 48, 49, 58, 59, 60, 61, 62, 63, 66, 68, 73, 75, 76, 77, 78, 79, 81, 87 (PT2841), 89 (PT4245), 90 (PT4831), and 92 (PT5118) (total, 30). Because of the difficulty of producing antisera for some M types, AOF typing has been frequently used to predict the M type (10, 22). While AOF typing does not always have the same type specificity as M typing, this approach is considered valid for the majority of strains from developed countries (3). In this study, isolates were assigned to eight different M types based on AOF typing. AOF typing was used to predict the following M types: 9, 11, 25, 28, 48, 77, 78, and 92 (PT5118).
sic gene analysis.
Sequencing of the sic gene was performed as previously described (13). One hundred and fourteen M1 isolates were selected from the years 1993 to 1999 (9.5% of the M1 collection) to represent different geographic regions in Canada, including British Columbia, Alberta, Saskatchewan, Manitoba, Ontario, Quebec, and the Maritime Provinces.
RESULTS AND DISCUSSION
In 1999 there were 86 serologically distinct GAS M types recognized worldwide, of which 54 were found in isolates submitted to the NCS (M types 1, 2, 3, 4, 5, 6, 9, 11, 12, 13, 14, 18, 22, 24, 25, 26, 27, 28, 29, 31, 33, 39, 41, 43, 44, 48, 49, 52, 53, 55, 58, 59, 60, 61, 62, 63, 65, 66, 68, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 83, 87, 89, 90, and 92). The most frequently identified M types in this collection are shown in Table 1. Of the top four M types, M1 accounted for the majority of isolates, representing over one-quarter (26.4%) of the collection, followed by M12 (9.8%), M28 (8.9%), and M3 (6.8%). The prevalence of M1 over other M types was expected, as similar findings have been reported by other investigators in North America and Europe (8, 10, 15, 23, 24). In a report of the GAS M typed by the Colindale Public Health Laboratory (CPHL) in England, M1 was the most frequently identified type, accounting for 10.4% of 16,909 GAS submitted during the 1980s (5). This percentage is lower than the 26.4% found at the NCS for this serotype, which suggests that M1 is not as prevalent in the United Kingdom as it is in Canada. While this discrepancy may reflect differences in the proportion of circulating strains, it could also be due to the geographical separation (Canada versus United Kingdom) and/or different proportions of submissions by anatomical site of isolation or the different period examined. Examination of the CPHL data shows that a large number of GAS isolates typed originated from throat swabs (46%), whereas only 16% of the isolates in the NCS collection originated from the throat. This higher percentage of noninvasive isolates (throat swabs) included in the CPHL collection may also account for some of the differences in M-type distribution observed between the two reports. Other differences in the proportion of M types found in the United Kingdom versus Canada include the large number of M49 isolates from the CPHL study (7.7%), whereas this type accounts for only 1.3% of the NCS collection (5). The two studies agree on the importance of M28, which ranks third in the United Kingdom and third in Canada, accounting for 8 and 9% of the circulating types, respectively.
TABLE 1.
M serotypes according to submitting region, 1993 to 1999a
| Serotype | No. of specimens from province(s)b |
Total no. (%) of specimen | |||||||
|---|---|---|---|---|---|---|---|---|---|
| BC | ALTA | SASK | MAN | ONT | QUE | MARIT/NFLD | YUK/NUN/NWT | ||
| M1 | 64 | 141 | 22 | 38 | 631 | 328 | 32 | 1 | 1,257 (26.4) |
| M12 | 18 | 72 | 6 | 24 | 243 | 85 | 16 | 1 | 465 (9.8) |
| M28 | 14 | 41 | 6 | 12 | 238 | 108 | 7 | 0 | 426 (8.9) |
| M3 | 5 | 70 | 5 | 24 | 133 | 76 | 13 | 0 | 326 (6.8) |
| M4 | 10 | 32 | 7 | 8 | 143 | 81 | 14 | 0 | 295 (6.2) |
| M11 | 1 | 37 | 8 | 7 | 115 | 57 | 3 | 2 | 230 (4.8) |
| M89 (PT4245) | 4 | 14 | 6 | 7 | 97 | 18 | 3 | 0 | 149 (3.1) |
| M6 | 2 | 17 | 6 | 2 | 74 | 28 | 16 | 0 | 145 (3.0) |
| M2 | 8 | 11 | 2 | 2 | 55 | 34 | 10 | 0 | 122 (2.6) |
| M77 | 4 | 30 | 3 | 1 | 36 | 9 | 6 | 0 | 89 (1.9) |
| M75 | 1 | 3 | 0 | 0 | 54 | 12 | 2 | 0 | 72 (1.5) |
| M22 | 2 | 7 | 1 | 0 | 34 | 23 | 2 | 0 | 69 (1.4) |
| M49 | 10 | 16 | 5 | 3 | 21 | 4 | 2 | 0 | 61 (1.3) |
| M61 | 2 | 1 | 1 | 1 | 27 | 5 | 0 | 0 | 37 (0.8) |
| M58 | 9 | 6 | 0 | 0 | 8 | 3 | 0 | 0 | 26 (0.5) |
| M33 | 0 | 3 | 0 | 0 | 18 | 0 | 0 | 0 | 21 (0.4) |
| M5 | 0 | 3 | 0 | 0 | 10 | 7 | 0 | 0 | 20 (0.4) |
| M29 | 0 | 15 | 1 | 0 | 3 | 1 | 0 | 0 | 20 (0.4) |
| All others | 7 | 25 | 7 | 22 | 98 | 31 | 5 | 1 | 196 (4.1) |
| M nontypeable | 30 | 119 | 18 | 63 | 393 | 101 | 9 | 1 | 734 (15.4) |
| Total no. (%) | 191 (4.0) | 663 (13.9) | 104 (2.2) | 214 (4.5) | 2,431 (51.1) | 1,011 (21.2) | 140 (2.9) | 6 (0.1) | 4,760 (100) |
All specimens were included.
Abbreviations: BC, British Columbia; ALTA, Alberta; SASK, Saskatchewan; MAN, Manitoba; ONT, Ontario; QUE, Quebec; MARIT/NFLD, Maritime Provinces and Newfoundland; YUK/NUN/NWT, Yukon, Nunavut Region, and Northwest Territories.
Recently, investigators in the United States performed a population-based surveillance study in five U.S. states from 1 July 1995 to 31 December 1999 in which all GAS, where possible, were emm typed (23). The four most common emm types identified in this surveillance study were emm1 (20.8%), emm28 (9.2%), emm12 (7.6%), and emm3 (7.1%). These are the same top four as were found in our study with only small differences in percentages, suggesting that little variation in the main circulating GAS M types exists between the two countries.
With respect to nontypeable isolates, we found 734 nontypeable M types, accounting for 15.4% of the collection. This is higher than has been reported in other studies such as the United Kingdom report in which 7.35% of the GAS isolates were nontypeable (5). The large percentage of nontypeable isolates maybe due to nontypeable clones appearing in Canada. Further studies grouping these strains into clusters by amplified fragment length polymorphism analysis followed by emm gene sequencing will allow us to determine if these nontypeable strains represent previously unrecognized types.
Fluctuations in submission rates for M1, M12, M28, and M3.
The yearly submission rate for all isolates more than tripled during the period examined. Analysis of the proportions of the top four M types received revealed some variation in their contribution each year. This variation is the most dramatic for M3 (Table 2). Increased numbers of M3 isolates were first evident in 1994, peaking in 1995 and then decreasing in 1996. A likely explanation for this sudden increase is the introduction of a new M3 strain into the Canadian population, followed by its gradual disappearance.
TABLE 2.
M type and numbers of isolates collected from various sites according to year
| Antigen and site(s) | No. of strains serotyped in: |
Total no. of strains serotyped | Antigen and site(s) | No. of strains serotyped in: |
Total no. of strains serotyped | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1993 | 1994 | 1995 | 1996 | 1997 | 1998 | 1999 | 1993 | 1994 | 995 | 1996 | 1997 | 1998 | 1999 | |||||
| M1 | ||||||||||||||||||
| Blood, brain, and CSF | 39 | 51 | 88 | 65 | 101 | 135 | 140 | 619 | ||||||||||
| Miscellaneous | 39 | 37 | 78 | 38 | 74 | 106 | 69 | 441 | ||||||||||
| Throat | 9 | 5 | 32 | 59 | 19 | 9 | 16 | 149 | ||||||||||
| Unidentified | 5 | 2 | 22 | 5 | 7 | 3 | 4 | 48 | ||||||||||
| M28 | ||||||||||||||||||
| Blood, brain, and CSF | 5 | 9 | 24 | 38 | 39 | 43 | 44 | 202 | ||||||||||
| Miscellaneous | 6 | 9 | 14 | 21 | 32 | 38 | 39 | 159 | ||||||||||
| Throat | 3 | 1 | 9 | 16 | 2 | 6 | 1 | 38 | ||||||||||
| Unidentified | 4 | 1 | 3 | 19 | 27 | |||||||||||||
| M12 | ||||||||||||||||||
| Blood, brain, and CSF | 7 | 15 | 37 | 31 | 29 | 34 | 43 | 196 | ||||||||||
| Miscellaneous | 18 | 9 | 16 | 23 | 25 | 26 | 19 | 136 | ||||||||||
| Throat | 15 | 12 | 38 | 32 | 8 | 9 | 3 | 117 | ||||||||||
| Unidentified | 2 | 3 | 2 | 2 | 7 | 16 | ||||||||||||
| M3 | ||||||||||||||||||
| Blood, brain, and CSF | 6 | 19 | 70 | 45 | 7 | 7 | 27 | 181 | ||||||||||
| Miscellaneous | 3 | 9 | 21 | 20 | 1 | 2 | 13 | 71 | ||||||||||
| Throat | 2 | 8 | 40 | 12 | 2 | 2 | 66 | |||||||||||
| Unidentified | 5 | 3 | 2 | 10 | ||||||||||||||
| M4 | ||||||||||||||||||
| Blood, brain, and CSF | 11 | 13 | 21 | 22 | 21 | 17 | 18 | 123 | ||||||||||
| Miscellaneous | 10 | 5 | 16 | 10 | 16 | 12 | 11 | 80 | ||||||||||
| Throat | 7 | 4 | 21 | 20 | 5 | 19 | 1 | 77 | ||||||||||
| Unidentified | 2 | 8 | 1 | 3 | 1 | 15 | ||||||||||||
| M11 | ||||||||||||||||||
| Blood, brain, and CSF | 3 | 7 | 21 | 19 | 25 | 27 | 102 | |||||||||||
| Miscellaneous | 1 | 1 | 3 | 14 | 28 | 25 | 14 | 86 | ||||||||||
| Throat | 1 | 4 | 3 | 10 | 17 | 35 | ||||||||||||
| Unidentified | 2 | 3 | 2 | 7 | ||||||||||||||
| M89(PT4245) | ||||||||||||||||||
| Blood, brain, and CSF | 6 | 2 | 6 | 20 | 18 | 17 | 24 | 93 | ||||||||||
| Miscellaneous | 3 | 3 | 9 | 9 | 11 | 14 | 49 | |||||||||||
| Throat | 3 | 1 | 2 | 6 | ||||||||||||||
| Unidentified | 1 | 1 | ||||||||||||||||
| M6 | ||||||||||||||||||
| Blood, brain, and CSF | 3 | 2 | 24 | 19 | 4 | 4 | 6 | 62 | ||||||||||
| Miscellaneous | 5 | 3 | 19 | 11 | 5 | 4 | 2 | 49 | ||||||||||
| Throat | 1 | 24 | 3 | 1 | 29 | |||||||||||||
| Unidentified | 2 | 1 | 2 | 5 | ||||||||||||||
| M2 | ||||||||||||||||||
| Blood, brain, and CSF | 1 | 6 | 3 | 8 | 9 | 9 | 8 | 44 | ||||||||||
| Miscellaneous | 3 | 2 | 8 | 9 | 5 | 13 | 7 | 47 | ||||||||||
| Throat | 4 | 1 | 5 | 10 | 1 | 2 | 3 | 26 | ||||||||||
| Unidentified | 1 | 3 | 1 | 5 | ||||||||||||||
| M77 | ||||||||||||||||||
| Blood, brain, and CSF | 1 | 1 | 3 | 4 | 6 | 12 | 12 | 39 | ||||||||||
| Miscellaneous | 1 | 4 | 3 | 2 | 12 | 6 | 28 | |||||||||||
| Throat | 6 | 3 | 2 | 3 | 14 | |||||||||||||
| Unidentified | 2 | 1 | 5 | 8 | ||||||||||||||
| M75 | ||||||||||||||||||
| Blood, brain, and CSF | 1 | 2 | 7 | 16 | 3 | 1 | 5 | 35 | ||||||||||
| Miscellaneous | 2 | 5 | 6 | 6 | 8 | 3 | 1 | 31 | ||||||||||
| Throat | 3 | 2 | 5 | |||||||||||||||
| Unidentified | 1 | 1 | ||||||||||||||||
| M49 | ||||||||
| Blood, brain, and CSF | 2 | 3 | 1 | 5 | 5 | 7 | 9 | 32 |
| Miscellaneous | 1 | 6 | 4 | 4 | 1 | 4 | 2 | 22 |
| Throat | 1 | 2 | 3 | |||||
| Unidentified | 2 | 1 | 1 | 4 | ||||
| M22 | ||||||||
| Blood, brain, and CSF | 3 | 2 | 2 | 5 | 4 | 9 | 5 | 30 |
| Miscellaneous | 2 | 2 | 8 | 4 | 3 | 5 | 2 | 26 |
| Throat | 8 | 2 | 1 | 11 | ||||
| Unidentified | 1 | 1 | 2 | |||||
| M61 | ||||||||
| Blood, brain, and CSF | 2 | 5 | 9 | 1 | 1 | 18 | ||
| Miscellaneous | 1 | 1 | 6 | 7 | 1 | 16 | ||
| Throat | 1 | 2 | 3 | |||||
| Unidentified | ||||||||
| M73 | ||||||||
| Blood, brain, and CSF | 4 | 2 | 1 | 2 | 2 | 11 | ||
| Miscellaneous | 1 | 1 | ||||||
| Throat | 1 | 1 | ||||||
| Unidentified | 1 | 1 | ||||||
| M87 (PT2841) | ||||||||
| Blood, brain, and CSF | 2 | 1 | 3 | 1 | 3 | 1 | 11 | |
| Miscellaneous | 1 | 1 | ||||||
| Throat | 1 | 2 | 3 | |||||
| Unidentified | ||||||||
| M5 | ||||||||
| Blood, brain, and CSF | 1 | 2 | 7 | 10 | ||||
| Miscellaneous | 1 | 1 | 1 | 1 | 5 | 9 | ||
| Throat | 1 | 1 | ||||||
| Unidentified | ||||||||
| M33 | ||||||||
| Blood, brain, and CSF | 2 | 4 | 1 | 2 | 9 | |||
| Miscellaneous | 2 | 3 | 1 | 1 | 2 | 2 | 11 | |
| Throat | 1 | 1 | ||||||
| Unidentified | ||||||||
| M29 | ||||||||
| Blood, brain, and CSF | 3 | 2 | 3 | 1 | 9 | |||
| Miscellaneous | 3 | 2 | 5 | 1 | 11 | |||
| Throat | ||||||||
| Unidentified | ||||||||
| Other M types | ||||||||
| Blood, brain, and CSF | 3 | 7 | 8 | 6 | 14 | 10 | 25 | 73 |
| Miscellaneous | 5 | 5 | 9 | 16 | 7 | 17 | 20 | 79 |
| Throat | 2 | 5 | 7 | 12 | 1 | 27 | ||
| Unidentified | 1 | 2 | 2 | 3 | 8 | |||
| M nontypeable | ||||||||
| Blood, brain, and CSF | 43 | 27 | 41 | 41 | 46 | 52 | 45 | 295 |
| Miscellaneous | 40 | 22 | 55 | 36 | 43 | 45 | 39 | 280 |
| Throat | 12 | 28 | 46 | 26 | 1 | 33 | 2 | 148 |
| Unidentified | 1 | 1 | 2 | 2 | 3 | 2 | 11 | |
| Total | 348 | 370 | 918 | 817 | 667 | 843 | 797 | 4,760 |
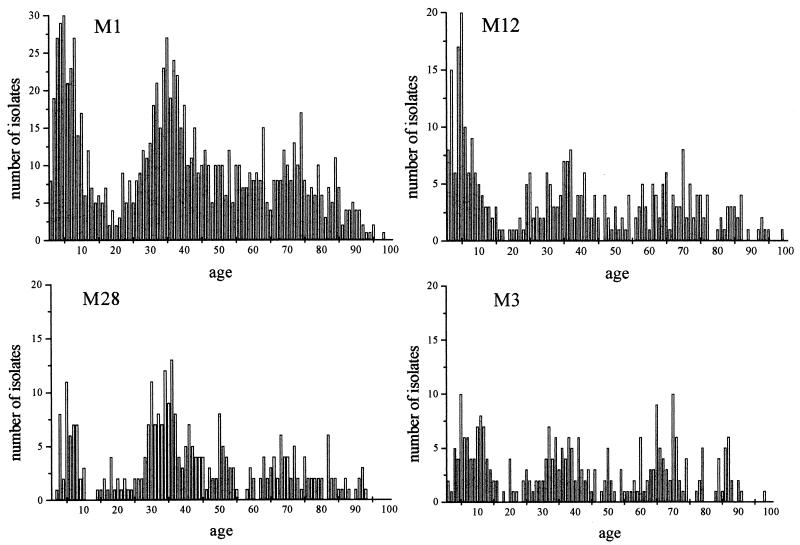
M1, M12, M28, and M3 versus age.
A total of 2,474 isolates of M1, M12, M28, and M3 were received, of which 2,287 (92%) were submitted with patient date of birth. Figure 1 shows the number of M1, M12, M28, and M3 isolates submitted versus age. There appear to be three clusters of isolates present for each M type dependent on age. The first is during the early years of life up to age 15 years. This can be expected, as clusters such as this one in this age group are mirrored in other bacterial pathogens. Interestingly, a second cluster occurs from age 25 to 45 years. Why this cluster occurs in this age group is unclear. One would assume that exposure to a particular M type early in life would provide protection later on in life. This appears to be the case for individuals 16 to 25 years old but not for those older than 25 years. A possible explanation is that individuals in the second cluster are members of households with children in the first cluster and are therefore constantly exposed to GAS. However, exposure of an individual that one would assume should be immune should, in theory, not result in infection. To understand why a second cluster appears in this age group will require more careful epidemiological and immunological investigation. Also, the presence of two distinct clusters suggests careful consideration will be needed in designing any future vaccine schedules against this pathogen. A final diffuse clustering occurs later on in life and is not as dramatic as the first two clusters. This is expected since, as individuals age, they become more susceptible to infections.
FIG. 1.
Numbers of M1, M12, M28, and M3 isolates identified from 1993 to 1999 as a function of age.
Geographic distribution of submitted GAS.
Greater than one-half of the collection originated from the province of Ontario (51.1%). This province represented 37.3% of the Canadian population (according to the 1996 population census [1]). The high percentage of isolates submitted from Ontario in comparison to the rest of Canada is probably due to the mandatory reporting of all invasive GAS infections that was instituted in this province in January 1992 (6, 7, 19). Isolates from Quebec and Alberta accounted for 21.2 and 13.9% of GAS, respectively, that were M typed. Together, these three provinces provided 86.2% of the GAS that were M typed from 1993 to 1999 and represent 71.3% of the Canadian population (according to the 1996 population census), suggesting that the three provinces are probably overrepresented in the sample (1). With no evidence of bias of M types submitted relative to the other submitters, the collection is likely representative of the M types circulating in Canada. Further removal of the Ontario isolates from the analysis results in very little change to the ranking of M types.
To examine and compare GAS M types isolated from different sites, we have separated isolates collected from blood, brain, and CSF from isolates collected from throats and miscellaneous (other anatomical) sites.
M serotypes of GAS isolated from blood, brain, and CSF.
The vast majority of isolates included in this category (2,126 of 2,195) were cultured from blood. Forty-five M types were identified, the most frequent of which for each of the seven years was M1 (28.2%) (Table 2). M1 is frequently associated with invasive infections; therefore, it was not unexpected that this serotype accounted for a quarter of the collection. M1 together with M28 (9.2%), M12 (8.2%), M3 (8.2%), and M4 (5.6%) made up the top five M types isolated from blood, brain, and CSF. No other M type accounted for more than 5% of isolates.
Davies et al. performed a 2-year study for 1992 and 1993 in Ontario in which only invasive isolates were collected (obtained from a normally sterile site) (6). The prevalence of M1 was 24%, that of M12 was 7.4%, that of M4 was 6.5%, that of M28 was 6.2%, and that of M3 was 5.8%. These percentages are comparable with our data for isolates from blood, brain, and CSF, suggesting that what is found in Ontario roughly approximates what is occurring in the rest of Canada.
Another study performed by Beall et al. identified the emm gene sequence and T-antigen type from 340 blood isolates collected from San Francisco, Calif.; Atlanta, Ga.; and Connecticut (2). As in our study and the United Kingdom study, M1 (emm1) accounted for the highest percentage of GAS types seen (14.7%). Interestingly, similar to the United Kingdom study, this percentage is substantially lower than that found in Canada. The recent study by O'Brien et al. found that 20.8% of isolates were emm1 (23). This is also lower than the 28.4% found in our study. Why this difference occurs between the Canadian, United Kingdom, and U.S. data is unclear and will require further investigation.
M serotypes of GAS isolated from miscellaneous sites and throats.
Consistent with isolates from blood, brain, and CSF, the most common serotype in this grouping was M1 for all of the years reported. There is very little difference in the five most common serotypes found in these sites in comparison to those found in invasive sites (Table 2). Together these five serotypes account for over half of the M types in this collection (65.8% for blood, brain, and CSF versus 57% for all others [Table 2]).
The greatest change in ranking between sites is seen in the differences in proportions of M89 (PT4245). For blood, brain, and CSF, M89 ranked sixth (93 isolates), and for the other sites, it ranked ninth (56 isolates). This may suggest that M89 (PT4245) is more prevalent in serious invasive disease; however, to accurately determine this, an active surveillance study is needed. Also, in the overall comparison of numbers of isolates (149/4,760 = 3.1%), the isolation of M89 is not overly significant.
sic gene variability.
To better characterize the M1 strains circulating in Canada from 1993 to 1999, we sequenced the sic gene in 119 M1 invasive isolates (9.5% of the M1 collection) (Table 3). The M1 GAS were chosen to be temporally and geographically representative of the collection. The distribution according to year was as follows: 7 isolates from 1993, 14 isolates from 1994, 19 isolates from 1995, 16 isolates from 1996, 27 isolates from 1997, 17 isolates from 1998, and 23 isolates from 1999. The geographical distribution was as follows: 10 isolates from British Columbia, 24 isolates from Alberta, 10 isolates from Saskatchewan, 8 isolates from Manitoba, 17 isolates from Ontario, 43 isolates from Quebec, and 7 isolates from the Maritime Provinces and Newfoundland. The collection from Ontario was deliberately undersampled, as the sic types from this province had been previously reported for the years 1992 to 1998 (13).
TABLE 3.
Distribution of sic variants from 9.5% of the M1 strains typed by the NCS
| sic typea | No. of sequenced isolates from yr |
Total no. of sequenced isolates | ||||||
|---|---|---|---|---|---|---|---|---|
| 1993 | 1994 | 1995 | 1996 | 1997 | 1998 | 1999 | ||
| 1.01 | 6 | 1 | 6 | 6 | 4 | 1 | 2 | 26 |
| 1.02 | 1 | 5 | 5 | 3 | 3 | 17 | ||
| 1.135 | 1 | 1 | 5 | 5 | 12 | |||
| 1.178 | 1 | 1 | 2 | 5 | 2 | 11 | ||
| 1.78 | 2 | 1 | 1 | 1 | 1 | 6 | ||
| 1.181 | 2 | 3 | 5 | |||||
| 1.36 | 1 | 2 | 1 | 4 | ||||
| 1.13 | 2 | 2 | ||||||
| 1.17 | 1 | 1 | 2 | |||||
| 1.34 | 1 | 1 | 2 | |||||
| 1.68 | 1 | 1 | 2 | |||||
| 1.88 | 1 | 1 | 2 | |||||
| 1.196 | 1 | 1 | 2 | |||||
For sic types 1.09, 1.12, 1.26, 1.35, 1.50, 1.66, 1.72, 1.111, 1.163, 1.197, 1.199, 1.253, 1.257, 1.277, 1.278, 1.279, 1.280, 1.281, 1.287, 1.298, 1.299, and 1.300, only one isolate each was identified.
The selected M1 isolates were all from invasive sites (blood, CSF, pleural fluid, knee fluid, and necrotic tissue). Thirty-six sic types were identified, with four accounting for nearly 60% of the isolates: sic1.01 (22.8%), sic1.02 (14.9%), sic1.135 (10.5%), and sic1.178 (9.6%). Besides being the most frequent allele in Canada, sic1.01 has also been shown to be the most abundant allele in Connecticut, Minnesota, and Finland (13). sic alleles 1.02 and 1.135 also were found in Finland and the United States as well as Canada.
Recently, sequencing of the emm gene, which codes for the GAS M protein, has been shown to have excellent correlation with M typing (3, 10). Sequencing of the emm gene was not used by this laboratory during the years 1993 to 1999. However, we have begun to use this assay for some of the nontypeable strains collected after 1999. The NCS currently has no plans to discontinue serological M typing but rather will complement this with emm gene sequencing for strains that are nontypeable serologically.
With the continued worldwide problem of diseases caused by GAS and the major interest in creating vaccines, it is important to continue to monitor the circulating M types in Canada and worldwide.
Acknowledgments
We thank all laboratories and attending physicians, nurses, and health care providers who submitted GAS isolates for serotyping from all provinces and territories.
We also thank the Population and Public Health Branch of Health Canada, Winnipeg, Canada, for their continued financial support to the NCS.
REFERENCES
- 1.Anonymous. 1996. 1996 census data. Ottawa: Census Operations Division, Statistics Canada. 1996. [Online.] http://www.statcan.ca/english/census96/table1.html.
- 2.Beall, B., R. R. Facklam, T. Hoenes, and B. Schwartz. 1997. Survey of emm gene sequences and T-antigen types from a systemic Streptococcus pyogenes infection isolates collected in San Francisco, California; Atlanta, Georgia; and Connecticut in 1994 and 1995. J. Clin. Microbiol. 35:1231-1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beall, B., G. Gherardi, M. Lovgren, R. R. Facklam, B. A. Forwick, and G. J. Tyrrell. 2000. emm and sof gene sequence variation in relation to serological typing of opacity-factor-positive group A streptococci. Microbiology 146:1195-1209. [DOI] [PubMed] [Google Scholar]
- 4.Bisno, A. L., and D. L. Stevens. 2000. Streptococcus pyogenes (including streptococcal toxic shock syndrome and necrotizing fasciitis), p. 2101-2117. In G. L. Mandel, J. E. Bennett, and R. Dolin (ed.), Mandell, Douglas and Bennett's principles and practice of infectious diseases. Churchill Livingstone, Philadelphia, Pa.
- 5.Coleman, G., A. Tanna, A. Efstratiou, and E. T. Gaworzewska. 1993. The serotypes of Streptococcus pyogenes present in Britain during 1980-1990 and their association with disease. J. Med. Microbiol. 93:165-178. [DOI] [PubMed] [Google Scholar]
- 6.Davies, H. D., A. McGeer, B. Schwartz, K. Green, D. Cann, A. Simor, D. Low, and the Ontario Group A Streptococcal Study Group. 1996. Invasive group A streptococcal infections in Ontario, Canada. N. Engl. J. Med. 335:547-554. [DOI] [PubMed] [Google Scholar]
- 7.Demers, B., A. E. Simor, H. Velland, P. M. Schlievert, S. Byrne, F. Jamieson, S. Walmsly, and D. E. Low. 1993. Severe invasive group A streptococcal infections in Ontario, Canada: 1987-1991. Clin. Infect. Dis. 16:792-800. [DOI] [PubMed] [Google Scholar]
- 8.Efstratiou, A. 2000. Group A streptococci in the 1990s. J. Antimicrob. Chemother. 45:3-12. [DOI] [PubMed] [Google Scholar]
- 9.Facklam, R., B. Beall, A. Efstratiou, P. Kriz, G. Tyrrell, M. Lovgren, D. Martin, and E. Kaplan. 2000. Nomenclature of new emm types of group A streptococci, p. 797-803. In D. R. Martin and J. R. Taggs (ed.). Streptococci and streptococcal diseases: entering the new millennium. Proceedings of the XIV Lancefield International Symposium on Streptococci and Streptococcal Disease. Securacopy, Porirua, New Zealand.
- 10.Facklam, R. R., B. Beall, A. Efstratiou, V. Fischetti, D. Johnson, E. Kaplan, P. Kriz, M. Lovgren, D. Martin, B. Schwartz, A. Totolian, D. Bessen, S. Hollingshead, F. Rubin, J. Scott, and G. Tyrrell. 1999. emm typing and validation of provisional M types for group A streptococci. Emerg. Infect. Dis. 5:247-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Facklam, R. R., D. R. Martin, M. Lovgren, D. R. Johnson, A. Efstratiou, T. A. Thompson, S. Gowan, P. Kriz, G. J. Tyrrell, E. Kaplan, and B. Beall. 2002. Extension of the Lancefield classification for group A streptococci by addition of 22 new M protein gene sequence types from clinical isolates: emm103 to emm124. Clin. Infect. Dis. 34:28-38. [DOI] [PubMed] [Google Scholar]
- 12.Fiscehtti, V. A. 1989. Streptococcal M protein: molecules design and biological behavior. Clin. Microbiol. Rev. 2:285-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoe, N. P., N. Kazumitsu, S. Lukomski, D. Grigsby, M. Liu, P. Kordari, S. Dou, X. Pan, J. Vuopio-Varkila, S. Salmelinna, A. McGeer, D. E. Low, B. Schwartz, A. Schuchat, S. Naidich, D. De Lorenzo, Y. Fu, and J. M. Musser. 1999. Rapid selection of complement-inhibiting protein variants in group A streptococcus epidemic waves. Nat. Med. 5:924-929. [DOI] [PubMed] [Google Scholar]
- 14.Hoe, N. P., R. M. Ireland, F. R. DeLeo, B. B. Gowe, D. W. Dorward, J. M. Voyich, M. Liu, E. H. Burns Jr., D. M. Culnan, A. Bretscher, and J. M. Musser. 2002. Insight into the molecular basis of pathogen abundance: group A Streptococcus inhibitor of complement inhibits bacterial adherence and internalization into human cells. Proc. Natl. Acad. Sci. USA 28:7646-7651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoge, C. W., B. Schwartz, D. F. Talkington, R. F. Breiman, E. M. MacNeil, and S. J. Englender. 1993. The changing epidemiology of invasive group A streptococcal infections and the emergence of streptococcal toxic shock-like syndrome. JAMA 269:384-389. [PubMed] [Google Scholar]
- 16.Holm, S. E., A. Norrby, A. Bergholm, and M. Norgen. 1992. Aspects of pathogenesis of serious group A streptococcal infections in Sweden, 1988-1989. J. Infect. Dis. 166:31-37. [DOI] [PubMed] [Google Scholar]
- 17.Johnson, D. R., D. L. Stevens, and E. L. Kaplan. 1992. Epidemiological analysis of group A streptococcal serotypes associated with severe systemic infections, rheumatic fever or uncomplicated pharyngitis. J. Infect. Dis. 166:374-382. [DOI] [PubMed] [Google Scholar]
- 18.Johnson, D. R., J. Sramek, E. L. Kaplan, R. Bicova, J. Havlicek, H. Havlickova, J. Motlova, and P. Kriz. 1996. Laboratory diagnosis of group A streptococcal infections. World Health Organization, Geneva, Switzerland.
- 19.Kaul, R., A. McGeer, D. E. Low, K. Green, B. Schwartz, et al. 1997. Population-based surveillance for group A streptococcal necrotizing fasciitis: clinical features, prognostic indicators and microbiologic analysis of seventy-seven cases. Am. J. Med. 103:18-24. [DOI] [PubMed] [Google Scholar]
- 20.Lancefield, R. C. 1928. The antigenic complex of Streptococcus haemolyticus. I. Demonstration of a type-specific substance in extracts of Streptococcus haemolyticus. J. Exp. Med. 47:91-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lancefield, R. C. 1962. Current knowledge of type-specific M antigen of group A streptococci. J. Immunol. 89:307-313. [PubMed] [Google Scholar]
- 22.Maxted, W. R., J. P. Widdowson, C. A. M. Fraser, L. C. Ball, and D. C. J. Bassett. 1973. The use of the serum opacity reaction in the typing of group A streptococci. J. Med. Microbiol. 6:83-90. [DOI] [PubMed] [Google Scholar]
- 23.O'Brien, K. L., B. Beall, N. L. Barrett, P. R. Cieslak, A. Reingold, M. M. Farley, R. Danila, E. R. Zell, R. Facklam, B. Schwartz, and A. Schuchat for the Active Bacterial Core Surveillance/Emerging Infections Program Network. 2002. Epidemiology of invasive group A Streptococcus disease in the United States 1995-1999. Clin. Infect. Dis. 35:268-276. [DOI] [PubMed] [Google Scholar]
- 24.Schwartz, B., R. R. Facklam, and B. F. Breiman. 1990. Changing epidemiology of group A streptococcal infection in the USA. Lancet 336:1167-1171. [DOI] [PubMed] [Google Scholar]