Abstract
The isothermal nucleic acid sequence-based amplification (NASBA) system was applied for the detection of rhinoviruses using primers targeted at the 5′ noncoding region (5′ NCR) of the viral genome. The nucleotide sequence of the 5′ NCRs of 34 rhinovirus isolates was determined to map the most conserved regions and design more appropriate primers and probes. The assay amplified RNA extracted from 30 rhinovirus reference strains and 88 rhinovirus isolates, it did not amplify RNA from 49 enterovirus isolates and other respiratory viruses. The assay allows one to discriminate between group A and B rhinoviruses. Sensitivities for the detection of group B and group A rhinoviruses was 20 and 200 50% tissue culture infective doses, respectively.
Rhinoviruses (RVs) are the most frequent cause of acute upper respiratory tract infections in humans and are usually associated with the common cold (22, 24). However, they can also cause lower respiratory tract infections resulting in severe disease in children, in the elderly (17, 19, 23, 26), and in immunosuppressed patients (11, 43; N. Rabella, M. Otegui, M. Gurgui, R. Labeaga, M. Herrero, J. M. Munoz, and G. Prats, Abstr. 9th Eur. Congr. Clin. Microbiol. Infect. Dis., abstr. P1006, 1999). RVs have been isolated in association with an underlying disease, such as cystic fibrosis (37), and in cases of otitis media (3) and sinusitis (31). In addition, RV infections may exacerbate chronic bronchitis and asthma (9, 15, 16, 28).
Andries et al., through evaluation of capsid-binding compounds, classified the RVs into two groups: A and B (2). This classification correlates with sequence similarities.
Detection of RVs by tissue culture is slow and cumbersome due to the specific culture conditions required. This limits diagnostic capabilities to a few reference laboratories (29). Serologic diagnosis is virtually impossible because of the existence of more than 100 serotypes.
Several studies have shown RT-PCR to be more sensitive than culture for the detection of RVs (1, 10, 14, 15, 16, 31, 33). These assays are based on known sequences of the RV serovars.
Nucleic acid sequence-based amplification (NASBA) (Organon Teknika, Boxtel, The Netherlands) might offer an interesting alternative to reverse transcriptase PCR (RT-PCR), because it directly amplifies RNA. It uses the simultaneous enzymatic activities of avian myeloblastosis virus (AMV) RT, RNase H, and T7 RNA polymerase under isothermal conditions. The NASBA technique has already been successfully applied for the detection of human immunodeficiency virus type 1 (HIV-1) RNA (18), citrus tristeza virus (20), human papillomavirus (35), Mycoplasma pneumoniae (21), malaria parasites (36), human hepatitis C virus (34), and Mycobacterium leprae (40) and for the detection and identification of Mycobacterium avium and M. tuberculosis (41).
We applied NASBA for the detection of RVs. Because the sensitivity of the assay based on the known sequences of RVs was unsatisfactory, we determined the sequence of the 5′ nocoding regions (NCRs) of 34 additional RV strains, selected new primers and probes, and assessed their specificities and sensitivities for RV strains.
MATERIALS AND METHODS
Virus strains.
The 30 RV reference strains (group A: 4, 6, 13, 14, 17, 26, 27, 43, 45, 52, 69, 70, 72, 84, 86, and 91; group B: 2, 15, 29, 30, 31, 39, 41, 44, 51, 56, 59, 63, 85, 89) were kindly provided by K. Andries, Janssen Research Foundation, Beerse, Belgium (2). RVs were cultured on MRC-5 cells, and the 50% tissue culture infective dose (TCID50) were determined according to the method of Kärber (13). Eighty-eight untyped RVs isolated between 1990 and 1998 from respiratory specimens at the University Hospital Antwerp were included as well as a clinical isolate of each of the following: coxsackie viruses B1 to B6; echoviruses 1, 3, 11, 20, and 30; polioviruses 1, 2, and 3; 35 untyped enterovirus isolates; influenza A and B virus; parainfluenza virus 2 and 3; human adenovirus; respiratory syncytial virus; and herpes simplex viruses types 1 and 2. Viruses were cultured and titrated in the appropriate cell lines (MRC-5, MDCK, or Hep-2 cells).
Sequence analysis of the 5′ NCR of 34 RV strains.
The 5′ NCRs of a panel of 34 RVs were sequenced. Eighteen reference strains of serovars 6, 13, 17, 27, 29, 39, 43, 45, 51, 52, 59, 69, 70, 72, 84, 85, 86, and 91 and 16 clinical isolates were chosen arbitrarily. Nucleic acids were isolated by the method described by Boom et al. (5). In brief, 100 μl of a virus suspension was added to a guanidinium thiocyanate (GuSCN) (Sigma-Aldrich, Bornem, Belgium) solution, pH 6.2 (4.7 M GuSCN; 46 mM Tris-HCl, pH 6.2; 20 mM EDTA, 1.2% [wt/vol] Triton X-100), and mixed vigorously for rapid lysis. Seventy microliters of activated silica (1-g/ml suspension in 0.1 M HCl; Sigma-Aldrich) was added. The mixture was washed twice with washing solution (5.25 M GuSCN; 50 mM Tris-HCl, pH 6.2), twice with 70% (vol/vol) ethanol, and once with acetone. After drying at 56°C, nucleic acids were eluted from the silica using 100 μl RNase-, DNase-free H2O and stored at −70°C for sequencing and analysis by NASBA.
Five microliters of the nucleic acid solutions of the RV strains were used in a 50-μl single-tube RT-PCR reaction using the Access RT-PCR System (Promega, Leiden, The Netherlands) according to the instructions of the manufacturer. RT-PCR conditions were 1× AMV/Tfl reaction buffer, a 0.2 mM concentration of each deoxynucleoside triphosphate, 0.02 pmol of primers OL-26 and OL-27 (10) per ml, 0.5 mM MgSO4, AMV RT (0.1 U/μl), and Tfl DNA polymerase (0.1 U/μl). After the RT reaction at 48°C for 45 min, the resulting 5′ NCR cDNA was amplified by one incubation step at 94°C for 2 min, 40 cycles at 94°C for 30 s, 60°C for 1 min, 68°C for 2 min each, and one final incubation step at 68°C for 7 min. To avoid contamination sample preparation, setup of the reactions and setup of the product analysis were done in separate rooms. As a control, negative samples were processed simultaneously. Sequencing of the resulting amplification products was performed by Eurogentec (Seraing, Belgium) using primer OL-26.
Primers and probes.
Primers and probes used are listed in Table 1. The primers initially used (pd 904, pd 905, pd 906, and pd 907) were based on the published sequences of the RV 5′ NCRs (4, 6, 27, 38). The 5′ end of the P1 primers is a T7 RNA polymerase promoter sequence followed by a stretch of nucleotides complementary to the sequence of the target RNA. The sequences of the P2 primers are identical to the target RNA sequence.
TABLE 1.
RV primer and probe sequences
| Oligonucleotide | Sequenceb | Positionc | Function |
|---|---|---|---|
| Primer | |||
| Rhino-P1.1 pd 904a | 5′ AATTCTAATACGACTCACTATAGGGAGAGAGCACACGGGGGTCTTCACA 3′ | 239-258 | Downstream primer group B RV |
| Rhino-P1.5 EG 59 | 5′ AATTCTAATACGACTCACTATAGGGAGAGAGCACACGGGGCTCTTC 3′ | 242-258 | Downstream primer group B RV |
| Rhino-P2.1 pd 906a | 5′ AGACCTGGCAGATGAGGCT 3′ | 131-149 | Upstream primer group B RV |
| Rhino-P1.2 pd 905a | 5′ AATTCTAATACGACTCACTATAGGGAGAGAGCACACGGGGCTCTTCACA 3′ | 239-258 | Downstream primer group A RV |
| Rhino-P1.6 pd 1275 | 5′ AATTCTAATACGACTCACTATAGGGAGAGAGCTCAGTGGGCTCTTC 3′ | 242-258 | Downstream primer group A RV |
| Rhino-P2.2 pd 907a | 5′ TAGTCTGGTCGATGAGGCT 3′ | 131-149 | Upstream primer group A RV |
| Rhino-P2.3 pd 1274 | 5′ TAGCTTAGGCTGATGAGTC 3′ | 131-149 | Upstream primer group A RV |
| OL-26d | 5′ GCACTTCTGTTTCCCC 3′ | RT-PCR primer | |
| OL-27d | 5′ CGGACACCCAAAGTAG 3′ | RT-PCR primer | |
| Probe | |||
| Rhino-BIO1 pd 908a | 5′ CCCCACTGGCGACAGTGT 3′ | 157-174 | Biotin capture probe group B RV |
| Rhino-BIO3 pd 1147 | 5′ CCCCACTGGYRACAGTGK 3′ | 157-174 | Biotin capture probe group B RV |
| Rhino-N1 pd 910a | 5′ AGCCTGCGTGGCTGCCTGC 3′ | 178-196 | ECL probe group B RV |
| Rhino-BIO2 pd 909a | 5′ CCCCACGGGCGACCGTGT 3′ | 157-174 | Biotin capture probe group A RV |
| Rhino-BIO8 pd 1277 | 5′ CCCCACTGGTGACAGTGG 3′ | 157-174 | Biotin capture probe group A RV |
| Rhino-N2 pd 911a | 5′ AGCCTGCGTGGCGGCCAGC 3′ | 178-196 | ECL probe group A RV |
| Rhino-N5 pd 1276 | 5′ AGGCTGCGTTGGGGCCTAC 3′ | 178-196 | ECL probe group A RV |
At first, primer mix 1, containing pd 904, pd 905, pd 906, and pd 907, was applied to all RVs. Primer mix 2, containing pd 905, pd 907, pd 1274, and pd 1275, was used for the amplification of group A viruses, and primer mix 3 containing EG 59 and pd 906 was used for the amplification of group B viruses.
For electrochemiluminescence (ECL) detection, biotin-labeled probes were used in combination with ruthenium-labeled detection probes. Probe combinations I (consisting of pd 909 and pd 911) and II (pd 909, pd 911, pd 1276, and pd 1277) were used to detect group A viruses and probe combinations III (pd 908 and pd 910) and IV (pd 908, pd 910, and pd 1147) were used to detect group B viruses.
The new primers and probes were chosen on the basis of the manual alignments of the P1 and P2 binding sites and on the hybridization sites of the ECL and biotin probes. No specific software was used.
NASBA and ECL detection.
Nucleic acids were extracted by the method of Boom et al. (5). NASBA reactions were performed as described by Ovyn et al. (30). In negative controls, target nucleic acid was replaced by 5 μl of RNase-, DNase-free H2O. The amplification products were processed immediately for ECL detection (42).
Amplicons were captured by hybridization with specific biotin-labeled oligonucleotide probes bound to streptavidin-coated magnetic particles and were detected with ruthenium labeled ECL probes (Table 1). For hybridization, 5 μl of the amplicons, diluted according to the instructions of the manufacturer, were added to a mixture consisting of 10 μl of biotin capture probe and 10 μl of ECL detection probe followed by a 30-min incubation at 41°C. Finally, 300 μl of NucliSens Reader assay buffer (Organon Teknika) were added and read in an ECL reader (Organon Teknika). To determine the cut off, 100 different individual truly negative samples were evaluated by both assays. In each run, a tube with the reference solution was included. The measured counts were recalculated to the same signal of the reference solution, i.e., 20,000 counts. The average and three times the standard deviation of the 100 measurements were calculated. The cutoff level is expressed relative to the signal of the reference solution, according to the following formula: [(3 × standard deviation) + average]/20,000. The results of both NASBA assays were considered positive above a cutoff of 0.017× and 0.015 times the signal of the reference solution (or above 2.01 and 1.96 times the signal of the negative control, correspond in both cases to the average of the negative samples plus 3 standard deviations) for RV group A and RV group B, respectively.
To measure cross hybridization, the amplicons obtained from the RV reference strains were hybridized with all four probe combinations.
Specificity and sensitivity of the NASBA assays.
The specificity of the assays was determined by testing the respiratory and enteric viruses listed above. The sensitivity of the NASBA assays was determined on serial 10-fold dilutions of the RV reference strains in tissue culture medium (Life Technologies, Merelbeke, Belgium). Amplifications were performed with primer mixes 1 and 2 for group A viruses, and primer mixes 1 and 3 for group B viruses, followed by detection with probe combinations I and II for group A viruses and combinations III and IV for group B viruses.
Typing of RV clinical isolates.
Nucleic acids from 88 clinical RV isolates were amplified with the three primer mixtures. The amplicons obtained with primer mixture 1 were hybridized with probe combinations I and III and the amplicons obtained with primer mixtures 2 and 3 were hybridized with probe combinations II and IV for the detection of group A and group B viruses, respectively.
Nucleotide sequence accession numbers.
The nucleotide sequence data reported in this paper have been deposited in the GenBank sequence database under accession no. AF542419 to AF542452.
RESULTS
Sensitivities of NASBA primers.
The sensitivity of the assay obtained with primer mix 1 (pd 904, pd 905, pd 906, and pd 907) and probe combinations I (pd 909 and pd 911) and III (pd 908 and pd 910) was not satisfactory for both groups of RVs (Tables 2 and 3). After sequencing of additional strains and synthesis of new primers (pd 1274, pd 1275, and EG59) and probes (pd 1147, pd 1276, and pd 1277) covering a broader spectrum of RV strains, primer mix 2, composed of pd 905, pd 907, pd 1274, and pd 1275, and probe combination II (pd 909, pd 911, pd 1276, and pd 1277) were used for the amplification and detection of group A viruses. Primer mix 3, composed of EG 59 and pd 906, and probe combination IV (pd 908, pd 910, and pd 1147) were used for the amplification and detection of group B viruses.
TABLE 2.
Group A RVs sensitivity of NASBA using the different primer and probe sets
| RV reference strain | Detection level (TCID50/ml)
|
|
|---|---|---|
| Primer mix 1, probe combination I | Primer mix 2, probe combination II | |
| 4 | 2,000 | |
| 6 | ||
| 13 | 2,000 | |
| 14 | ||
| 17 | 20,000 | 200 |
| 26 | ||
| 27 | 2,000 | |
| 43 | 2,000 | |
| 45 | 2,000 | |
| 52 | 1,800 | |
| 69 | 2,000 | |
| 70 | 20,000 | 2,000 |
| 72 | ||
| 84 | ||
| 86 | ||
| 91 | 2,000 | |
TABLE 3.
Group B RVs sensitivity of NASBA using the different primer and probe sets
| RV reference strain | Detection level (TCID50/ml)
|
|
|---|---|---|
| Primer mix 1, probe combination III | Primer mix 3, probe combination IV | |
| 2 | NDa | 200 |
| 15 | 20 | 20 |
| 29 | 0.2 | 2 |
| 30 | ND | 20 |
| 31 | ND | 20 |
| 39 | 200,000 | 20 |
| 41 | 200,000 | 200 |
| 44 | ND | 20 |
| 51 | 200,000 | 200 |
| 56 | ND | 20 |
| 59 | 2,000 | 20 |
| 63 | 200 | 20 |
| 85 | 200,000 | 20 |
| 89 | 2,000 | 20 |
ND, not done.
For group B viruses maximal sensitivity (2 to 200 TCID50/ml) was obtained with primer mixture 3 in combination with probe combination IV for RV serovars 2, 15, 29, 30, 31, 39, 41, 44, 51, 56, 59, 63, 85, and 89 (Table 3). For group A RVs, the sensitivity obtained with primer mix 2 and probe combination II was lower for RV serovars 4, 13, 27, 43, 45, 52, 69, 70, and 91; a negative result was obtained for serovars 6, 14, 26, 72, 84, and 86 (Table 2).
Specificity of the NASBA RV assay.
Primer mixture 1 directed the amplification of group A and group B RVs, whereas primer mixtures 2 and 3 directed the amplification of group A and group B RVs, respectively. Probe combinations I and II hybridized specifically with group A RV amplicons and probe combinations III and IV hybridized specifically with group B RV amplicons.
NASBA performed with these primers and probes on RNA extracted from other respiratory viruses remained negative except for one untyped enterovirus clinical isolate.
Typing of RV clinical isolates.
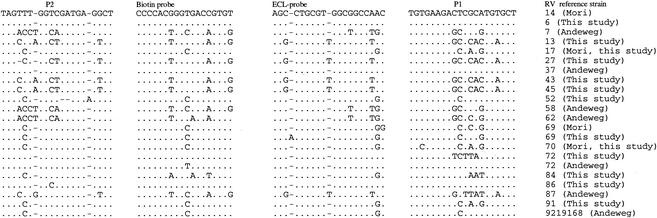
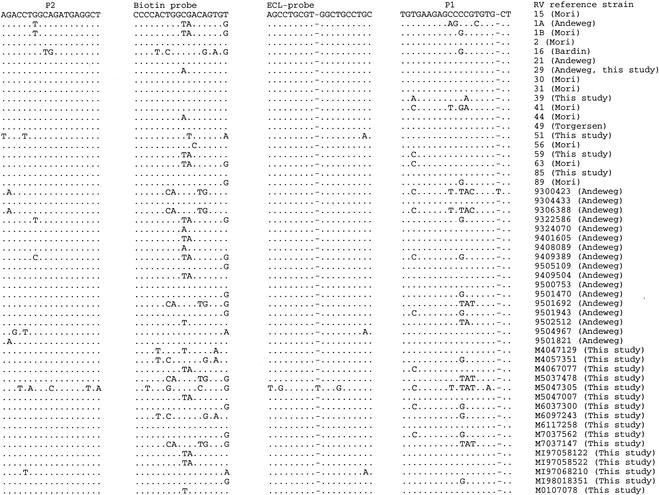
NASBA using primer mix 1 and probe combinations I and III classified 80 of 88 (90.9%) of clinical isolates as group B RVs. Primer mix 3 and probe combination IV allowed the classification of one more isolate (M7037562). For seven isolates (M0107266, M2107365, M4057351, M5037478, M5047305, M6097243, and M7037147) no positive ECL signal was obtained. For five of these (M4057351, M5037478, M5047305, M6097243, and M7037147) this is probably due to the presence of a high number of mismatches in the regions targeted for amplification and detection (Fig. 1). We were unable to sequence isolates M0107266 and M2107365 because of failure of the RT-PCR.
FIG.1.
5′ NCR subregion sequences of group A RVs.
Conserved 5′ NCR subregions.
As a result of the different sensitivity levels, with primer mix 1 and probe combinations I and III (Table 2 and 3), the 5′ NCR of 34 RV strains or isolates was sequenced.
The 5′ NCR regions we choose for primer and probe design are fairly conserved among the different RV strains (Fig. 1 and 2). For group B RVs (Fig. 2), the 5′ NCR showed little sequence variation in the subregion of the P2 primer and the ECL detection probe. There was more sequence variation, however, in the subregion of the P1 primer (pd 904) and the biotin capture probe (pd 908). The length of this P1 primer (pd 904) was reduced by 3 nucleotides at its 3′ end (EG 59) and additional versions of the biotin labeled capture probe (pd 1147) were developed to improve the performance of the assay. There is more sequence variation in the 5′NCR of the group A viruses (Fig. 1). To obtain an assay with a better sensitivity three nucleotides at the 3′ terminus of the initial P1 primer (pd 905) were removed. Additional versions of biotin capture (pd 1277) and ECL (pd 1276) probes were developed to cover mismatches, including an insertion located in the region of the ECL detection probe and observed in the 5′ NCR sequence of RVs 13, 27, 43 and 45 (Fig. 1).
FIG. 2.
5′ NCR subregion sequences of group B RVs.
DISCUSSION
The aim of this study was to develop a NASBA assay to detect RVs and classify them among group A and group B. Assays with primers and probes based on published 5′ NCR sequences (27) were negative for many RVs. Therefore, the 5′ NCR of 34 serovars was sequenced, revealing mismatches between the 5′ NCRs and the primers and probes used. These mismatches could be expected since the original primers were based on the nucleotide sequences of 14 strains only.
Based on these results, new oligonucleotide primers and probes specific for group A and group B viruses were synthesized. In comparison with the primers used initially (pd 904, pd 905, pd 906, and pd 907) only slight modifications had to be introduced. For the amplification of group B viruses, only 2 primers are required. The omission of the first three nucleotides at the 3′ terminus of the P1 primer and the use of probe combination IV considerably improved the analytical sensitivity of the assay. For the amplification of group A viruses, four primers are still required. In this case also, the omission of the first three nucleotides at the 3′ terminus of the P1 primer and the synthesis of new probes improved the analytical sensitivity of the assay. Gobbers et al. (12) reported that single point mutations near the 3′ end of the primers, closely followed by a second mismatch, seem to hamper amplification. This could explain why no amplification was obtained with RNA obtained from reference strains 14, 72, and 84 and why the seven clinical isolates (M0107266, M2107365, M4057351, M5037478, M5047305, M6097243, and M7037147) could not be typed (Fig. 2). Five strains (4, 41, 26, 56, and 89) and two clinical isolates (M0107266 and M2107365) could not be sequenced as no RT-PCR amplicons could be obtained, probably also resulting from mismatches at critical positions in the RT-PCR primers. Coste et al. (7) mentioned that the presence of more than four point mutations in a primer decreases the amplification efficiency of the RT-PCR and that a number of mismatches in combination with one or two critical mismatches results in a complete absence of amplification.
Andeweg et al. (1) reported that RV 87 causes a special problem because it has an enterovirus-specific insertion at position 359. We found the same extra nucleotide in the 5′ NCR sequence of reference RV strains 13, 27, 43, and 45. To avoid misclassification, an ECL detection probe, pd 1276 (Table 1), containing this extra nucleotide was designed.
The sensitivity for the detection of group B viruses was 20 TCID50/ml but was lower for group A RVs. This should not constitute a serious diagnostic problem because group A viruses seem to be less prevalent.
Since the NASBA primers we designed are based on the sequences of a large set of RVs, including recent isolates belonging to both groups A and B (2, 39), it is expected that the new assay will detect the majority of the group B RVs currently circulating as well as some group A RVs. Moreover, RVs circulating at present as well as prototype RVs, isolated many years ago, still appear to have the same conserved sequences.
Our new assay is highly specific, since a panel of other respiratory viruses was not amplified except one enterovirus isolate. Of about 35 enterovirus isolates tested, only one untyped enterovirus isolate was positive when tested undiluted in the NASBA assay. However, when this isolate was grown semiquantitatively, it was culture positive in a range from undiluted to 10−8 and thus contained high concentrations of virus. The ECL signal was only weakly positive (ECL counts below 25,000) in the undiluted sample and contained a titer of enteroviruses that, in our opinion, is higher than any ever reached in a respiratory clinical specimen, and therefore will not influence the specificity of our RV amplification test.
In this study, all NASBA-positive clinical isolates belonged to group B, confirming the serotyping-based observations of Andries et al. (2), Monto et al. (25), Krilov et al. (19), and Fox et al. (8) and the sequence-based observations of Andeweg et al. (1).
The sensitivity of our NASBA assay compares favorably with those of the assays developed by Samuelson et al. (32), Gama et al. (10), and Johnston et al. (16), who detected selected RV strains at levels between 1 and 10 TCID50/ml.
In conclusion, through the synthesis of a new set of primers and probes, a sensitive and specific NASBA assay for the detection of group B RVs was developed.
Acknowledgments
We thank S. R. Pattyn for critical review of the manuscript.
REFERENCES
- 1.Andeweg, A. C., T. M. Bestebroer, M. Huybreghs, T. G. Kimman, and J. C. de Jong. 1999. Improved detection of rhinoviruses in clinical samples by using a newly developed nested reverse transcrition assay. J. Clin. Microbiol. 37:524-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andries, K., B. Dewindt, J. Snoeks, L. Wouters, H. Moereels, P. J. Lewi, and P. A. Janssen. 1990. Two groups of rhinoviruses revealed by a panel of antiviral compounds present sequence divergence and differential pathogenicity. J. Virol. 64:1117-1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arola, M., T. Ziegler, O. Ruuskanen, J. Mertsola, K. Näntö-Salonen, and P. Halonen. 1988. Rhinovirus in acute otitis media. J. Pediatr. 113:693-695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bardin, P. G., S. L. Johnston, G. Sanderson, B. S. Robinson, M. A. Pickett, D. J. Fraenkel, and S. T. Holgate. 1994. Detection of rhinovirus infection of the nasal mucosa by oligonucleotide in situ hybridization. Am. J. Respir. Cell. Mol. Biol. 10:207-213. [DOI] [PubMed] [Google Scholar]
- 5.Boom, R., C. J. A. Sol, M. M. M. Salimans, C. L. Jansen, P. M. E. Werthum-Van Dillen, and J. Van der Noordaa. 1990. Rapid and simple method for purification of nucleic acids. J. Clin. Microbiol. 28:495-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Callahan, P. L., S. Mizutani, and R. J. Colonno. 1985. Molecular cloning and complete sequence determination of RNA genome of human rhinovirus type 14. Proc. Natl. Acad. Sci. USA 82:732-736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coste, J., B. Montes, J. Reynes, M. Peeters, C. Segarra, J. P. Vendrell, E. Delaporte, and M. Segondy. 1996. Comparative evaluation of three assays for the quantitation of human immunodeficiency virus type 1 RNA in plasma. J. Med. Virol. 50:293-302. [DOI] [PubMed] [Google Scholar]
- 8.Fox, J. P., M. K. Cooney, C. E. Hall, and H. M. Foy. 1985. Rhinoviruses in Seattle families. Am. J. Epidemiol. 122:830-846. [DOI] [PubMed] [Google Scholar]
- 9.Fraenkel, D. J., D. G. Bardin, G. Sanderson, F. Lompe, S. L. Johnston, and S. T. Holgate. 1995. Lower airway inflammation during rhinovirus colds in normal and in asthmatic subjects. Am. J. Resp. Crit. Care Med. 151:879-886. [DOI] [PubMed] [Google Scholar]
- 10.Gama, R. E., P. R. Horsnell, P. J. Hughes, C. North, C. B. Bruce, W. Al-Nakib, and G. Stanway. 1989. Amplification of rhinovirus specific nucleic acids from clinical samples using the polymerase chain reaction. J. Med. Virol. 28:73-77. [DOI] [PubMed] [Google Scholar]
- 11.Ghosh, S., R. Champlin, R. Couch, J. Englund, I. Raad, S. Malik, M. Luna, and E. Whimbey. 1999. Rhinovirus infections in myelosuppressed adult blood and marrow transplant recipients. Clin. Infect. Dis. 29:528-532. [DOI] [PubMed] [Google Scholar]
- 12.Gobbers, E., K. Fransen, T. Oosterlaken, W. Janssens, L. Heyndrickx, T. Ivens, K. Vereecken, R. Schoones, P. van de Wiel, and G. van der Groen. 1997. Reactivity and amplification efficiency of the NASBA HIV-1 RNA amplification system with regard to different HIV-1 subtypes. J. Virol. Methods 66:293-301. [DOI] [PubMed] [Google Scholar]
- 13.Grist, N. R., E. J. Bell, E. A. C. Follet, and G. E. D. Urquhart. 1979. Diagnostic methods in clinical virology, 3rd ed., p. 86-87. Blackwell Scientific Publications, Oxford, England.
- 14.Halonen, P., E. Rocha, J. Hierholzer, B. Holloway, T. Hyypia, P. Huuskainer, and M. Pallansik. 1995. Detection of enteroviruses and rhinoviruses in clinical specimens by PCR and liquid-phase hybridization. J. Clin. Microbiol. 33:648-653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ireland, D. C., J. Kent, and K. G. Nicholson. 1993. Improved detection of rhinoviruses in nasal and throat swabs by seminested RT-PCR. J. Med. Virol. 40:96-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnston, S. L., G. Sanderson, P. K. Pattemore, S. Smith, P. G. Bardin, C. B. Bruce, P. R. Lambden, D. A. J. Tyrrell, and S. T. Holgate. 1993. Use of polymerase chain reaction for diagnosis of picornavirus infection in subjects with and without respiratory symptoms. J. Clin. Microbiol. 31:111-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kellner, G., T. Popow-Kraupp, M. Kundi, C. Binder, and C. Kuntz. 1989. Clinical manifestations of respiratory tract infections due to respiratory syncytial virus and rhinoviruses in hospitalized children. Acta Paediatr. Scand. 78:390-394. [DOI] [PubMed] [Google Scholar]
- 18.Kievits, T., B. van Gemen, D. van Strijp, R. Schukkink, M. Dircks, H. Adriaanse, L. Malek, R. Sooknanan, and P. Lens. 1991. NASBA ™ isothermal enzymatic in vitro nucleic acid amplification optimized for the diagnosis of HIV-1 infection. J. Virol. Methods 35:273-286. [DOI] [PubMed] [Google Scholar]
- 19.Krilov, L., L. Pierik, E. Kellner, K. Mahan, D. Watson, M. Hirsch, V. Hamparian, and K. McIntosh. 1986. The association of rhinoviruses with lower respiratory tract disease in hospitalized patients. J. Med. Virol. 19:345-352. [DOI] [PubMed] [Google Scholar]
- 20.Lair, S. V., T. E. Mirkow, J. A. Dodds, and M. F. Murphy. 1993. A single temperature amplification technique applied to the detection of citrus tristeza viral RNA in plant nucleic acid extracts. J. Virol. Methods 47:141-152. [DOI] [PubMed] [Google Scholar]
- 21.Loens, K., D. Ursi, M. Ieven, P. van Aarle, P. Sillekens, P. Oudshoorn, and H. Goossens. 2002. Detection of Mycoplasma pneumoniae in spiked clinical samples by nucleic acid sequence-based amplification. J. Clin. Microbiol. 40:1339-1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mäkelä, M. J., T. Puhakka, O. Ruuskanen, M. Leinonen, P. Saikku, M. Kimpimaki, S. Blomqvist, T. Hyypiä, and P. Arstilla. 1998. Viruses and bacteria in the etiology of the common cold. J. Clin. Microbiol. 36:539-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McMillan, J. A., L. B. Weiner, A. M. Higgins, and K. MacKnight. 1993. Rhinovirus infection associated with serious illness among pediatric patients. Pediatr. Infect. Dis. J. 12:321-325. [DOI] [PubMed] [Google Scholar]
- 24.Mertsola, J., T. Ziegler, O. Ruuskanen, T. Vanto, A. Kiovikko, and P. Halonen. 1991. Recurrent wheezy bronchitis and viral respiratory infections. Arch. Dis. Child. 66:124-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Monto, A. S., E. R. Bryan, and S. Ohmit. 1987. Rhinovirus infections in Tecumseh, Michigan: frequency of illness and number of serotypes. J. Infect. Dis. 156:43-49. [DOI] [PubMed] [Google Scholar]
- 26.Monto, A. S., and A. Aulor. 1995. Viral respiratory infections in the community: epidemiology, agents and interventions. Am. J. Med. 89:245-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mori, J., and J. P. Clewley. 1994. Polymerase chain reaction and sequencing for typing rhinovirus RNA. J. Med. Virol. 44:323-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nicholson, K. G., J. Kent, V. Hammersley, and E. Cancio. 1997. Acute viral infections of upper respiratory tract in elderly people living in the community: comparative, prospective, population based study of disease burden. BMJ. 316:1060-1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Niederman, M. S., J. B. Bass, G. D. Campbell, A. M. Fein, R. F. Grossman, L. A. Mandell, T. J. Marrie, G. A. Sarosi, A. Torres, and V. L. Yu. 1993. Guidelines for the initial management of adults with community acquired pneumonia: diagnosis, assessment of severity and initial antimicrobial therapy. Am. Rev. Resp. Dis. 148:1418-1426. [DOI] [PubMed] [Google Scholar]
- 30.Ovyn, C., D. Van Strijp, M. Ieven, D. Ursi, B. Van Gemen, and H. Goossens. 1996. Typing of Mycoplasma pneumoniae by nucleic acid sequence-based amplification, NASBA®. Mol. Cell. Probes 10:319-324. [DOI] [PubMed] [Google Scholar]
- 31.Pitkaränta, A., E. Arruda, H. Malmberg, and F. G. Hayden. 1997. Detection of rhinovirus in sinus brushings of patients with acute community-acquired sinusitis by reverse transcription-PCR. J. Clin. Microbiol. 35:1791-1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Samuelson, A., D. Westmoreland, R. Eccles, and J. D. Fox. 1998. Development and application of a new method for amplification and detection of human rhinovirus RNA. J. Virol. Methods 71:179-209. [DOI] [PubMed] [Google Scholar]
- 33.Santti, J., T. Hyypiä, and P. Halonen. 1997. Comparison of PCR primer pairs in the detection of human rhinoviruses in nasopharyngeal aspirates. J. Virol. Methods 66:139-147. [DOI] [PubMed] [Google Scholar]
- 34.Sillekens, P., W. Kok, B. van Gemen, P. Lens, H. Huisman, T. Cuypers, and T. Kievits. 1994. Specific detection of HCV RNA using NASBA as a diagnostic tool, p. 71-82. In Hepatitis C virus GEMHEP. John Libbey Eurotext, Paris, France.
- 35.Smits, H. L., B. van Gemen, R. Schukkink, J. van der Velden, S. P. Tjong-A-Hung, M. F. Jebbink, and J. ter Schegget. 1995. Application of the NASBA nucleic acid amplification method for the detection of human papillomavirus type 16 E6-E7 transcripts. J. Virol. Methods 54:75-81. [DOI] [PubMed] [Google Scholar]
- 36.Smits, H. L., G. C. Gussenhoven, W. Terpstra, R. A. F. Schukkink, B. van Gemen, and T. van Gool. 1997. Detection, identification and semi-quantification of malaria parasites by NASBA amplification of small subunit ribosomal RNA sequences. J. Microbiol. Methods 28:65-75. [Google Scholar]
- 37.Smyth, A. R., R. L. Smyth, C. Y. W. Tong, C. A. Hart, and D. P. Heaf. 1995. Effect of respiratory virus infections including rhinovirus on clinical status in cystic fibrosis. Arch. Dis. Child. 73:117-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Torgersen, H., T. Skern, and D. Blaas. 1989. Typing of human rhinoviruses based on sequence variations in the 5′ non-coding region. J. Gen. Virol. 70:3111-3116. [DOI] [PubMed] [Google Scholar]
- 39.Uncapher, C. R., C. M. DeWitt, and R. J. Colonno. 1991. The major and minor group receptor families contain all but one human rhinovirus serotype. Virology 180:814-817. [DOI] [PubMed] [Google Scholar]
- 40.van der Vliet, G. M. E., S.-N. Cho, K. Kampirapap, J. van Leeuwen, R. A. F. Schukkink, B. van Gemen, P. K. Das, W. R. Faber, G. P. Walsh, and P. R. Klatser. 1996. Use of NASBA® RNA amplification for detection of Mycobacterium leprae in skin biopsies from untreated and treated leprosy patients. Int. J. Lepr. 64:396-403. [PubMed] [Google Scholar]
- 41.van der Vliet, G. M. E., R. A. F. Schukkink, B. van Gemen, P. Schepers, and P. R. Klatser. 1993. Nucleic acid sequence based amplification (NASBA) for the identification of Mycobacteria. J. Gen. Microbiol. 139:2423-2429. [DOI] [PubMed] [Google Scholar]
- 42.Van Gemen, B., R. van Beuning, and A. Nabbe. 1994. A one tube quantitative HIV-1 RNA NASBA nucleic acid amplification assay using electrochemiluminescent (ECL) labeled probes. J. Virol. Methods 49:157-168. [DOI] [PubMed] [Google Scholar]
- 43.Whimbey, E., R. E. Champlin, R. B. Couch, J. A. Englund, J. M. Goodrich, L. Raad, D. Przepiorka, V. A. Lewis, N. Mirza, H. Yousuf, J. J. Tarrand, and G. P. Bodey. 1996. Community respiratory virus infections among hospitalized adult bone marrow transplant recipients. Clin. Infect. Dis. 22:778-782. [DOI] [PubMed] [Google Scholar]