Hepatitis C virus (HCV) is a single-stranded RNA virus that belongs to the family Flaviviridae (6). HCV was initially recognized as non-A, non-B hepatitis virus (NANBH) in 1974 until cloning of the etiologic agent in 1989 (2, 10). The global prevalence of HCV infection is approximately 3% (170 million people) (21). In the United States, approximately 3.9 million people (1.8% of the population) are HCV seropositive (19).
The primary mode of HCV transmission is exposure to infected human blood via intravenous drug use or unscreened transfusions (21). The practice of screening donors for HCV antibodies in developed countries since 1990 has substantially lowered the risk of acquiring HCV infection from a transfusion to approximately 1 in 263,000 (39). This is based on results from nucleic amplification testing (NAT) of pooled donor samples that has been performed in North America since March 1999, in which by July 2000, 62 donations from 16.3 million seronegative donors were identified by NAT to be positive for HCV (39). Nosocomial HCV transmission during dialysis, colonoscopy, and surgery has also been reported (21). The rate of HCV seroconversion among health care workers after a needlestick injury is 0 to 7% (9). Perinatal and sexual transmission of the virus is inefficient, but occurs more frequently if the HCV-infected mother or sexual partner is also infected with human immunodeficiency virus type 1 (HIV-1) (9, 43).
Most people with acute HCV infection are asymptomatic or have mild symptoms (fatigue, nausea, jaundice) but are unable to clear the virus, leading to chronic infection in approximately 80% of cases (21). Chronic HCV infection progresses at a variable rate to cirrhosis in 15 to 20% of patients, who then have a 1 to 4% annual risk of developing hepatocellular carcinoma (21). HCV-associated end-stage liver disease is the leading indication for liver transplantation in American adults (19).
Screening of the general population for HCV infection is not recommended. In addition to blood donors, diagnostic testing should be performed for individuals with risk factors for HCV infection who may need medical care (9). Detailed recommendations for identifying those individuals have been outlined by the Centers for Disease Control and Prevention (9).
Although advances have been made, a reliable culture system for HCV is not yet available (6). Laboratory assays that are available for the diagnosis and management of HCV infection include (i) serologic tests to detect HCV antibodies, (ii) molecular tests to detect and quantitate HCV RNA, and (iii) genotyping techniques. Assays to detect and quantify HCV core antigen have also been developed. Performance characteristics and clinical use of these assays will be discussed.
SEROLOGIC ASSAYS
Screening EIAs.
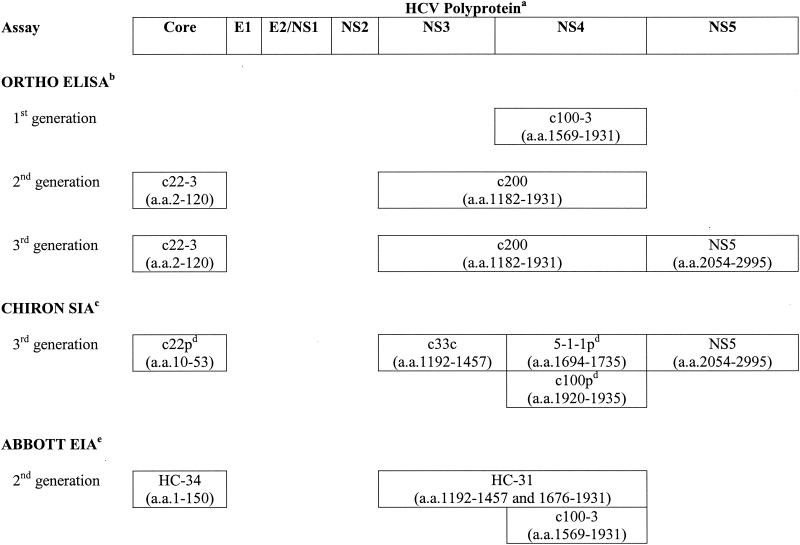
The initial test used to diagnose HCV is an enzyme immunoassay (EIA) for anti-HCV immunoglobulin G (IgG). The HCV genome encodes a polyprotein of 3,011 to 3,033 amino acids that is processed into 10 structural and nonstructural (NS) proteins (6). Three generations of screening EIAs have been developed to detect antibodies against various epitopes of these proteins (Fig. 1).
FIG. 1.
HCV antigens used for serologic assays. a, E, envelope; NS, nonstructural protein; a.a., amino acid sequence of recombinant protein or synthetic peptide antigen. b, Ortho HCV ELISA (version 3.0; Ortho-Clinical Diagnostics, Inc.). c, Chiron RIBA HCV strip immunoassay (SIA; version 3.0; Chiron Corporation). d, p, synthetic peptide. e, Abbott HCV EIA (version 2.0; Abbott Laboratories).
First-generation EIAs (EIAs 1.0) used the c100-3 epitope of an NS protein (NS4) (20). The sensitivities of these EIAs were low for a high-prevalence population (approximately 80%), and the fraction of positive results that were false positive was as high as 70% for a low-prevalence population (blood donors) (15). This led to the development of more sensitive and specific second-generation EIAs (EIAs 2.0) that incorporated additional antigens from NS (c33c) and structural (c22-3) proteins that were approved for use by the Food and Drug Administration (FDA) in 1992. Second-generation assays detect HCV antibodies in 20% more patients with acute NANBH and in 10% more patients with chronic cases of infection than EIAs 1.0 do and detect HCV antibodies 30 to 90 days sooner than EIAs 1.0 do (3). The mean window of seroconversion was reduced from 16 weeks with EIAs 1.0 to 10 weeks with EIAs 2.0 (15). The sensitivities of EIAs 2.0 in a high-prevalence population are approximately 95% (based on HCV RNA detection by PCR) (15).
In 1996, FDA approved a third-generation EIA (EIA 3.0) that added a fourth antigen (NS5) to those in EIAs 2.0. EIA 3.0 detected antibodies an average of 26 days earlier in 5 of 21 individuals with transfusion-transmitted HCV (4), and the sensitivity is slightly better than that of EIA 2.0 in a high-prevalence population (as high as 97%) (18). Both the Abbott HCV EIA (version 2.0; Abbott Laboratories, Abbott Park, Ill.) and the Ortho HCV enzyme-linked immunosorbent assay (ELISA; version 3.0; Ortho-Clinical Diagnostics, Inc., Rochester, N.Y.) are used in the United States.
Confirmatory anti-HCV assays.
Chiron Corporation (Emeryville, Calif.) developed a strip immunoassay to help resolve true-positive from false-positive EIA results. FDA approved the second-generation recombinant immunoblot assay (RIBA) in 1993, followed by approval of the third-generation RIBA in 1999. The strip immunoassays include the EIA antigens and human superoxide dismutase (hSOD). Because the recombinant antigens c33c and NS5 used for EIA are made as fusion proteins with hSOD, recombinant hSOD is included on the RIBA strip to detect nonspecific antibodies. RIBA is considered positive if there are reactions with at least two antigens with intensities greater than or equal to that for the weak IgG control and no reactivity with hSOD. Indeterminate RIBAs are those in which there are reactions with only one antigen or the hSOD plus one or more HCV antigens. Replacement of the c100 and c22 recombinant proteins with synthetic peptides in the version 3.0 RIBA has significantly reduced the number of indeterminate RIBA results (31, 32).
Use of serologic assays.
Of the 25 to 35% of patients with acute infection who develop symptoms, only 50 to 70% will have detectable antibodies at that time, but 90% will have measurable antibodies after 3 months (29). Serologic assays detect HCV antibodies that indicate present or previous infection, but they cannot discriminate acute from chronic or resolved infection. Anti-HCV IgM antibodies can be detected in 50 to 93% of patients with acute HCV infections and 50 to 70% of chronic cases, so they are not a reliable indicator of acute infection (30).
Confirmation by RIBA is needed only for low-risk patients (healthy blood donors) or if a high-risk patient is HCV RNA negative (25, 33). Confirmation by RIBA has not been very useful for resolving weakly positive samples (optical density ratios between 1 and 2), and molecular HCV RNA detection is recommended instead (33). Individuals with indeterminate RIBA results should be evaluated by a sensitive HCV RNA detection test (29).
Patients with acute hepatitis of uncertain origin and negative hepatitis serology panels should undergo qualitative HCV RNA testing (12). Occasionally, immunocompromised patients, patients undergoing hemodialysis, and patients with mixed cryoglobulinemia have false-negative serology results and may require HCV RNA testing for diagnosis (13, 21, 42). Passively transferred maternal anti-HCV antibodies may be detected in the children of HCV-infected mothers for up to 1 year; however, defined diagnostic criteria for HCV RNA detection are not available (9, 43).
HCV RNA DETECTION AND QUANTITATION
The presence of HCV RNA in plasma defines active infection, and HCV RNA can be detected 1 to 3 weeks postexposure (29). A single negative HCV RNA assay result does not exclude the possibility of active infection with a transient drop in the level of viremia below the assay's limit of detection (29).
Laboratories detect HCV RNA with commercially available assay kits (Table 1) or by in-house home-brewed methods. Because of the limited amount of HCV RNA in infected individuals, a target or signal amplification step is needed. Reverse transcriptase (RT) PCR (RT-PCR) and transcription-mediated amplification (TMA) are target amplification methods. The branched DNA (bDNA) assay is a signal amplification technique.
TABLE 1.
Commercially available HCV RNA detection assaysa
| Test | Method | Manufacturera | Lower limit of detection (IU/ml) | Linear range of quantification (IU/ml) | Primary application |
|---|---|---|---|---|---|
| Qualitative | |||||
| AMPLICOR HCV test, version 2.0 | RT-PCR | Roche | 50 | Evidence of active infection and response to therapy | |
| VERSANT HCV RNA qualitative assay | TMA | Bayer | 5 | Evidence of active infection and response to therapy | |
| Ampliscreen HCV test, version 2.0 | RT-PCR | Roche | <50 | Blood screening | |
| Procleix HIV-1/HCV assay | TMA | Chiron | <50 | Blood screening | |
| Quantitative | |||||
| AMPLICOR HCV MONITOR, version 2.0 | RT-PCR | Roche | 600 | 600-500,000 | Determination of viral load and length of therapy |
| VERSANT HCV RNA assay, version 3.0 | bDNA | Bayer | 615 | 520-8,300,000 | Determination of viral load and length of therapy |
Roche, Roche Diagnostics; Bayer, Bayer Diagnostics; Chiron, Chiron Corporation.
For RT-PCR, an RT step converts RNA to cDNA, which is used as a template for the PCR (41). Primers whose sequences correspond to the 5′ untranslated region (5′ UTR) are commonly used because this is the most conserved region of the genome (8). The Roche AMPLICOR HCV test (Roche Diagnostics, Branchburg, N.J.) includes 37 amplification cycles followed by hybridization to an HCV-specific oligonucleotide probe. The semiautomated version of the AMPLICOR HCV test uses the COBAS instrument to reduce the hands-on time required for detection and calculation by the technologist (1). The qualitative AMPLICOR HCV test (version 2.0) received FDA approval in 2001 and has a lower limit of detection of 50 IU/ml (23). The less sensitive quantitative HCV RNA assays lack FDA approval and are available only for research purposes.
TMA involves a more complex set of reactions with T7 RNA polymerase and RT under isothermal conditions to form detectable levels of RNA (41). TMA uses primers that contain a T7 RNA polymerase binding site so that RT synthesizes cDNA that becomes a template from which T7 RNA polymerase can synthesize numerous copies of RNA. The RNA amplicons reenter the TMA cycle and become templates for the next replication cycle. The TMA-based VERSANT HCV RNA qualitative assay (Bayer Diagnostics, Tarrytown, N.Y.) is not approved by FDA, but it is able to detect very low levels of HCV RNA (5 IU/ml) that are undetectable with RT-PCR systems (37, 38). The Procleix HIV-1/HCV assay also uses TMA technology and was approved by FDA in February 2002 for the screening of blood donations to identify HCV-positive donors who are antibody negative (17). A PCR-based blood screening assay (Ampliscreen HCV Test, version 2.0) is also expected to attain FDA approval.
The bDNA method of RNA detection uses solid-phase oligonucelotide probes that capture target RNA, followed by hybridization of a branched secondary (bDNA) probe (41). The bDNA amplifiers bind to enzyme-conjugated tertiary probes, and after substrate is added, the chemiluminescence produced is proportional to the amount of target RNA. The VERSANT HCV RNA assay (version 3.0; Bayer Diagnostics, Tarrytown, N.Y.) is a semiautomated quantitative bDNA test with a sensitivity better than that of the previous manual Quantiplex HCV RNA assay (version 2.0) that is attributed to improved probe numbers and an improved design. There is also a lower level of background activity due to redesigned label extenders and the use of nonnatural synthetic nucleotides. A study comparing the COBAS AMPLICOR HCV MONITOR assay (version 2.0) to the VERSANT HCV RNA assay (version 3.0) reported a correlation coefficient of 0.941 (5). It was reported that the AMPLICOR test had a higher level of quantitation than the VERSANT assay for samples with 500 to 100,000 IU/ml and underestimated the amount of RNA in specimens with >100,000 IU/ml (5). Another comparative study reported overall substantial agreement between the results of the VERSANT and AMPLICOR assays but reported significant differences when the assays were used to determine whether the quantity was above or below the 800,000-IU/ml threshold that has been proposed for adjustment of the length of treatment with combination agents (14).
Because of the higher sensitivities of commercially available qualitative assays in comparison to those of quantitative assays (Table 1), the value of quantitative assays has been limited to pretreatment evaluations. Commercial assays no longer report results in numbers of copies of RNA per milliliter, which represent different amounts of RNA, depending on the assay. The World Health Organization international standard has provided a common unit of measure that allows comparison between results from different assays (35). Qualitative assays should be used to confirm viremia and assess the therapeutic response until quantitative assays with comparable sensitivities are available.
GENOTYPING
On the basis of its extensive genetic heterogeneity, HCV has been divided into six major genotypes (represented by Arabic numerals) and at least 80 subtypes (represented by lowercase letters) (11). Different genotypes share approximately 65% sequence homology (11). Genotypes 1, 2, and 3 are found throughout the world; but the other genotypes are common in particular geographic regions (genotype 4 is common in North Africa and the Middle East, genotype 5 is common in South Africa, and genotype 6 is common in Southeast Asia) (44). The predominant genotype in patients with chronic HCV infection in the United States is genotype 1 (72% of patients), followed by genotype 2 (16%) and genotype 3 (10%) (28).
Molecular and serologic methods may be used to determine HCV genotypes. Commercial assays are available, but none are approved by FDA. The predominant molecular techniques used (hybridization and direct DNA sequencing) are based on nucleotide differences in the highly conserved 5′ UTR among genotypes.
The widely used INNO-LiPA HCV II assay (Innogenetics, Ghent, Belgium) uses PCR products from the 5′ UTR that hybridize to type-specific probes embedded on a nitrocellulose strip (40). The Trugene HCV 5′ noncoding region genotyping kit (Visible Genetics, Inc., Toronto, Ontario, Canada) determines genotypes by direct sequencing of the 5′ UTR (16). The Trugene and INNO-LiPA assays can be performed with amplification products obtained from the Roche AMPLICOR assays, and a recent comparison reported that the two assays have similar abilities to determine genotypes (16). The two assays have difficulty differentiating subtypes, with accuracies of 76 and 74%, respectively, compared to that of the “gold standard” method that sequences a different region of the genome, NS5B (16). Since management decisions have been made on the basis of genotype, not subtype, the overall accuracies of both assays have been considered acceptable.
Serologic methods rely on the detection of antibodies to genotype-specific epitopes in the NS4 or core region. Compared to molecular assays, serologic genotyping is easier to perform and less expensive, but it has lower sensitivity and specificity (22, 34).
THERAPY
The results of HCV RNA detection and genotyping assays have been incorporated into algorithms that can be used to make management decisions, but guidelines must be reassessed as new therapeutic agents become available. Indications for the treatment of patients with chronic HCV infection include detectable HCV, increased alanine aminotransferase levels, and histologic evidence of liver fibrosis and moderate inflammation (29). The efficacy of therapy is measured by whether a sustained virologic response (SVR) occurs. SVR is defined as undetectable HCV RNA 24 weeks after the cessation of treatment (12). Treatment for chronic HCV infection has evolved from interferon monotherapy, which results in a disappointing SVR rate of 10 to 20% (29), to combination therapy with interferon plus ribavirin, which is associated with a higher SVR rate of nearly 40% (27, 36). Optimal therapy is now considered to be weekly pegylated interferon plus daily ribavirin, with SVRs reported in more than half of the patients treated (SVR rate, 54%) (26). Infection with a non-genotype 1 strain has been identified as the strongest independent predictor of SVRs for all of these therapeutic regimens (24).
The duration of standard interferon plus ribavirin therapy has been based on the viral genotype and the pretreatment viral load. The SVR rates for patients infected with genotype 2 or 3 are essentially the same for 24 and 48 weeks of therapy, showing no benefit for the longer course of therapy (28, 36). In 1999, an international consensus panel recommended interferon plus ribavirin therapy for 24 weeks in patients infected with genotype 2 or 3 isolates (12). For patients infected with genotype 1 isolates, the panel recommended 48 weeks of interferon plus ribavirin therapy for those with a high pretreatment viral load (>800,000 IU/ml) and only 24 weeks of therapy for patients with those with a low pretreatment viral load (<800,000 IU/ml) (12, 35). A recent 48-week trial of pegylated interferon plus ribavirin reported a 42% SVR rate for patients with genotype 1 infections and an 82% SVR rate for patients with genotype 2 and 3 infections (26). Trials are under way to assess whether the duration of pegylated interferon therapy can be reduced on the basis of genotype and pretreatment viral load.
The more sensitive qualitative HCV RNA assays are recommended to make an early prediction of whether SVR will ultimately be achieved if patients complete the full course of therapy. For patients receiving interferon monotherapy, American and European consensus conferences recommended the cessation of treatment for patients with detectable HCV RNA after 12 weeks of therapy (12, 29). For patients receiving interferon plus ribavirin, week 24 is considered the most accurate time to assess the response and consider the cessation of therapy, since 10% of patients with detectable HCV RNA at 12 weeks will attain an SVR (27). A recent analysis of patients participating in three trials of pegylated alfa-2a interferon suggested that a decision to stop treatment could be made as early as week 12 if there is detectable HCV RNA or a <2-log10 drop in RNA levels (24).
ANTIGEN DETECTION
An accurate and specific ELISA for the detection and quantitation of HCV core antigen (Total HCV Core Ag assay; Ortho-Clinical Diagnostics, Rochester, N.Y.) has recently been developed (7). The assay has better sensitivity than an earlier version because of an immune complex dissociation step prior to antigen detection with a monoclonal antibody (7). The performance of the assay correlates well with those of molecular HCV RNA detection methods, but the lower level of detection (20,000 IU/ml) is significantly higher (7). A more sensitive assay is under development. Antigen detection may be useful in regions where the cost of molecular HCV testing has been prohibitive.
SUMMARY
It was noted in 1974 that 75% of cases of transfusion-associated hepatitis were caused by NANBH, but 15 years passed before advances in molecular biology led to the cloning of the NANBH agent and anti-HCV assays were developed (2). In a little more than a decade, our knowledge of and ability to diagnose and treat HCV infections have increased dramatically. In 1990, mass screening of blood donors was implemented in the United States and was estimated to have prevented 40,000 HCV infections in the first year (2). Improvements in EIAs and the performance of NAT on donations prior to transfusion have further lowered the incidence of transfusion-transmitted HCV (39).
Although the incidence of new HCV infections in the United States has declined, the population of individuals infected for ≥20 years who are at risk for serious complications is projected to increase until about 2015 (19). Improvements in therapy have resulted in better virologic response rates, and molecular HCV tests have proved useful in making management decisions. As new therapeutic options evolve and laboratory assays change, the clinical relevance and use of laboratory testing for HCV will require frequent reassessments. Consensus conferences that include experts from a variety of disciplines provide an ideal environment where these issues can be debated (12, 29).
REFERENCES
- 1.Albadalejo, J., R. Alonso, R. Antinozzi, M. Bogard, A.-M. Bourgault, G. Colucci, T. Fenner, H. Petersen, E. Sala, J. Vincelette, and C. Young. 1998. Multicenter evaluation of the COBAS AMPLICOR HCV assay, an integrated PCR system for rapid detection of hepatitis C virus RNA in the diagnostic laboratory. J. Clin. Microbiol. 36:862-865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alter, H. 1999. Discovery of non-A, non-B hepatitis and identification of its etiology. Am. J. Med. 107(Suppl. 6B):16S-20S. [DOI] [PubMed] [Google Scholar]
- 3.Alter, H. J. 1992. New kit on the block: evaluation of second-generation assays for detection of antibody to the hepatitis C virus. Hepatology 15:350-353. [DOI] [PubMed] [Google Scholar]
- 4.Barrera, J. M., B. Francis, G. Ercilla, M. Nelles, D. Achord, J. Darner, and S. R. Lee. 1995. Improved detection of anti-HCV in post-transfusion hepatitis by a third-generation ELISA. Vox Sang. 68:15-18. [DOI] [PubMed] [Google Scholar]
- 5.Beld, M., R. Sentjens, S. Rebers, C. Weegink, J. Weel, C. Sol, and R. Boom. 2002. Performance of the new Bayer VERSANT HCV RNA 3.0 assay for quantitation of hepatitis C virus RNA in plasma and serum: conversion to international units and comparison with the Roche COBAS Amplicor HCV Monitor, version 2.0, assay. J. Clin. Microbiol. 40:788-793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bendinelli, M., M. Pistello, F. Maggi, and M. Vatteroni. 2000. Blood-borne hepatitis viruses: hepatitis B, C, D, and G viruses and TT virus, p. 306-337. In S. Specter, R. L. Hodinka, and S. A. Young (ed.), Clinical virology manual, 3rd ed. ASM Press, Washington, D.C.
- 7.Bouvier-Alias, M., K. Patel, H. Dahari, S. Beaucourt, P. Larderie, L. Blatt, C. Hezode, G. Picchio, D. Dhumeaux, A. U. Neumann, J. G. McHutchison, and J.-M. Pawlotsky. 2002. Clinical utility of total HCV core antigen quantification: a new indirect marker of HCV replication. Hepatology 36:211-218. [DOI] [PubMed] [Google Scholar]
- 8.Bukh, J., R. H. Purcell, and R. H. Miller. 1992. Importance of primer selection for the detection of hepatitis C virus RNA with the polymerase chain reaction assay. Proc. Natl. Acad. Sci. USA 89:187-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. 1998. Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. Morb. Mortal. Wkly. Rep. 47(RR-19):1-39. [Google Scholar]
- 10.Choo, Q.-L., G. Kuo, A. J. Weiner, L. R. Overby, D. W. Bradley, and M. Houghton. 1989. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science 244:359-362. [DOI] [PubMed] [Google Scholar]
- 11.Davis, G. L. 1999. Hepatitis C virus genotypes and quasispecies. Am. J. Med. 107(Suppl. 6B):21S-26S. [DOI] [PubMed] [Google Scholar]
- 12.European Association for the Study of the Liver Consensus Panel. 1999. European Association for the Study of the Liver International Consensus Conference on Hepatitis C. J. Hepatol. 30:956-961. [PubMed] [Google Scholar]
- 13.Fabrizi, F., F. F. Poordad, and P. Martin. 2002. Hepatitis C infection and the patient with end-stage renal disease. Hepatology 36:3-10. [DOI] [PubMed] [Google Scholar]
- 14.Germer, J. J., P. J. Heimgartner, D. M. Ilstrup, W. S. Harmsen, G. D. Jenkins, and R. Patel. 2002. Comparative evaluation of the VERSANT HCV RNA 3.0, QUANTIPLEX HCV RNA 2.0, and COBAS AMPLICOR HCV MONITOR version 2.0 assays for quantification of hepatitis C virus RNA in serum. J. Clin. Microbiol. 40:495-500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gretch, D. R. 1997. Diagnostic tests for hepatitis C. Hepatology 26(3 Suppl. 1):43S-47S. [DOI] [PubMed] [Google Scholar]
- 16.Halfon, P., P. Trimoulet, M. Bourliere, H. Khiri, V. De Ledinghen, P. Couzigou, J. M. Feryn, P. Alcaraz, C. Renou, H. J. A. Fleury, and D. Ouzan. 2001. Hepatitis C virus genotyping based on 5′ noncoding sequence analysis (Trugene). J. Clin. Microbiol. 39:1771-1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jackson, J. B., K. Smith, C. Knott, A. Korpela, A. Simmons, E. Piwowar-Manning, S. McDonough, L. Mimms, and J. M. Vargo. 2002. Sensitivity of the Procleix HIV-1/HCV assay for detection of human immunodeficiency virus type 1 and hepatitis C virus RNA in a high-risk population. J. Clin. Microbiol. 40:2387-2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kao, J.-H., M.-Y. Lai, Y.-T. Hwang, P.-M. Yang, P.-J. Chen, J.-C. Sheu, T.-H. Wang, H.-C. Hsu, and D.-S. Chen. 1996. Chronic hepatitis C without anti-hepatitis C antibodies by second-generation assay: a clinicopathologic study and demonstration of the usefulness of a third-generation assay. Dig. Dis. Sci. 41:161-165. [DOI] [PubMed] [Google Scholar]
- 19.Kim, W. R., R. S. Brown, N. A. Terrault, and H. El-Serag. 2002. Viral hepatitis: hepatitis C. In Burden of liver disease in the United States: summary of a workshop. Hepatology 36:227-242. [DOI] [PubMed] [Google Scholar]
- 20.Kuo, G., Q.-L. Choo, H. J. Alter, G. L. Gitnick, A. G. Redeker, R. H. Purcell, T. Miyamura, J. L. Dienstag, et al. 1989. An assay for circulating antibodies to a major etiologic virus of human non-A, non-B hepatitis. Science 244:362-364. [DOI] [PubMed] [Google Scholar]
- 21.Lauer, G. M., and B. D. Walker. 2001. Hepatitis C virus infection. N. Engl. J. Med. 345:41-52. [DOI] [PubMed] [Google Scholar]
- 22.Lee, J.-H., W. K. Roth, and S. Zeuzem. 1997. Evaluation and comparison of different hepatitis C virus genotyping and serotyping assays. J. Hepatol. 26:1001-1009. [DOI] [PubMed] [Google Scholar]
- 23.Lee, S. C., A. Antony, N. Lee, J. Leibow, J.-Q. Yang, S. Soviero, K. Gutekunst, and M. Rosenstraus. 2000. Improved version 2.0 qualitative and quantitative AMPLICOR reverse transcription-PCR tests for hepatitis C virus RNA: calibration to international units, enhanced genotype reactivity, and performance characteristics. J. Clin. Microbiol. 38:4171-4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee, S. S., E. J. Heathcote, K. R. Reddy, S. Zeuzem, M. W. Fried, T. L. Wright, P. J. Pockros, D. Haussinger, C. I. Smith, A. Lin, and S. C. Pappas. 2002. Prognostic factors and early predictability of sustained viral response with peginterferon alfa-2a (40KD). J. Hepatol. 37:500-506. [DOI] [PubMed] [Google Scholar]
- 25.Lott, J. A., F. S. Nolte, D. R. Gretch, R. S. Koff, and L. B. Seeff. 2000. Laboratory guidelines for screening, diagnosis and monitoring hepatic injury, p. 21-23. In D. R. Dufour (ed.), Laboratory medicine practice guidelines. National Academy of Clinical Biochemistry, Washington, D.C.
- 26.Manns, M. P., J. G. McHutchison, S. C. Gordon, V. K. Rustgi, M. Shiffman, R. Reindollar, Z. D. Goodman, K. Koury, et al. 2001. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomized trial. Lancet 358:958-965. [DOI] [PubMed] [Google Scholar]
- 27.McHutchison, J. G., J. A. Shad, S. C. Gordon, T. R. Morgan, M.-H. Ling, J.-J. Garaud, J. K. Albrecht, and J. L. Dienstag. 2001. Predicting response to initial therapy with interferon plus ribavirin in chronic hepatitis C using serum HCV RNA results during therapy. J. Viral Hepat. 8:414-420. [DOI] [PubMed] [Google Scholar]
- 28.McHutchison, J. G., S. C. Gordon, E. R. Schiff, M. L. Shiffman, W. M. Lee, V. K. Rustgi, Z. D. Goodman, M.-H. Ling, et al. 1998. Interferon alfa-2b alone or in combination with ribavirin as initial treatment for chronic hepatitis C. N. Engl. J. Med. 339:1485-1492. [DOI] [PubMed] [Google Scholar]
- 29.National Institutes of Health Consensus Development Conference Panel. 1997. Management of hepatitis C. Hepatology 26(Suppl. 1):2S-10S. [DOI] [PubMed] [Google Scholar]
- 30.Pawlotsky, J.-M. 1999. Diagnostic tests for hepatitis C. J. Hepatol. 31(Suppl.1.):71-79. [DOI] [PubMed] [Google Scholar]
- 31.Pawlotsky, J.-M., A. Bastie, C. Pellet, J. Remire, F. Darthuy, L. Wolfe, C. Sayada, J. Duval, and D. Dhumeaux. 1996. Significance of indeterminate third-generation hepatitis C virus recombinant immunoblot assay. J. Clin. Microbiol. 34:80-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pawlotsky, J.-M., A. Fleury, V. Choukroun, L. Deforges, F. Roudot-Thoraval, P. Aumont, J. Duval, and D. Dhumeaux. 1994. Significance of highly positive c22-3 “indeterminate” second-generation hepatitis C virus (HCV) recombinant immunoblot assay (RIBA) and resolution by third-generation HCV RIBA. J. Clin. Microbiol. 32:1357-1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pawlotsky, J.-M., I. Lonjon, C. Hezode, B. Raynard, F. Darthuy, J. Remire, C.-J. Sousy, and D. Dhumeaux. 1998. What strategy should be used for diagnosis of hepatitis C virus infection in clinical laboratories? Hepatology 27:1700-1702. [DOI] [PubMed] [Google Scholar]
- 34.Pawlotsky, J.-M., L. Prescott, P. Simmonds, C. Pellet, P. Laurent-Puig, C. Labonne, F. Darthuy, J. Remire, et al. 1997. Serological determination of hepatitis C virus genotype: comparison with a standardized genotyping assay. J. Clin. Microbiol. 35:1734-1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pawlotsky, J.-M., M. Bouvier-Alias, C. Hezode, F. Darthuy, J. Remire, and D. Dhumeaux. 2000. Standardization of hepatitis C virus RNA quantification. Hepatology 32:654-659. [DOI] [PubMed] [Google Scholar]
- 36.Poynard, T., P. Marcellin, S. S. Lee, C. Niederau, G. S. Minuk, G. Ideo, V. Bain, J. Heathcote, S. Zeuzem, C. Trepo, and J. Albrecht. 1998. Randomised trial of interferon α2b plus ribavirin for 48 weeks or for 24 weeks versus interferon α2b plus placebo for 48 weeks for treatment of chronic infection with hepatitis C virus. Lancet 352:1426-1432. [DOI] [PubMed] [Google Scholar]
- 37.Ross, R. S., S. O. Viazov, S. Hoffmann, and M. Roggendorf. 2001. Performance characteristics of a transcription-mediated nucleic acid amplification assay for qualitative detection of hepatitis C virus RNA. J. Clin. Lab. Anal. 15:308-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sarrazin, C., D. A. Hendricks, F. Sedarati, and S. Zeuzem. 2001. Assessment, by transcription-mediated amplification, of virologic response in patients with chronic hepatitis C virus treated with peginterferon α-2a. J. Clin. Microbiol. 39:2850-2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stramer, S. L., S. Caglioti, and D. M. Strong. 2000. NAT of the United States and Canadian blood supply. Transfusion 40:1165-1168. [DOI] [PubMed] [Google Scholar]
- 40.Stuyver, L., R. Rossau, A. Wyseur, M. Duhamel, B. Vanderborght, H. Van Heuverswyn, and G. Maertens. 1993. Typing of hepatitis C virus isolates and characterization of new subtypes using a line probe assay. J. Gen. Virol. 74:1093-1102. [DOI] [PubMed] [Google Scholar]
- 41.Tang, Y.-W., and D. H. Persing. 1999. Molecular detection and identification of microorganisms, p. 215-244. In P. R. Murray, E. J. Baron, M. A. Pfaller, F. C. Tenover, and R. H. Yolken, ed., Manual of clinical microbiology, 7th ed. ASM Press, Washington, D.C.
- 42.Thio, C. L., K. R. Nolt, J. Astemborski, D. Vlahov, K. E. Nelson, and D. L. Thomas. 2000. Screening for hepatitis C virus in human immunodeficiency virus-infected individuals. J. Clin. Microbiol. 38:575-577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zanetti, A. R., E. Tanzi, and M. L. Newell. 1999. Mother-to-infant transmission of hepatitis C virus. J. Hepatol. 31(Suppl. 1):96-100. [DOI] [PubMed] [Google Scholar]
- 44.Zein, N. N. 2000. Clinical significance of hepatitis C virus genotypes. Clin. Microbiol. Rev. 13:223-235. [DOI] [PMC free article] [PubMed] [Google Scholar]