Abstract
The drug susceptibility testing performance of a broth-based method with microscopic reading of bacillary growth, the microscopic observation drug susceptibility (MODS) assay, was compared to that of the reference 7H10 agar method of proportion by using 53 isolates of Mycobacterium tuberculosis from persons at risk for multidrug-resistant TB. For isoniazid (0.1 μg/ml) and rifampin (2.0 μg/ml), there was 100% agreement between MODS results read at day 11 and the reference method. Levels of agreement for ethambutol tested at 2.5 and 7.5 μg/ml were 70 and 58%, respectively. Levels of agreement for streptomycin tested at 2.0 and 6.0 μg/ml were 77 and 51%, respectively. For isoniazid and rifampin drug susceptibility testing, MODS is as accurate as and more rapid than the reference method.
Multidrug-resistant (MDR) tuberculosis (TB) threatens the success of global TB control. Detection of drug resistance is important, but widely used drug susceptibility tests on Lowenstein-Jensen slants or agar-containing plates are slow (4, 5, 6, 9). Drug susceptibility tests performed by automated liquid culture systems are more rapid but expensive and therefore not widely used in resource-limited settings (3, 5). An accurate, rapid, inexpensive, and technically simple method for M. tuberculosis drug susceptibility testing is needed for areas with high rates of MDR TB.
The microscopic observation drug susceptibility assay (MODS) is a liquid culture method based on microscopic detection of characteristic M. tuberculosis morphology. Caviedes et al. compared the performance of MODS to that of the microwell Alamar blue assay for isoniazid (INH) and rifampin (RIF) drug susceptibility testing (3). The reported levels of concordance were 99% for INH and 90% for RIF. Moreover, direct MODS results were available at a median of 9.5 days after inoculation of patient sputum samples into the culture medium.
While promising, the performance of the MODS drug susceptibility test has not yet been compared to that of a reference method. The objectives of this study were to compare MODS with the reference 7H10 method of proportion for indirect drug susceptibility testing using INH, RIF, ethambutol (EMB), and streptomycin (SM) and to assess the MODS critical drug concentrations that best agreed with the reference method.
Fifty-three archived clinical isolates of M. tuberculosis obtained from patients at high risk for MDR TB were used for this study. M. tuberculosis strain CDC1551 was used as a drug-susceptible control. All isolates were stored frozen, thawed, and subcultured in Middlebrook 7H9 broth (Difco, Sparks, Md.) containing 0.2% glycerol, 10% albumin-dextrose-catalase (ADC; Becton Dickinson, Sparks, Md.), and 0.05% Tween 80 (Sigma, St. Louis, Mo.). Mid-log-phase cultures were diluted to a turbidity equivalent to McFarland 1.0 and then diluted to 10−3 and 10−5 in phosphate-buffered saline with 0.01% Tween 80. INH, RIF, EMB, and SM were obtained in chemically pure form (Sigma), and stock solutions were prepared according to National Committee for Clinical Laboratory Standards guidelines (8).
MODS liquid medium was prepared by using Middlebrook 7H9 broth base (Difco; 5.9 g per liter), 0.31% glycerol, 1.25 g of casein hydrolysate (Sigma), and 10% oleic ADC. Antibiotic stock solutions were added to give the following final critical drug concentrations: INH, 0.1 and 0.4 μg/ml; RIF, 2.0 μg/ml; EMB, 2.5 and 7.5 μg/ml; SM, 2.0 and 6.0 μg/ml. These critical concentrations were adopted from recommended 7H12 liquid critical concentrations (5). One milliliter of each drug medium was distributed into wells of a sterile 24-well plate (Costar, Corning, N.Y.). One hundred-microliter aliquots of diluted bacterial samples were inoculated into wells of drug-containing medium and also into control wells containing drug-free medium. Each plate contained one additional well of drug-free medium; no bacteria were inoculated into this well, which served as a control for cross contamination. Plates were sealed with polyethylene tape (Fisher, Springfield, N.J.), incubated at 37°C in an atmosphere of 5% CO2, and daily observed for 15 days with an inverted light microscope at ×40. After day 15, the wells were observed two times per week for a total observation time of 3 weeks. For the purposes of this study, growth was defined as the emergence of visually detectable (×40 magnification) serpentine clusters of bacteria (3). A sample was considered susceptible if growth was visible in the drug-free well but not in the drug-containing well. A sample was considered resistant if both the drug-free well and the drug-containing well showed visible growth; any growth observed in the drug-containing well was considered to represent resistance to that drug.
For the reference drug susceptibility test, antibiotic stock solutions were diluted and added to Middlebrook 7H10 agar (Difco) containing 10% oleic ADC to give the following critical concentrations in quadrant plates: INH, 0.2 μg/ml; RIF, 1.0 μg/ml; EMB, 5.0 μg/ml; SM, 2.0 μg/ml. One hundred-microliter aliquots of diluted bacterial samples were inoculated onto quadrants of drug-containing or drug-free media. Drug resistance in the 7H10 method of proportion was defined as 1% or more growth of colonies on the drug-containing agar quadrant compared to growth on the drug-free quadrant (5, 6, 8). Results were recorded at 21 days after inoculation. For both MODS and the reference method, readers of results were blind to the isolate identification.
According to the reference 7H10 method of proportion, 43 (81%) isolates were resistant to INH, 42 (79%) isolates were resistant to RIF, 31 (58%) isolates were resistant to EMB, and 39 (74%) isolates were resistant to SM. Six test isolates and the CDC1551 control strain were susceptible by the reference method to all tested drugs. Twenty-nine isolates were resistant to all four drugs. Thirteen other isolates were resistant to two or three drugs (12 isolates were resistant to both INH and RIF). Five isolates had single drug resistance, including one isolate with RIF monoresistance. All isolates found by MODS to have high-level resistance to SM or EMB were also resistant to low-level SM or EMB. There was no evidence of cross contamination in the wells without an inoculum.
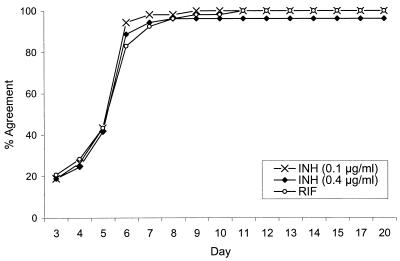
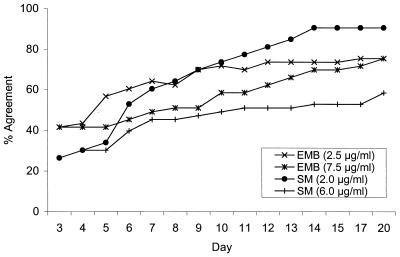
Figures 1 and 2 show the percentages of agreement between MODS results at the 10−5 dilution and the reference method results in relation to the duration of the liquid culture. Agreement between MODS and the reference method results was slightly better for the MODS 10−5 dilution than for the MODS 10−3 dilution, and consequently only MODS 10−5 dilution results are presented. By day 7, there was 93% agreement between MODS and the reference method RIF results, 98% agreement between the MODS 0.1-μg/ml INH results and the reference method INH results, and 94% agreement between the MODS 0.4-μg/ml INH results and the reference method INH results (Fig. 1). By day 11, there was 100% agreement between MODS and the reference method for both 0.1-μg/ml INH and RIF and 96% agreement for 0.4-μg/ml INH. Among isolates determined to be resistant by the reference method and by MODS, the mean time in days (±1 standard deviation) for detection of resistance by MODS was 5.7 ± 0.8 for INH at 0.1 μg/ml, 5.8 ± 0.8 for INH at 0.4 μg/ml, and 6.0 ± 1.3 for RIF. Over 90% of the INH- and RIF-resistant isolates were identified within 7 days. The percentages of agreement between MODS day 11 results and those of the reference method for EMB at 2.5 μg/ml, EMB at 7.5 μg/ml, SM at 2.0 μg/ml, and SM at 6.0 μg/ml were 70, 58, 77, and 51%, respectively (Fig. 2). Table 1 shows the number and type of disagreements between MODS and the reference method and summarizes the percentages of agreement between the two tests.
FIG. 1.
Agreement of daily MODS drug susceptibility test results with the reference method results at day 21 for INH and RIF.
FIG. 2.
Agreement of daily MODS drug susceptibility test results with the reference method results at day 21 for EMB and SM.
TABLE 1.
Characterization of agreement and disagreement between the reference method and MODS (10−5 dilution) read at day 11
| Drug | Reference method resulta | No. of isolates with indicated MODS (day 11) result at: |
Agreement (%) with MODS at: |
||||
|---|---|---|---|---|---|---|---|
| Low concn |
High concn |
||||||
| S | R | S | R | Low concn | High concn | ||
| INH | S | 10 | 0 | 10 | 0 | 100 | 96 |
| R | 0 | 43 | 2 | 41 | |||
| RIF | S | 11 | 0 | NAb | NA | 100 | NA |
| R | 0 | 42 | NA | NA | |||
| EMB | S | 16 | 6 | 22 | 0 | 70 | 58 |
| R | 10 | 21 | 22 | 9 | |||
| SM | S | 14 | 0 | 14 | 0 | 77 | 51 |
| R | 12 | 27 | 26 | 13 | |||
S, susceptible; R, resistant.
NA, not applicable.
These results indicate that for INH and RIF, MODS drug susceptibility testing is as accurate as and more rapid than the reference method. This study builds on earlier analyses of MODS drug susceptibility testing (3) by comparing MODS to a reference drug susceptibility test, namely, the 7H10 method of proportion. For EMB and SM, correlation between MODS and the reference method was poor at the two tested critical concentrations. Discrepant results for EMB and SM drug susceptibility testing have also been observed in other liquid culture systems (1, 2, 7, 9, 10).
MODS is a qualitative test in which the observer determines resistance by visualizing growth through the microscope. For MODS, unlike the reference method, there are no discrete colonies to count and therefore a proportion cannot be calculated. This is one potential explanation for disagreement between MODS and the reference method for EMB and SM, although this did not apply for INH and RIF. Another possible explanation for discrepant results is slightly different inoculum bacterial concentrations due to sampling error. Because in MODS any growth in a drug-containing well was deemed to reflect resistance to that drug, we anticipated that MODS would tend to “overcall” resistance, in that isolates read as susceptible by the reference method would be read as resistant by MODS. However, this was observed only for EMB at the low MODS drug concentration and was not the apparent reason for any other disagreements. Further evaluation of MODS is needed to better understand whether resolution of discrepant results is possible for EMB and SM.
Accurate detection of INH and RIF resistance is clinically significant since resistance to these two agents defines per se MDR TB, which requires substantially different treatment than non-MDR TB. However, if RIF resistance is detected, drug susceptibility testing of other first-line and second-line anti-TB drugs is still needed to help the clinician select the most effective treatment regimen.
Potential advantages of MODS include rapidity (compared with solid agar culture methods) and potential low cost (compared with automated liquid culture systems). Sample preparation for MODS is similar to that required for preparation of a sputum sample for smear and culture. The microscopic detection of mycobacteria by MODS is technically similar to microscopic examination of a smear. These are features that make MODS suitable for use in resource-poor settings. Aspects that limit its use in these kinds of settings are the need for appropriate biological hazard containment facilities, electricity, carbon dioxide, and aseptic techniques. Despite these barriers, the advantages of relatively rapid, inexpensive, and accurate detection of INH and RIF resistance still make MODS an attractive drug susceptibility test with potential benefits at the individual patient and public health levels.
Acknowledgments
We thank Robert Gilman for his generous gift of M. tuberculosis clinical isolates. We also thank Jacques Grosset for his critical review of the manuscript.
This work was supported by a grant from the United States Agency for International Development Gorgas Tuberculosis Initiative and by grants from the National Institute of Allergy and Infectious Diseases, National Institutes of Health (IK24 AI01637 and U19-AI45432 to R.E.C).
REFERENCES
- 1.Bergmann, J. S., and G. L. Woods. 1997. Reliability of mycobacteria growth indicator tube for testing susceptibility of Mycobacterium tuberculosis to ethambutol and streptomycin. J. Clin. Microbiol. 35:3325-3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bergmann, J. S., and G. L. Woods. 1998. Evaluation of the ESP culture system II for testing susceptibilities of Mycobacterium tuberculosis isolates to four primary antituberculous drugs. J. Clin. Microbiol. 36:2940-2943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caviedes, L., T. S. Lee, R. Gilman, P. Sheen, E. Spellman, E. H. Lee, D. E. Berg, S. Montenegro-James, and the Tuberculosis Working Group in Peru. 2000. Rapid, efficient detection and drug susceptibility testing of Mycobacterium tuberculosis in sputum by microscopic observation of broth cultures. J. Clin. Microbiol. 38:1203-1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crofton, J., P. Chaulet, and D. Maher. 1997. Guidelines for the management of drug-resistant tuberculosis. World Health Organization, Geneva, Switzerland.
- 5.Inderlied, C. B., and M. Salfinger. 1999. Antimycobacterial agents and susceptibility tests, p. 1601-1623. In P. R. Murray, E. J. Baron, M. A. Pfaller, F. C. Tenover, and R. H. Yolken (ed.), Manual of clinical microbiology, 7th ed. American Society for Microbiology, Washington, D.C.
- 6.Kent, P. T., and G. P. Kubica. 1985. Public health mycobacteriology—a guide for the level III laboratory. Centers for Disease Control, U.S. Department of Health and Human Services, Atlanta, Georgia.
- 7.Laszlo, A., P. Gill, V. Handzel, M. M. Hodgkin, and D. M. Helbecque. 1983. Conventional and radiometric drug susceptibility testing of Mycobacterium tuberculosis complex. J. Clin. Microbiol. 18:1335-1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Committee for Clinical Laboratory Standards. 2000. Susceptibility testing of mycobacteria, Nocardia, and other aerobic actinomycetes, 2nd ed. Tentative standard M24-T2. National Committee for Clinical Laboratory Standards, Wayne, Pa. [PubMed]
- 9.Siddiqi, S. H., J. P. Libonati, and G. Middlebrook. 1981. Evaluation of a rapid radiometric method for drug susceptibility testing of Mycobacterium tuberculosis. J. Clin. Microbiol. 13:908-912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Siddiqi, S. H., J. E Hawkins, and A. Laszlo. 1985. Interlaboratory drug susceptibility testing of Mycobacterium tuberculosis by a radiometric procedure and two conventional methods. J. Clin. Microbiol. 22:919-923. [DOI] [PMC free article] [PubMed] [Google Scholar]