Abstract
Urine PCR has been used for the diagnosis of Borrelia burgdorferi infection in recent years but has been abandoned because of its low sensitivity and the irreproducibility of the results. Our study aimed to analyze technical details related to sample preparation and detection methods. Crucial for a successful urine PCR were (i) avoidance of the first morning urine sample; (ii) centrifugation at 36,000 × g; and (iii) the extraction method, with only DNAzol of the seven different extraction methods used yielding positive results with patient urine specimens. Furthermore, storage of frozen urine samples at −80°C reduced the sensitivity of a positive urine PCR result obtained with samples from 72 untreated erythema migrans (EM) patients from 85% in the first 3 months to <30% after more than 3 months. Bands were detected at 276 bp on ethidium bromide-stained agarose gels after amplification by a nested PCR. The specificity of bands for 32 of 33 samples was proven by hybridization with a GEN-ETI-K-DEIA kit and for a 10 further positive amplicons by sequencing. By using all of these steps to optimize the urine PCR technique, B. burgdorferi infection could be diagnosed by using urine samples from EM patients with a sensitivity (85%) substantially better than that of serological methods (50%). This improved method could be of future importance as an additional laboratory technique for the diagnosis of unclear, unrecognized borrelia infections and diseases possibly related to Lyme borreliosis.
Laboratory methods for the diagnosis of Lyme borreliosis (LB) are still a matter of debate. Serological enzyme-linked immunosorbent assay (ELISA) and immunoblotting (IB) methods are now the tests of choice for confirming the diagnosis of a Borrelia burgdorferi infection (21). In early LB, however, serologic testing is not suitable because of its low sensitivity of ∼40%, especially since erythema migrans (EM) is a clinical diagnosis (11, 19). Recent studies compared several diagnostic modalities for early LB. The most sensitive method was quantitative PCR assay of skin biopsies (80.9%), followed by two-stage serologic testing of convalescent-phase samples (66.0%), nested PCR assay of skin biopsies (63.8%), and culture (51.1%) (11). In several other studies, PCR assays for B. burgdorferi had been performed worldwide with various organs, cultures, culture supernatants, and body fluids in clinical studies, with animal models, and with ticks by using different targets for amplification derived from chromosome- and plasmid-related molecules (17). Whereas amplification of borrelia DNA from synovial fluid and cerebrospinal fluid has become an important diagnostic tool (7, 8, 10, 14, 18), the urine PCR assay is now done only sporadically (14). In previous work, we found that ∼90% of EM patients showed a positive urine PCR assay result before treatment (15, 16). These results obtained by using the same technique, however, could not be confirmed either by us or by other researchers (personal communications; 2, 8). Recent recommendations thus rule out the urine PCR assay as a diagnostic method (2, 21). As our previous experience indicated that this method is valuable and advantageous for confirming typical and atypical borrelia infections (1), we aimed to reproduce previous data, to analyze sample preparation, handling, and storage conditions, and to optimize the detection method by hybridization of amplicons.
MATERIALS AND METHODS
Strains and spiked urine samples.
The method we used had been systematically developed previously by using cultures of B. burgdorferi sensu stricto B31, B. afzelii, and B. garinii and spiked urine samples. The specificity of the PCR method had been tested on 13 borrelia isolates and proved negative with Treponema pallidum, T. denticola, B. hermsii, and several other microorganisms that colonize the genitourethral area (15, 16). Our new experiments were performed with cultures of B. burgdorferi sensu stricto B31, B. afzelii König, and B. garinii VSBM (a gift from B. Crowe, Baxter, Orth/Donau, Austria) and spiked urine samples from healthy persons.
Patients and urine sampling.
Seventy-two urine samples from 32 females and 40 males (mean age, 41.5 [range, 7 to 81] years) with clinically diagnosed EM by the criteria of the European concerted action of risk assessment in Lyme borreliosis (19) were investigated. The incubation time was known for 68 of 72 patients and averaged 36.6 days (median, 21 days; range, 1 to 365 days). Sixty-four of the patients were investigated before treatment, and eight were investigated in the first 1 to 15 days of treatment. Extraction was performed with 10 ml of urine stored at 4°C within 3 days, or 10-ml aliquots of urine were frozen at −80°C until use and then thawed at room temperature. Urine was collected three times a day from three patients, i.e., morning, noon, and evening. Antibodies were investigated in our 72 patients by IDEIA flagellum B. burgdorferi ELISA (Dakopatts, Copenhagen, Denmark) and in 34 patients by a B. garinii IB assay (MRL Diagnostics, Cypress, Calif.) as well.
Extraction experiments.
The following seven extraction methods were used as directed by the manufacturers and compared: lysing methods with 5% Chelex (15) and alkaline lysis (14) and extraction by GeneClean (2, 5), a DNeasy tissue kit (Qiagen, Hilden, Germany), puregene-proteinase K digestion (3) (Gentra Systems Inc., Minneapolis, Minn.), and magnetic beads (Dynabeads; Dynal, Oslo, Norway).
Extraction by DNAzol.
Ten-milliliter aliquots of urine were centrifuged at 36,000 × g and 4°C for 30 min. Genomic DNA was isolated from the pellet with 1 ml of a guanidine-detergent lysing solution (DNAzol; Molecular Research Center, Inc., Cincinnati, Ohio) that hydrolyzes RNA and allows selective precipitation of DNA. The solution was then mixed with 6 μl of a polyacrylamide carrier (microcarrier Gel-TR; MRC). After 10 min, DNA was precipitated from the lysate with 500 μl of ethanol p.a. and centrifuged as described above for 20 min. The pellet was washed twice with 1 ml of 75% ethanol, the supernatant was discarded in each case, and the DNA was then solubilized in 100 μl of ultrapure water.
Nested PCR assay.
The total volume of the PCR mixture was 50 μl. Ten microliters of the sample was subjected to the first PCR. AmpliTaq Gold Polymerase (1.25 U/reaction mixture; Perkin-Elmer Cetus) and Gold buffer (Applied Biosystems, Foster City, Calif.) were used. A nested PCR was performed with a set of one outer primer pair and one inner primer pair (BBSCH1-BBSCH2 and FL7-FL59) targeting portions of the inner part of the B. burgdorferi flagellin gene (15). The flagellin PCR cycler program consisted of 30 cycles of denaturation at 94°C for 30 s, annealing at 52°C for 30 s, and extension at 72°C for 30 s. Thermocycling was preceded by a 10-min phase at 95°C. The final extension phase lasted for 10 min at 72°C. Four microliters of the amplicons obtained was used for the second PCR with the same cycler program but an annealing temperature of 58°C. To avoid cross contamination and sample carryover, pre- and post-PCR sample processing and PCR amplification were performed in separate rooms and plugged pipette tips were used for all fluid transfers. Amplicons were visualized at 276 bp on 3% agarose gels stained with ethidium bromide.
Hybridization.
Positive amplicons were verified in 33 samples by GEN-ETI-K-DEIA (DiaSorin Inc., Stillwater, Minn.) in accordance with the manufacturer's protocol. This DNA enzyme immunoassay is based on the hybridization of amplified DNA with an oligonucleotide probe (Fl 15 and Fl 16) (12) complementary to a sequence of the amplified DNA that is used to coat the wells of a microtiter plate by a streptavidin-biotin bridge. Single-stranded sample DNA (heat denatured) is added to the well, and hybrids formed are detected by a mouse anti-DNA monoclonal antibody that only reacts with double-stranded, not single-stranded, DNA; the absorbance (optical density [OD]) is then measured with a spectrophotometer at 450 and 620 nm.
Sequencing.
Ten samples of amplification products were sequenced with an ABI Prism 310 automated sequencer (Applied Biosystems). Sequence analysis was done with the BLAST server at the National Center for Biotechnology Information website.
Inhibition studies.
Thirty-six of 46 negative urine samples were spiked with 10−4 B. afzelii bacteria before performance of a PCR to determine inhibition.
Controls.
A culture of B. afzelii at a dilution of 10−4 (∼50 cells/PCR mixture with a concentration of 0.5 × 108/ml of culture) was used as a positive control, and 5 μl of water was used as a negative control. Urine samples from 59 healthy persons were used for control studies.
RESULTS AND DISCUSSION
Urine PCR was found to be a sensitive method for diagnosis of B. burgdorferi infection. However, several factors were found to be crucial for successful amplification of Borrelia DNA from patient urine samples.
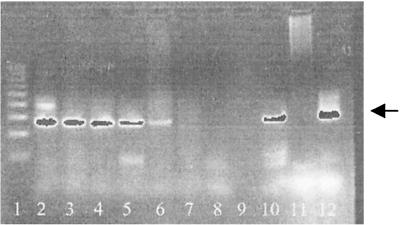
The sensitivity of the assay for cultures and spiked urine samples after DNAzol extraction was the same (about five cells per PCR mixture), indicating that all of the spiked borrelia cells could be recovered after extraction with DNAzol. In Fig. 1, the PCR results obtained with different dilutions of spiked urine after DNAzol extraction are shown. The results of PCR assays performed with cultures and spiked urine after the use of different extraction methods, outlined in Table 1, show the superiority of DNAzol extraction.
FIG. 1.
PCR after extraction with DNAzol from spiked urine samples. Lanes: 1, DNA molecular size ladder; 2 to 6, 10−1 to 10−5 dilutions of B. afzelii material; 7 to 9, 10−6 to 10−8 dilutions of B. afzelii material; 10, positive control; 11, negative control; 12, 276-bp standard.
TABLE 1.
Sensitivity of PCR assay of cultures and spiked urine samples after application of different isolation methods
| Extraction method and organism | Sensitivity (no. of cells/PCR mixture) |
|
|---|---|---|
| Culture | Spiked urine | |
| Puregene + proteinase K | NDa | |
| B. burgdorferi s.s.b | 2,500 | |
| B. garinii | 1,250 | |
| B. afzelii | 1,250 | |
| GeneClean | ND | |
| B. burgdorferi s.s. | 2,500 | |
| B. garinii | 1,250 | |
| B. afzelii | 1,250 | |
| 5% Chelex | ||
| B. burgdorferi s.s. | 10 | 1,000 |
| B. garinii | 5 | 500 |
| B. afzelii | 5 | 500 |
| Alkaline lysis | ||
| B. burgdorferi s.s. | 10 | 1,000 |
| B. garinii | 5 | 500 |
| B. afzelii | 5 | 500 |
| Qiagen | ND | |
| B. burgdorferi s.s. | 25,000 | |
| B. garinii | 12,500 | |
| B. afzelii | 12,500 | |
| Dynabeads | ND | |
| B. burgdorferi s.s. | <10,000 (neg.)c | |
| B. garinii | <5,000 (neg.) | |
| B. afzelii | <5,000 (neg.) | |
| DNAzol | ||
| B. burgdorferi s.s. | 10 | 10 |
| B. garinii | 5 | 5 |
| B. afzelii | 5 | 5 |
ND, not done.
s.s., sensu stricto.
neg., negative.
Sensitivity of urine PCR assay and inhibition.
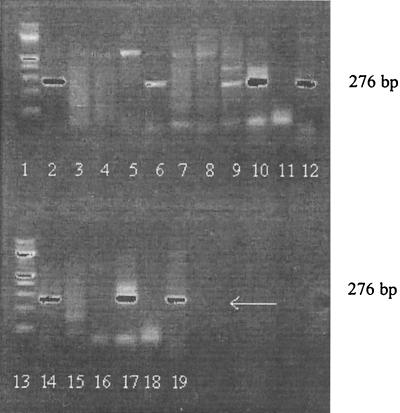
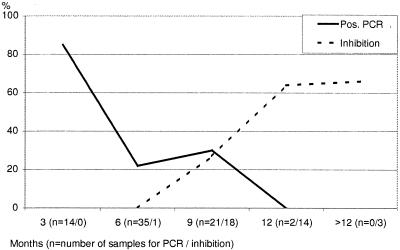
Only after DNAzol extraction were patient urine samples positive. When urine specimens were investigated immediately or after being frozen for only 3 months, 12 (85%) of 14 EM patients were positive, but after freezing for >3 months, only 14 (24.8%) of 58 patients were positive (Fig. 2 and 3). In 15 of 26 positive samples, strong bands were present; in 11 samples, bands were faint but distinct. PCR data could be reproduced by agarose gel electrophoresis when sufficient DNA was present and when electrophoresis was performed within 3 months. Only 1 of 59 healthy control individuals (mean age, 35 [range, 14 to 55] years) was PCR positive when investigated within 3 months. Positive control samples gave positive results and negative control samples gave negative results in every single experiment. None of 37 urine specimens from EM patients investigated by the other six extraction methods was PCR positive. Only after DNAzol extraction were specific bands detected on agarose gels for 5 of 12 samples reinvestigated after storage for more than 6 months.
FIG. 2.
Urine PCR after extraction of samples from EM patients by DNAzol. Lanes: 1 and 13, DNA molecular size ladder; 2, 6, 9, 10, and 14, positive patient samples; 17, positive control; 3, 4, 5, 7, 8, 11, 15, and 16, negative patient samples; 18, negative control; 12 and 19, 276-bp marker.
FIG. 3.
Correlation of sample storage time with a positive PCR assay result or inhibition.
PCR inhibition behaved similarly to the loss of PCR assay sensitivity seen after long-term freezing (Fig. 3).
Inhibition increased with storage time. Since inhibition experiments were performed with urine specimens stored for more than 6 months, the rate of inhibition could not be determined in samples frozen for shorter periods. Altogether, 17 (44%) of 36 urine specimens were inhibited. In order to find out which substances might be inhibitory in urine, we performed urinalysis of 14 specimens from EM patients in this study and 96 further specimens (urine PCR assay data not shown), or 110 urine specimens in all. Fifty-six of the 110 specimens showed pathological findings (protein, glucose, ketone, nitrite, leukocytes, erythrocytes, hemoglobulin). However, the chi-square test showed no significant difference (P > 0.05) between PCR-positive and PCR-negative urine samples. Whereas freezing and thawing of an already extracted sample of spiked urine did not influence the result, the PCR assay result was not reproducible after an extracted patient sample had been frozen. Serial sampling of urine samples from three PCR-positive patients showed that sensitivity was highest in the evening and that the result was negative in the morning for two of the three patients (Table 2).
TABLE 2.
Daily profile of B. burgdorferi DNA in urinea
| Patient no. | Morning | Noon | Evening |
|---|---|---|---|
| 1b | −/− | +/− | +/+ |
| 2 | − | + | + |
| 3 | + | + | ++ |
−, negative; +, positive; ++, strongly positive.
Investigations done in duplicate.
The amount of Borrelia DNA in the urine of EM patients seems to be small.
Urine samples centrifuged at less than 14,000 × g did not yield positive PCR results. In parallel experiments, extraction was performed with positive patients' whole urine, the supernatant, and the pellet. The PCR result was only positive with the pellet after centrifugation at 36,000 × g. Therefore, it can be assumed that DNA is lost during centrifugation at low speeds.
Hybridization and sequencing.
Hybridization with GEN-ETI-K-DEIA allowed identification of B. afzelii and/or B. garinii in 32 of 33 positive samples. Twenty-nine showed B. afzelii sequences (mean OD, 0.82; range, 0.115 to 1.858; cutoff value, 0.254), and 31 showed B. garinii sequences (mean OD, 2.5; range, 0.026 to 3.0; cutoff value, 0.199, respectively). Only one EM patient was negative by both hybridizations. Since the amplified flagellin gene sequence is highly conserved, the Fl 15 (B. garinii) and Fl 16 (B. afzelii) complementary oligonucleotide probes could not really differentiate between B. afzelii and B. garinii. Picken even reported cross-reactions of Fl 16 (B. afzelii strain P/Sto) with Borrelia strains of the DN127 group (12). Hybridization by GEN-ETI-K-DEIA nonetheless seems to be a useful semiquantitative method for confirming amplification products, as also indicated by Lebech et al.; the possible exclusion of gel electrophoresis is a further advantage (8).
A BLAST search revealed several identities for B. burgdorferi sensu lato genes for flagellin protein in all 10 of the samples investigated further (e.g., GenBank accession no. AB035617), which also confirms the specificity of our bands on agarose gels.
Serology of EM patients.
Thirty-nine of 72 patients were immunoglobulin M (IgM) and/or IgG positive. In 4 of 39 samples, the positive IgM ELISA could not be confirmed by IB; one sample that was negative by IgG ELISA was positive by IB. In summary, 36 (50%) of 72 patients were seropositive before treatment, in contrast to 85% when the urine PCR was performed within 3 months. Since LB is endemic in our area and 7.7% of healthy persons are seropositive (13), positive serology is not diagnostic of EM or other manifestations of LB. Therefore, additional diagnostic methods should be used not necessarily for typical but rather for atypical cases of LB.
Comparable with urine PCR would be PCR on skin biopsies, which was found positive for 80.9% of the samples tested (11). However, performance of skin biopsies is a more laborious and, above all, invasive method, apart from the fact that the skin is often uninvolved in the various manifestations of LB. The sensitivity of PCR testing of whole blood performed by Goodman et al. in a prospective, controlled, and blinded study yielded positive results with only 18.4% of the samples tested. This method can therefore not be used routinely (4).
Conditions for handling, storage, and extraction have been found to be crucial to the performance of PCR assays of patient material (6, 9, 20). Our study demonstrated that B. burgdorferi DNA can be detected in urine by PCR assay when certain conditions are fulfilled, such as avoidance of the first morning urine, centrifugation at 36,000 × g, extraction by DNAzol, and avoidance of freezing for >3 months. For confirmation of the PCR products, hybridization by GEN-ETI-K-DEIA can be recommended.
Acknowledgments
We thank G. Gorkiewicz, Institute of Molecular Biology, Biochemistry, and Microbiology, Karl Franzens University, Graz, Austria, for sequencing.
This work was supported by the Austrian Science Foundation (project P13668).
REFERENCES
- 1.Aberer, E., B. L. Schmidt, F. Breier, T. Kinaciyan, and A. Luger. 1999. Amplification of flagellar and RNA polymerase C gene sequences of Borrelia burgdorferi in urine of patients with granuloma anulare and lichen sclerosus et atrophicus. Arch. Dermatol. 135:210-212. [DOI] [PubMed] [Google Scholar]
- 2.Brettschneider, S., H. Bruckbauer, N. Klugbauer, and H. Hofmann. 1998. Diagnostic value for detection of Borrelia burgdorferi in skin biopsy and urine samples from patients with skin borreliosis. J. Clin. Microbiol. 36:2658-2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goodman, J. E., P. Jurkovich, J. M. Kramber, and R. C. Johnson. 1991. Molecular detection of persistent Borrelia burgdorferi in the urine of patients with active Lyme disease. Infect. Immun. 59:269-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goodman, J. E. Bradley, A. E. Ross, P. Goellner, A. Lagus, B. Vitale, B. W. Berger, S. Luger, R. C. Johnson. 1995. Bloodstream invasion in early Lyme disease: results from a prospective, controlled, blinded study using the polymerase chain reaction. Am. J. Med. 99:6-12. [DOI] [PubMed] [Google Scholar]
- 5.Karch, H., H. I. Huppertz, M. Böhme, H. Schmidt, D. Wiebecke, and A. Schwarzkopf. 1994. Demonstration of Borrelia burgdorferi DNA in urine samples from healthy humans whose sera contain B. burgdorferi-specific antibodies. J. Clin. Microbiol. 32:2312-2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kessler, H. H., E. Stelzl, R. B. Raggam, J. Haas, F. Kirchmeir, K. Hegenbarth, E. Daghofer, B. I. Santner, E. Marth, and R. E. Stauber. 2001. Effects of storage and type of blood collection tubes on hepatitis C virus level in whole blood sample. J. Clin. Microbiol. 39:1788-1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lebech, A.-M., and K. Hansen. 1992. Detection of Borrelia burgdorferi DNA in urine samples and cerebrospinal fluid samples from patients with early and late Lyme neuroborreliosis by polymerase chain reaction. J. Clin. Microbiol. 30:1646-1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lebech, A.-M., K. Hansen, F. Brandrup, O. Clemmensen, and L. Halkier-Sorensen. 2000. Diagnostic value of PCR for detection of Borrelia burgdorferi DNA in clinical specimens from patients with erythema migrans and Lyme neuroborreliosis. Mol. Diagn. 5:139-150. [DOI] [PubMed] [Google Scholar]
- 9.Liedtke, W., B. Opalka, C. W. Zimmermann, and E. Schmid. 1994. Different methods of sample preparation influence sensitivity of Mycobacterium tuberculosis and Borrelia burgdorferi PCR. PCR Methods Appl. 3:301-304. [DOI] [PubMed] [Google Scholar]
- 10.Nocton, J. J., F. Dressler, B. J. Rutledge, P. N. Rys, D. H. Persing, and A. C. Steere. 1994. Detection of Borrelia burgdorferi DNA by polymerase chain reaction in synovial fluid from patients with Lyme arthritis. N. Engl. J. Med. 330:229-234. [DOI] [PubMed] [Google Scholar]
- 11.Nowakowski, J., I. Schwartz, D. Liveris, G. Wang, M. E. Aguero-Rosenfeld, G. Girao, D. McKenna, R. B. Nadelmann, L. F. Cavaliere, and G. P. Wormser. 2001. Laboratory diagnostic techniques for patients with early Lyme disease associated with erythema migrans: a comparison of different techniques. Clin. Infect. Dis. 333:2023-2027. [DOI] [PubMed] [Google Scholar]
- 12.Picken, R. N. 1992. Polymerase chain reaction primers and probes derived from flagellin gene sequences for specific detection of the agents of Lyme disease and North American relapsing fever. J. Clin. Microbiol. 30:99-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pierer, K., T. Koeck, W. Freidl, D. Stuenzner, G. Pierer, E. Marth, H. Lechner, and J. R. Moese. 1993. Prevalence of antibodies to B. burgdorferi flagellin in Styrian blood donors. Zentbl. Bakteriol. 279:239-243. [DOI] [PubMed] [Google Scholar]
- 14.Priem, S., M. G. Rittig, T. Kamradt, G. R. Burmester, and A. Krause. 1997. An optimized PCR leads to rapid and highly sensitive detection of Borrelia burgdorferi in patients with Lyme borreliosis. J. Clin. Microbiol. 35:685-690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schmidt, B. L., E. Aberer, C. Stockenhuber, H. Klade, F. Breier, and A. Luger. 1995. Detection of Borrelia burgdorferi DNA by polymerase chain reaction in the urine and breast milk of patients with Lyme borreliosis. Diagn. Microbiol. Infect. Dis. 21:121-128. [DOI] [PubMed] [Google Scholar]
- 16.Schmidt, B. L., R. R. Muellegger, C. Stockenhuber, H. P. Soyer, S. Hoedl, S., A. Luger, and H. Kerl. 1996. Detection of Borrelia burgdorferi-specific DNA in urine specimens from patients with erythema migrans before and after antibiotic therapy. J. Clin. Microbiol. 4:1359-1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmidt, B. L. 1997. PCR in laboratory diagnosis of human Borrelia burgdorferi infections. Clin. Microbiol. Rev. 10:185-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schnarr, S., N. Putschky, M. C. Jendro, H. Zeidler, M. Hammer, J. G. Kuipers, and J. Wollenhaupt. 2001. Chlamydia and Borrelia DNA in synovial fluid of patients with early undifferentiated oligoarthritis. Arthritis Rheum. 44:2679-2685. [DOI] [PubMed] [Google Scholar]
- 19.Stanek, G., S. O. O'Conell, M. Cimmino, E. Aberer, W. Kristoferitsch, M. Granström, E. Guy, and J. Gray. 1996. European concerted action of risk assessment in Lyme borreliosis: clinical case definitions for Lyme borreliosis. Wien. Klin. Wochenschr. 108:741-747. [PubMed] [Google Scholar]
- 20.Villanueva, A. V., R. P. Podzorski, and M. P. Reyes. 1998. Effects of various handling and storage conditions on stability of Treponema pallidum DNA in cerebrospinal fluid. J. Clin. Microbiol. 36:2117-2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilske, B., L. Zoeller, V. Brade, H. Eiffert, U. B. Goebel, G. Stanek, and H. W. Pfister. 2001. MiQ 12,20 Lyme Borreliose. Qualitätsstandards in der mikrobiologisch-infektiologischen Diagnostik, p. 23-25. Urban & Fischer, Munich, Germany.