Abstract
Conventional light microscopy has been the established method for malaria diagnosis. However, recently several nonmicroscopic rapid diagnostic tests have been developed for situations in which reliable microscopy may not be available. This study was conducted to evaluate the diagnostic performance of a recently introduced ICT Malaria Pf/Pv test. This assay detects Plasmodium falciparum histidine-rich protein 2 antigen (PfHRP-2) for P. falciparum diagnosis and pan-malarial antigen for P. vivax diagnosis. In this study we compared the performance of ICT Malaria Pf/Pv with microscopy of Giemsa-stained blood films and with an OptiMAL test that detects Plasmodium lactate dehydrogenase (pLDH) antigen. A total of 750 clinically suspected malaria patients were examined at local health centers in Kuwait. Both the antigen tests had a high degree of specificity (>98%) for detection of malaria infection. However, they were less sensitive than microscopy. Compared with microscopy the ICT Malaria PF/pf test failed to detect malaria infection in 93 (34%) of 271 malaria patients (11% of patients with P. falciparum and 37% of patients with P. vivax) and the OptiMAL test failed to detect malaria infection in 41 (15%) of 271 malaria patients (7% of patients with P. falciparum and 13% of patients with P. vivax). The sensitivities of the ICT Malaria Pf/Pv and OptiMAL tests for detection of P. falciparum infection were 81 and 87%, and those for detecting P. vivax were 58 to 79%, respectively. The sensitivity of the ICT Malaria Pf/Pv and OptiMAL tests decreased significantly to 23 and 44%, respectively, at parasite densities of <500/μl. Both of the tests also produced a number of false-positive results. Overall, the performance of the OptiMAL test was better than that of the ICT Malaria Pf/Pv test. However, our results raise particular concern over the sensitivity of the ICT Malaria Pf/Pv test for detection of P. vivax infection. Further developments appear necessary to improve the performance of the ICT Malaria Pf/Pv test.
In malaria patients, a prompt and accurate diagnosis is the key to effective disease management. The two diagnostic approaches currently used most often, clinical diagnosis and microscopic diagnosis, however, do not allow a satisfactory diagnosis of malaria. Clinical diagnosis is the most widely used approach; however, the symptoms of malaria are very nonspecific and overlap those of other febrile illnesses (32). A diagnosis of malaria based on clinical grounds alone is therefore unreliable and, when possible, should be confirmed by laboratory tests. Microscopic examination of thick blood film is currently the standard method for malaria diagnosis. This method is relatively simple and has low direct costs, but its reliability is questionable, particularly at low levels of parasitemia and in the interpretation of mixed infection (17, 31).
Recently, rapid antigen detection methods have been developed for situations in which reliable microscopy may not be available. These tests are based on the detection of antigen(s) released from parasitized red blood cells (19). In the case of Plasmodium falciparum, these new methods are based on detection of P. falciparum histidine-rich protein 2 (HRP-2) (ParaSight F by Becton Dickinson, Cockeysville, Md., and the ICT Malaria Pf by ICT Diagnostics, Sydney, Australia) (2, 5, 26, 30; M. Garcia, S. Kirimoama, D. Marlborough, J. Leafasia, and K. H. Rieckmann, Letter, Lancet 347:1549, 1996) or Plasmodium-specific lactate dehydrogenase (pLDH) (OptiMAL by Flow Inc., Portland, Oreg.) (12, 13, 18, 20). Species-specific pLDH isoforms have been used to develop a test for Plasmodium vivax (OptiMAL).
The sensitivity and specificity of each of these tests have been assessed in a range of clinical situations (2, 5, 12-14, 18-20, 26, 27; Garcia et al., letter). Although the overall sensitivity and specificity of all these assays for detection of P. falciparum infection are high (usually >90%), the sensitivity falls off at parasite densities of <350/μl. Both the ICT Malaria Pf and ParaSight F tests do not detect P. vivax infection. Recently, a new test kit capable of detecting antigen of P. falciparum and P. vivax has been introduced: ICT Malaria Pf/Pv (AMRAD ICT, Frenchs Forest, New South Wales, Australia) (3, 6, 10, 15). The performance of this test has yet not been fully assessed.
In this study we compared the sensitivities of the OptiMAL and ICT Malaria Pf/Pv tests in symptomatically diagnosed malaria patients against microscopy of thick or thin blood films.
MATERIALS AND METHODS
Patients.
Seven hundred fifty patients with a clinical suspicion of malaria attending the local district hospitals and health centers participated in this study. The study was conducted during the period September 1999 to March 2002 in Kuwait. The majority of these patients were workers from tropical countries where malaria infection is endemic. There is no malaria transmission in Kuwait; however, each year more than 800 imported malaria cases are reported (11).
The symptomatic diagnosis of malaria was based on the presence of fever (temperature > 37.5°C) at the time of presentation to the health centers, or within the previous 48 h, coupled with a recent history of living in or visiting a malaria-prone country. Patients were between 2 and 50 years old. All patients who had been treated for malaria in the previous 4 weeks were excluded from the study. For each patient, a finger prick was made and the following was collected: 50 μl of blood in a preheparinized Eppendorf tube for the ICT Malaria Pf/Pv and OptiMAL tests and one thick or thin blood smear for Giemsa stain microscopy. The microscopists, ICT reader, and OptiMAL reader were all blinded to each other's diagnoses. Informed consent to participate in the study was obtained from participants, and the Ethical Committee of the Faculty of Medicine, University of Kuwait, approved the study.
Microscopy of Giemsa-stained blood films.
Thick and thin blood films were stained with 10% Giemsa stain for 10 min and examined by two experienced microscopists who had no knowledge of patient disease status or nationality to avoid any bias in blood film readings. The microscopist counted a minimum of 200 consecutive fields in the thick blood film before classifying a slide as negative.
Parasites in thick blood film were counted against 200 to 500 white blood cells. The parasite density was estimated assuming 8,000 white blood cells/μl of blood (19, 29, 31).
OptiMAL test.
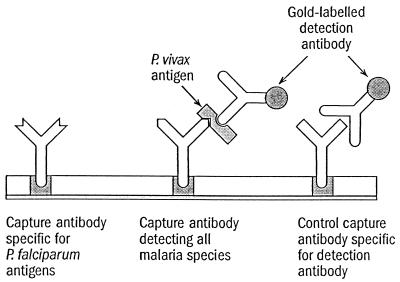
The OptiMAL test was performed following manufacturer's instructions. Briefly, 1 drop of whole blood was mixed with 2 drops of lysis buffer A, which disrupts the red blood cells and releases the pLDH, and the specimens were allowed to migrate to the top of the pLDH strip. After 8 min the strip was placed in washing buffer B, which cleared the hemoglobin from the strip. Interpretation of the test results was performed immediately. A negative control sample taken from an individual who had not been exposed to malaria for 3 years was included with each batch tested. In the pLDH assay there are two diagnostic zones of reaction containing different antibodies (Fig. 1). A monospecific antibody that recognizes only P. falciparum is present in the bottom reaction zone. A second, pan-specific antibody is present immediately above this zone. This monoclonal antibody recognizes the pLDH isoforms of P. vivax. A third reaction zone containing a pan-specific monoclonal antibody is present at the top of the test strip and serves as a procedural control for the assay.
FIG. 1.
Schematic representation of immunologic reaction on a positive strip (P. vivax infection).
ICT Malaria Pf/Pv test.
The procedural principle of the ICT Malaria Pf/Pv test is the same as that shown in Fig. 6. This test is based on detection of the P. falciparum-specific HRP-2 antigen and a pan-malarial antigen (P. vivax). The test was performed according to the manufacturer's instructions. Briefly, 10 μl of whole blood was added to a sample pad. A buffer reagent was added to induce cell lysis and allow P. falciparum HRP-2 antigen (PfHRP-2) and pan-malarial antigens to bind to colloidal gold-labeled antibody. Additional buffer caused the blood and immune complexes to migrate up the test strip and cross monoclonal antibody lines. Finally, more buffer was added to clear hemoglobin from the strip and facilitate reading. Tests were counted as valid if a control line was observed. They were considered P. falciparum positive if PfHRP-2-specific and pan-malarial antigen lines were visible or if only PfHRP-2-specfic lines were seen. If only the control and pan-malarial antigen lines were observed, the sample was counted as positive for a malaria parasite other than P. falciparum.
Statistical analysis.
Data were collected and analyzed using the SPSS statistical program. For sensitivity and specificity the test kits were compared with Giemsa stain-microscopy results. The sensitivity was calculated as the proportion of positive test results obtained among samples containing malaria parasites by microscopy; the specificity was calculated as the proportion of negative test results obtained among samples whose thick blood films were negative. Positive and negative predictive values were calculated as the proportion of true-positive results among all positively reacting samples and as the proportion of true-negative results among all negatively reacting samples, respectively.
RESULTS
Among the 750 symptomatically diagnosed malaria patients included in this study, 187 (25%) patients presented with microscopically confirmed vivax malaria. A further 54 (7%) were infected with P. falciparum. Twenty-four patients had mixed infections with P. falciparum and P. vivax, two were infected with P. malariae, and four patients had P. ovale infections (Table 1).
TABLE 1.
Parasite species detected in 750 clinically suspected malaria patients
| Species | No. of patients infected |
||
|---|---|---|---|
| Microscopy | ICT malaria Pf/Pv test | OptiMAL test | |
| P. falciparum | 54 | 48 | 50 |
| P. vivax | 187 | 118 | 162 |
| Mixed (P. falciparum and P. vivax) | 24 | 12 | 18 |
| P. malariae | 2 | 0 | 0 |
| P. ovale | 4 | 0 | 0 |
| Total positives | 271 | 178 | 230 |
| Total negatives | 479 | 572 | 619 |
The results of the detection of malaria by the two rapid antigen detection tests in comparison with microscopy are shown in Table 1. The ICT Malaria Pf/Pv test detected malarial antigen in 178 patients: 48 patients with P. falciparum infection, 118 with P. vivax infection, and 12 with mixed (P. falciparum and P. vivax) infection. The OptiMAL test detected malarial antigen in 230 patients: 50 patients with P. falciparum infection and 162 patients with P. vivax infection (Table 1). P. malariae and P. ovale were not detected by either of the antigen assays. Overall, the OptiMAL test was a better test than the ICT Malaria Pf/Pv test for detecting both P. falciparum and P. vivax infection. Using microscopy as the standard test, the ICT Malaria Pf/Pv test failed to detect malaria infection in 93 (34%) of 271 malaria patients (11% of patients with P. falciparum and 37% of patients with P. vivax), and the OptiMAL test failed to detect infection in 41 (15%) of 271 malaria patients (7% of patients with P. falciparum infection and 13% of patients with P. vivax). Compared with microscopy the performance of the ICT Malaria Pf/Pv test for P. falciparum and P. vivax parasites was as follows: sensitivity, 81 and 58%; specificity, 99 and 98%; positive predictive value, 92 and 92%; negative predictive value, 98 and 88%, respectively. The comparative performance of the OptiMAL test for P. falciparum and P. vivax parasites was as follows: sensitivity, 87 and 79%; specificity, 99 and 97%; positive predictive value, 94 and 91%; negative predictive value, 99 and 93%, respectively.
The results of sensitivity testing according to parasitemia are shown in Table 2. As expected, both the antigen tests performed suboptimally at parasitemias of <500 parasites/μl (ICT Malaria Pf/Pv test, 23% performed suboptimally; OptiMAL test, 44% performed suboptimally), but they had good sensitivity above this cutoff (ICT Malaria Pf/Pv test, 77% sensitivity; OptiMAL test, 96% sensitivity). However, it was of particular concern that both the tests failed to detect at least five patients (two by ICT Malaria test and three by OptiMAL test) that had parasitemias of >5,000/μl (Table 2). In addition, both the ICT Malaria Pf/Pv and OptiMAL tests yielded positive tests for P. falciparum and P. vivax infection with samples which were negative by microscopy (ICT Malaria test, four cases of falciparum infection and 9 cases of vivax infection; OptiMAL test, three cases of falciparum infection and 14 cases of vivax infection).
TABLE 2.
Parasite densities of specimens from patients with microscopy-confirmed malaria infections
| Parasite density (parasites/μl) | No. of specimens with indicated density as determined by: |
||
|---|---|---|---|
| Microscopy | ICT Malaria Pf/Pv test | OptiMAL test | |
| <500 | 48 | 11 | 21 |
| 500-5,000 | 192 | 144 | 188 |
| >5,000 | 25 | 23 | 21 |
| Total | 265 | 178 | 230 |
DISCUSSION
The development of easy, rapid, and accurate tests for the detection of plasmodial infection is highly desirable. In this study we investigated the performance of the ICT Malaria Pf/Pv test and OptiMAL test, relative to microscopy, with samples from symptomatically diagnosed malaria patients. Previous studies of OptiMAL and ICT tests have shown variable results (2, 5, 12-14, 18-20, 26; Garcia et al., letter).
Compared with microscopy, the ICT Malaria Pf/Pv test performed well with P. falciparum (sensitivity 81%); however, it failed to detect P. vivax infection in 69 (42%) patients.
The results of this study show that both ICT Malaria and OptiMAL tests detect falciparum malaria with high sensitivities: 81 and 87%, respectively. Detection of falciparum antigen by both the tests was very specific (99%). Similar sensitivities and specificities have been recorded for other test kits detecting the HRP-2 antigen of P. falciparum (5, 12-14, 18-20, 26; Garcia et al., letter). However, data regarding the detection of P. vivax using the ICT Malaria Pf/Pv test are scarce and variable. The sensitivity of the ICT Malaria Pf/Pv test for vivax malaria conducted at the village level in Myanmar was 66.7 to 79% (3), and that within the Network TropNet Europ was 76% (15). Compared with microscopy the performance of this test to detect vivax infection was less satisfactory (sensitivity, 58%; specificity. 98%) in our study, and the sensitivity fell to only 23% in patients with parasitemias of <500/μl. Further studies with a large set of patients with P. vivax infection from various areas of endemicity need to be investigated before definitive recommendations for the use of the ICT Malaria Pf/Pv test kit in diagnosing vivax malaria are possible.
Both the ICT Malaria Pf/Pv and OptiMAL tests detected P. falciparum and P. vivax infections in samples which were negative by microscopic examination (ICT Malaria Pf/Pv test, four patients with falciparum infection and nine patients with vivax infection; OptiMAL test, three patietns with falciparum infection and 14 patients with vivax infection). False-positive tests have been reported in earlier investigations (3, 12, 13), but they appear to play a minor role in evaluating the usefulness of malaria tests for clinical settings. False-positive reactions may occur in individuals who have been recently treated for malaria as reported earlier (2, 24-26) or if patients have circulating rheumatoid factors (3, 15; M. P. Grobusch, U. Alpermann, S. Schwenkl, T. Jelinek, and D. C. Warhurst, Letter, Lancet 353:297, 1999). The preliminary data show that PfHRP-2 antigen, which is detected by the ICT Malaria Pf/Pv test, may persist for up to 7 to 10 days after asexual parasite clearance (7, 24; Garcia et al., letter), whereas circulating pLDH activity, which is detected by the OptiMAL test, drops profoundly immediately after the parasites are cleared from the peripheral blood (21). Thus, the OptiMAL test may provide the potential to monitor the effectiveness of antimalarial therapy and thus assist in the detection of drug-resistant infections.
In addition, false-negative results by microscopy can occur if patients have undertaken self-medication prior to presentation. It is likely that some of our patients with false-positive results may have performed self-medication with antimalarial drugs during an attack of fever. However, it is unlikely that these factors account for the entire set of false-positive cases. It is more probable that most of the false-positive cases were true positives which were not detected by microscopy, due to sequestration limiting the number of circulating parasites at the time of blood collection or due to the parasitemia being below the detection limit of approximately 50/μl by microscopy.
The performance of both the ICT Malaria Pf/Pv and OptiMAL tests was greatly influenced by the level of parasitemia in peripheral blood. The sensitivity of the OptiMAL test was 96% and that of the ICT Malaria test was 77% at parasitemias of >500/μl; however, the sensitivity dropped to 44 and 23%, respectively, at parasitemias of <500/μl. Our findings are consistent with earlier findings (8, 12, 17, 19, 22, 23, 27, 32). This can potentially be dangerous, as to miss the diagnosis of malaria in an ambulant, febrile patient may mean that complications develop because appropriate treatment was not instituted in time. The assessment of a negative result in this situation will be clearly influenced by the clinical features. In this study both the antigen test kits missed at least five cases of malaria in samples with high-level parasitemia (>5,000 parasites/μl). False-negative dipstick test results in samples with higher parasitemias have been observed in earlier studies, but the underlying reason is not known (1, 4, 9, 16, 20, 28).
In conclusion, our results add to the evidence that these nonmicroscopical rapid tests for the detection of plasmodial antigens may develop into important diagnostic tools and can prove to be a valuable adjunct to clinical assessment of the patient and blood film microscopy under certain circumstances. These tests are rapid and simpler to perform and to interpret. However, their sensitivity indicates that they should not yet be regarded as first-line diagnostic tests. In our study the performance of the OptiMAL test to detect P. falciparum and P. vivax infection in symptomatically diagnosed patients was better than that of the ICT Malaria Pf/Pv test. Our results raise particular concern over the sensitivity of the ICT Malaria Pf/PV test for nonfalciparum (P. vivax) infection, which was only 58%. Thick blood film examination is still the standard method for diagnosing malaria because it detects all Plasmodium species and offers the clear distinctions between parasite growth stages, which are essential for therapeutic decisions.
Acknowledgments
The financial support provided by Kuwait University (grants MI 109 and MI 113) is gratefully acknowledged.
We thank the Ministry of Health of Kuwait for allowing access to malaria patients.
REFERENCES
- 1.Anonymous. 1996. A rapid dipstick antigen capture assay for the diagnosis of falciparum malaria. W. H. O. informal consultation on recent advances in diagnostic techniques and vaccines for malaria. Bull. W. H. O. 74:47-54. [PMC free article] [PubMed] [Google Scholar]
- 2.Beadle, C., G. W. Long, W. R. Weiss, P. D. McElroy, S. M. Maret, A. J. Oloo, and S. L. Hoffman. 1994. Diagnosis of malaria by detection of Plasmodium falciparum HRP-2 antigen with a rapid dipstick antigen-capture assay. Lancet 343:564-568. [DOI] [PubMed] [Google Scholar]
- 3.Cho-Min-Naing, and M. L. Gatton. 2002. Performance appraisal of rapid on-site malaria diagnosis (ICT Malaria Pf/Pv test) in relation to human resources at village level in Myanmar. Acta Trop. 81:13-19. [DOI] [PubMed] [Google Scholar]
- 4.Cooke, A., H., P. L. Chiodini, T. Doherty, A. H. Moody, J. Ries, and M. Pinder. 1999. Comparison of a parasite lactate dehydrogenase-based immunochromatographic antigen detection assay (OptiMAL) with microscopy for the detection of malaria parasites in human blood samples. Am. J. Trop. Med. Hyg. 60:173-176. [DOI] [PubMed] [Google Scholar]
- 5.Durrheim, D., N., J. J. P. la Grange, J. Govere, and N. M. Mngomezulu. 1998. Accuracy of a rapid immunochromatographic card test for Plasmodium falciparum in a malaria control programme in South Africa. Trans. R. Soc. Trop. Med. Hyg. 92:32-33. [DOI] [PubMed] [Google Scholar]
- 6.Dyer, M. E., E. Tjitra, B. J. Currie, and N. M. Anstey. 2000. Failure of ‘pan malarial' antibody of the ICT Malaria Pf/Pv immunochromatographic test to detect symptomatic Plasmodium malariae infection. Trans. R. Soc. Trop. Med. Hyg. 94:518. (Erratum, 95:80, 2001.) [DOI] [PubMed] [Google Scholar]
- 7.Eisen, D. P., and A. Saul. 2000. Disappearance of pan-malarial antigen reactivity using the ICT Malaria Pf/Pv kit parallels decline of patent parasitemia as shown by microscopy. Trans. R. Soc. Trop. Med. Hyg. 94:169-170. [DOI] [PubMed] [Google Scholar]
- 8.Fryauff, D., J., Purnomo, and M. A. Sutamihardja. 2000. Performance of the OptiMAL assay for detection and identification of malaria infections in asymptomatic residents of Irian Java, Indonesia. Am. J. Trop. Med. Hyg. 63:139-145. [DOI] [PubMed] [Google Scholar]
- 9.Humar, A., M. A. Harrington, D. Pillai, and K. C. Kain. 1997. ParaSight-F test compared with the polymerase chain reaction and microscopy for the diagnosis of P. falciparum malaria in travellers. Am. J. Trop. Med. Hyg. 56:44-48. [DOI] [PubMed] [Google Scholar]
- 10.Huong, N., M., T. M. E. Davis, S. Hewitt, N. V. Huong, T. T. Uyen, D. H. Nhan, and L. D. Cong. 2002. Comparison of three antigen detection methods for diagnosis and therapeutic monitoring of malaria: a field study from southern Vietnam. Trop. Med. Int. Health 7:304-308. [DOI] [PubMed] [Google Scholar]
- 11.Iqbal, J., A. Sher, and P. R. Hira. 1998. Malaria in non-endemic Kuwait: detection of very low level of Plasmodium falciparum infections using polymerase chain reaction. Med. Principles Practice 7:277-282. [Google Scholar]
- 12.Iqbal, J., A. Sher, P. R. Hira, and R. Al-Owaish. 1999. Comparison of the OptiMAL test with PCR for diagnosis of malaria in immigrants. J. Clin. Microbiol. 39:3644-3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iqbal, J., P. R. Hira, A. Sher, and A. A. Al-Enezi. 2001. Diagnosis of imported malaria by Plasmodium lactate dehydrogenase (pLDH) and histidine-rich protein 2 (PfHRP-2)-based immunocapture assays. Am. J. Trop. Med. Hyg. 64:20-23. [DOI] [PubMed] [Google Scholar]
- 14.Jelinek, T., M. P. Grobusch, and H. Nothdurft. 2000. Use of dipstick test for the rapid diagnosis of malaria in non-immune travelers. J. Travel. Med. 7:175-179. [DOI] [PubMed] [Google Scholar]
- 15.Jelinek, T., M. P. Grobusch, and G. Harms. 2001. Evaluation of a dipstick test for the rapid diagnosis of imported malaria among patients presenting within the Network TropNetEurop. Scand. J. Infect. Dis. 33:752-754. [DOI] [PubMed] [Google Scholar]
- 16.Karbwang, J., O. Tasanor, T. Kanda, Y. Wattanagoon, M. Ibrahim, K. Na-Bangchang, A. Thanavibul, and W. Rooney. 1996. ParaSight F test for the detection of treatment failure in multidrug resistant Plasmodium falciparum malaria. Trans. R. Soc. Trop. Med. Hyg. 90:513-515. [DOI] [PubMed] [Google Scholar]
- 17.Molyneux, M., and R. Fox. 1993. Diagnosis and treatment of malaria in Britain. BMJ 306:1175-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moody, A., A. Hunt-Cooke, E. Gabbett, and P. Chiodini. 2000. Performance of the OptiMAL malaria antigen capture dipstick for malaria diagnosis and treatment monitoring at the Hospital for Tropical Diseases, London. Br. J. Haematol. 109:891-894. [DOI] [PubMed] [Google Scholar]
- 19.Moody, A. 2002. Rapid diagnostic tests for malaria parasites. Clin. Microbiol. Rev. 15:66-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Palmer, C., J., J. F. Lindo, W. I. Klaskala, J. A. Quesada, R. Kaminsky, M. K. Baum, and A. L. Ager. 1998. Evaluation of the OptiMAL test for rapid diagnosis of Plasmodium vivax and Plasmodium falciparum malaria. J. Clin. Microbiol. 36:203-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Piper, R., J. Lebras, L. Wentworth, A. Hunt-Cooke, S. Houze, P. Chiodini, and M. Makler. 1999. Immunocapture diagnostic assays for malaria using Plasmodium lactate dehydrogenase (pLDH). Am. J. Trop. Med. Hyg. 60:109-118. [DOI] [PubMed] [Google Scholar]
- 22.Proux, S., L. Hkirijareon, C. Ngamngonkiri, S. McConnell, and F. Nosten. 2001. Paracheck-Pf: a new, inexpensive and reliable rapid test for P. falciparum malaria. Trop. Med. Int. Health 6:99-101. [DOI] [PubMed] [Google Scholar]
- 23.Ricci, L., I. Viani, and G. Piccolo. 2000. Evaluation of OptiMAL assay test to detect imported malaria in Italy. N. Microbiol. 23:391-398. [PubMed] [Google Scholar]
- 24.Shiff, C. J., Z. Premji, and J. N. Minjas. 1993. The rapid manual ParaSight F test: a new diagnostic tool for Plasmodium falciparum infection. Trans. R. Soc. Trop. Med. Hyg. 87:646-648. [DOI] [PubMed] [Google Scholar]
- 25.Shiff, C. J., J. N. Minjas, and Z. Premji. 1994. The ParaSight F test: a simple rapid manual dipstick test to detect Plasmodium falciparum infection. Parasitol. Today 10:494-495. [DOI] [PubMed] [Google Scholar]
- 26.Singh, N., N. Valecha, and V. P. Sharma. 1997. Malaria diagnosis by field workers using an immunochromatographic test. Trans. R. Soc. Trop. Med. Hyg. 91:396-397. [DOI] [PubMed] [Google Scholar]
- 27.Singh, N., A. Saxena, and N. Valecha. 2000. Field evaluation of the ICT malaria Pf/Pv immunochromatographic test for diagnosis of Plasmodium falciparum and P. vivax infection in forest village of Chhindwara, central India. Trop. Med. Int. Health 5:765-770. [DOI] [PubMed] [Google Scholar]
- 28.Van den, Ende, J., T. Vervoort, A. Van Gompel, and L. Lynen. 1998. Evaluation of two tests based on the detection of histidine rich protein 2 for the diagnosis of imported Plasmodium falciparum malaria. Trans. R. Soc. Trop. Med. Hyg. 92:285-288. [DOI] [PubMed] [Google Scholar]
- 29.Warhurst, D., C., and J. E. Williams. 1996. Laboratory diagnosis of malaria: ACP broadsheet no. 148. J. Clin. Pathol. 49:533-538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wongsrichanalai, C., N. Chuanak, S. Tulyayon, N. Thanoosingha, A. Laoboonchai, K. Thaimasarn, T. G. Brewer, and D. G. Happner. 1999. Comparison of a rapid field immunochromatographic test to expert microscopy for the detection of Plasmodium falciparum asexual parasitemia in Thailand. Acta Trop. 73:263-273. [DOI] [PubMed] [Google Scholar]
- 31.World Health Organization. 1996. Management of uncomplicated malaria and the use of antimalarial drugs for the protection of travellers, p. 98. Report of an informal consultation. W.H.O./MAL/96. World Health Organization, Geneva, Switzerland.
- 32.World Health Organization. 1999. New perspectives: malaria diagnosis. Report of a joint W.H.O./USAID informal consultation. W. H. O./MAL/2000.1091. World Health Organization, Geneva, Switzerland