Abstract
Human group B rotavirus was detected in 12 of 220 adult patients and 2 of 67 child patients with severe diarrhea in Bangladesh. Group B rotavirus may be virulent in both adults and children, and the virus may be an especially serious diarrheal agent in Bangladesh.
Rotaviruses are the most important etiological agents of severe diarrheal illness in infants, children, and adults throughout the world (1, 2, 7). These viruses have been classified into seven groups (A to G) by means of VP6 serology (14), genomic RNA electrophoretic patterns (21), and group-specific PCR (9). Group A rotavirus causes diarrhea in infants and has been detected in many countries since 1973 (3). On the other hand, group B rotavirus was found in China in 1983 (11). This virus is responsible predominantly for adult diarrhea and causes cholera-like diarrhea in adults, infecting more than a million people in a single epidemic (6, 12). Although group B rotavirusinfection was found not only in China (19) but also in Hong Kong, Australia, the United States, and the United Kingdom (4, 5, 8, 16-18, 20) through seroepidemiological studies, group B rotavirus detection has not been reported for a long time. After a gap of nearly 15 years, patients infected with human group B rotavirus were found in Calcutta, India, in 1998 (15).
During the course of molecular epidemiological surveillance of the patients with diarrhea residing in Mymensingh, Bangladesh, between December 2000 and July 2001, 14 human group B rotaviruses were detected in the stools of 287 patients with severe diarrhea.
Stool samples were collected from the patients within 3 days of the onset of the disease and were stored at −20°C until examined. The patients' ages ranged from 2 to 60 years.
Rotavirus detection in stool samples was carried out by RNA-polyacrylamide gel electrophoresis and silver nitrate staining (10). Group B rotavirus classification was carried out by reverse transcription (RT)-PCR with group B rotavirus-specific primers as described Gouvea et al. (9). The primer sequences (5′ to 3′) are as follows: B1, CTATTCAGTGTGTCGTGAGAGG; B3, CGAAGCGGGCTAGCTTGTCTGC; and B4, CGTGGCTTTGGAAAATTCTTG. The RT-PCRs were performed as described previously (25) with 25 cycles of 94°C for 1 min, 55°C for 1 min, and 72°C for 3 min and final incubation at 72°C for 7 min.
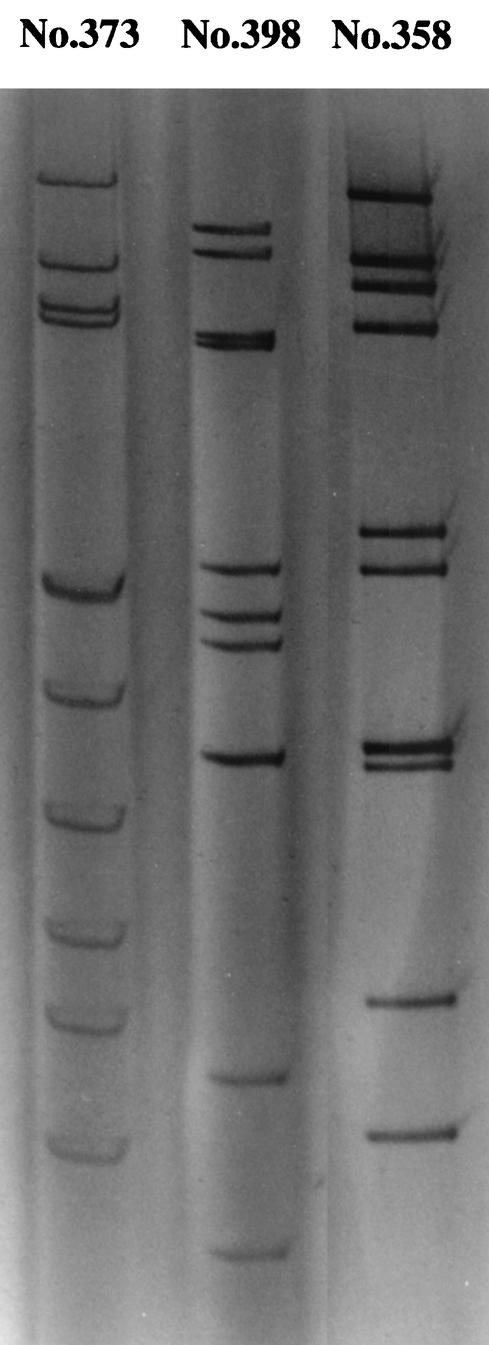
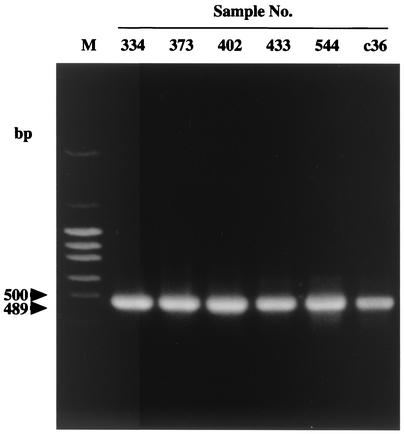
The electrophoresis pattern of genomic double-stranded RNA of the group B rotavirus (no. 373) was the same as that of the CAL-1 strain (15) but was different from those of group A (no. 398) and group C (no. 385) rotaviruses (Fig. 1). The rotaviruses found in 6 samples were examined by RT-PCR and classified as group B rotaviruses (Fig. 2). The migration patterns of these viruses were completely identical (data not shown).
FIG. 1.
Gel electrophoresis showing the genomic double-stranded RNA patterns of group B (no. 373), group A (no. 398), and group C (no. 385) rotaviruses. The viral RNAs were analyzed by electrophoresis in a 10% polyacrylamide gel and visualized by staining with silver nitrate. Sample no. 373, 398, and 358 showed the specific electrophoresis patterns of group B, A, and C rotaviruses, respectively.
FIG. 2.
RT-PCR products of the group B rotavirus in adult and infant patients' stools. RT-PCR was performed with the primers for group B rotavirus reported by Gouvea et al. (B1 to B4). Sample no. 334, 373, 402, 433, and 544 and sample c36 were collected from adult and infant patients, respectively. Only one PCR product, the expected 489-bp DNA fragment, was produced. Lane M is a 100-bp DNA ladder.
Group B rotavirus was detected in 12 of 220 (5.5%) adult patient stool samples and 2 of 67 (3.0%) child stool samples. The patients with group B rotavirus did not belong to the same family. Group A rotavirus was detected in 9 (4.1%) adults and 7 (10.4%) children, and group C rotavirus was detected in 2 (0.9%) adults. No mixed infection by two or more rotavirus groups was detected.
Electron microscopic observation of group B rotavirus-positive patients' stool samples was also carried out with 6% uranyl acetate. Rotavirus was detected in 5 of 5 samples. No other enteropathogens were detected (data not shown).
Fourteen patients with group B rotavirus were found from December 2000 to June 2001. The patients had very severe diarrhea, vomiting, and severe dehydration, with diarrhea occurring 8 to 25 times a day. Seven of 14 (50.0%) patients had a fever of more than 39°C. Eleven of these 14 (78.6%) patients passed watery stools. The color of the patients' stools was dark brown, white, or green (Table 1). These symptoms and characteristics of the patients' stools were almost the same as in the case of cholera.
TABLE 1.
Clinical symptoms and characteristics of stool samples of patients with group B rotavirus
| Patient no. | Age (yr) | Sexa | Date of onset of diarrhea (mo. day. yr) | Result for clinical symptomb
|
Presence of fever ≥ 39°C | Characteristics of stools | ||
|---|---|---|---|---|---|---|---|---|
| Vomiting | Diarrhea (no. of times/day) | Dehydration | ||||||
| 334 | 28 | F | 12.02.00 | + | 8 | + | No | Watery, dark brown |
| 335 | 30 | M | 12.01.00 | + | 12 | + | No | Watery, white |
| 342 | 35 | M | 12.09.00 | + | 13 | +++ | No | Watery, white |
| 348 | 38 | F | 12.11.00 | + | 13 | +++ | No | Watery with solid, dark brown |
| 373 | 30 | M | 12.16.00 | + | 12 | + | Yes | Soft, green |
| 379 | 28 | M | 12.16.00 | + | 15 | + | No | Watery, dark brown |
| 402 | 27 | F | 02.07.01 | + | 25 | +++ | Yes | Watery with solid, dark brown |
| 431 | 60 | M | 02.28.01 | + | 25 | +++ | Yes | Watery with solid, dark brown |
| 433 | 16 | F | 03.03.01 | + | 15 | + | Yes | Watery with solid, white |
| 470 | 56 | M | 06.12.01 | + | 25 | +++ | Yes | Watery, white |
| 488 | 60 | F | 06.27.01 | + | 20 | + | No | Soft, dark brown |
| 544 | 35 | F | 06.18.01 | + | 25 | +++ | Yes | Watery, dark brown |
| c21 | 2 | F | 05.15.01 | + | 14 | + | No | Soft, green |
| c36 | 2 | F | 05.20.01 | + | 17 | + | Yes | Watery, white |
M, male; F, female.
+ to +++, degree of symptoms.
These findings indicate that group B rotavirus may be highly virulent in both children and adults, and the virus may be an especially serious diarrheal agent in Bangladesh. Group B rotavirus may be widespread and may have caused the epidemic infection in Mymensingh. Since there has been no case reported of group B rotavirus being detected in 2-year olds, the diagnosis of diarrheal illness of children is very important.
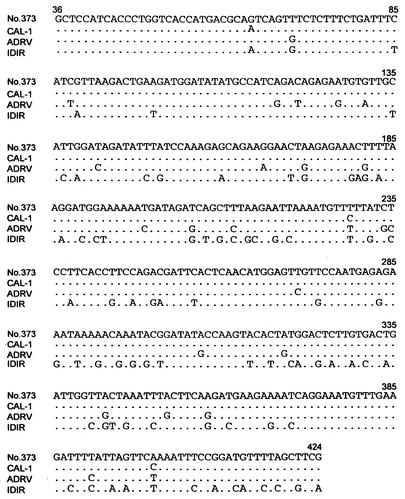
Mymensingh is located almost 300 km from Calcutta, India, where a group B rotavirus infection occurred in 1997 to 1998 (15). We determined partial sequences of the VP7 and NSP2 genes for some rotaviruses by PCR and direct sequencing with the dideoxynucleotide chain termination method. The V7 gene was amplified with primers corresponding to nucleotide no. 1 to 22 (forward primer) and no. 640 to 620 (reverse primer). A 350-bp sequence of the VP7 gene corresponding to nucleotide no. 201 to 550 of the adult diarrheal rotavirus (ADRV) sequence (7) was determined for specimen numbers 334, 373, and 402. This sequence of the three viruses was identical and showed high sequence identity to the same sequence regions of human group B rotaviruses CAL-1 (13) (99%) and ADRV (92%), whereas the identities with those of bovine virus WD653 (26) and murine virus IDIR (20) were considerably low (62 and 56%, respectively) (data not shown). The partial NSP2 gene (gene 8) sequence (nucleotide no. 36 to 424) of specimen no. 373 was determined by using the second PCR product that had been obtained by group B rotavirus detection described above and aligned with those of other group B rotaviruses (Fig. 3). Similar to the case of the VP7 gene, the sequence of no. 373 showed a higher sequence identity to those of CAL-1 (99%) and ADRV (94%) than to that of murine virus IDIR (80%). These results indicated that the Bangladeshi rotaviruses with unusual RNA patterns were group B rotaviruses that are genetically close to the previously reported human group B rotaviruses CAL-1 and ADRV.
FIG. 3.
Comparison of the partial NSP 2 gene (gene 8) sequences of no. 373, CAL-1, ADRV, and IDIR. Partial NSP 2 gene sequences (no. 36 to 424) of group B rotaviruses were sequenced and aligned. Dots indicate the same nucleotides as in Bangladesh virus no. 373. Only three nucleotides in the sequence of no. 373 were found to be different from that of CAL-1.
Group B rotavirus infection has occurred in three Asian countries, China in 1982, India in 1998, and Bangladesh in 2000. The three strains, ADRV in China, CAL-1 in India, and no. 373 in Bangladesh were genetically very close. Neverthless, Sen et al. (24) predicated that CAL-1 might be distinct from ADRV and that the sequences of VP4 and NSP3 of CAL-1 might not have genetically diverged from those of ADRV recently. Therefore, to confirm the origin of group B rotavirus in Bangladesh, genetic data of the Bangladesh strain and other group B rotavirus strains were needed.
In order to understand the distribution and transmission of group B rotavirus, more evidence based on surveillances of the virus is required. For the diagnosis of group B rotavirus infection, it is very important to develop a new instant and rapid method for group B rotavirus detection such as a latex agglutination test (22). Since there is no human group B rotavirus that can grow in cell culture, a porcine group B rotavirus, the SAK-1 strain, which grows in SKL cells and reacts with anti-ADRV antibodies (23), may facilitate seroepidemiological study for large-scale worldwide surveillance.
REFERENCES
- 1.Ahmed, M. U., S. Urasawa, K. Taniguchi, T. Urasawa, N. Kobayashi, F. Wakasugi, A. I. Islam, and H. A. Sahikh. 1991. Analysis of human rotavirus strains prevailing in Bangladesh in relation to nationwide floods brought by the 1988 monsoon. J. Clin. Microbiol. 29:2273-2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bern, C., J. Martines, I. de Zoysal, and R. I. Glass. 1992. The magnitude of the global problem of diarrhoeal disease: a ten-year update. Bull. W. H. O. 70:705-714. [PMC free article] [PubMed] [Google Scholar]
- 3.Bishop, R. F., G. P. Davidson, I. H. Holmes, and B. J. Ruck. 1973. Virus particles in epithelial cell of duodenal mucosa from children with viral gastroenteritis. Lancet ii:1281-1283. [DOI] [PubMed]
- 4.Brown, D. W., G. M. Beards, G. M. Chen, and T. H. Flewett. 1987. Prevalence of antibody to group B (atypical) rotavirus in humans and animals. J. Clin. Microbiol. 25:316-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang, K. O., A. V. Parwani, D. Smith, and L. J. Saif. 1997. Detection of group B rotaviruses in fecal samples from diarrheic calves and adult cows and characterization of their VP7 genes. J. Clin. Microbiol. 35:2107-2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen, C. M., T. Hung, J. C. Bridger, and M. A. McCrae. 1985. Chinese adult rotavirus is a group B rotavirus. Lancet i:1123-1124. [DOI] [PubMed]
- 7.Chen, G. M., T. Hung, and E. R. Mackow. 1990. Identification of the gene encoding the group B rotavirus VP 7 equivalent: primary characterization of the ADRV segment 9 RNA. Virology 178:311-315. [DOI] [PubMed] [Google Scholar]
- 8.Gatheru, Z., N. Kobayashi, N. Adachi, S. Chiba, J. Mail, P. Ogaa, J. Nyangao, E. Kiplagat, and P. M. Tukei. 1993. Characterization of human rotavirus strains causing gastroenteritis in Kenya. Epidemiol. Infect. 110:419-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gouvea, V., J. R. Allen, R. I. Glass, Z. Y. Fang, M. Bremont, J. Cohen, M. A. McCrae, L. J. Saif, P. Sinarachatanant, and E. O. Caul. 1991. Detection of group B and C rotaviruses by polymerase chain reaction. J. Clin. Microbiol. 29:519-523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herring, A. J., N. F. Inglis, C. K. Ojieh, D. R. Snodgrass, and J. D. Menzies. 1982. Rapid diagnosis of rotavirus infection by direct detection of viral nucleic acid in silver stained polyacrylamide gels. J. Clin. Microbiol. 16:473-477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hung, T., G. Chen, C. Wang, Z. Chou, T. Chao, W. Ye, H. Yao, and K. Meng. 1983. Rotavirus-like agent in adult non-bacterial diarrhoea in China. Lancet ii:1078-1079. [PubMed]
- 12.Hung, T., C. Wang, C. Z. Fang, Z. Chou, X. Chang, X. Liong, G. Chen, H. Yao, T. Chao, W. Ye, S. Den, and W. Chang. 1984. Waterborne outbreak of rotavirus diarrhoea in adults in China caused by a novel rotavirus. Lancet i:1139-1142. [PubMed]
- 13.Kobayashi, N., T. N. Naik, Y. Kusuhara, T. Krishnan, A. Sen, S. K. Bhattacharya, K. Taniguchi, M. M. Alam, T. Urasawa, and S. Urasawa. 2001. Sequence analysis of genes encoding structural and nonstructural proteins of a human group B rotavirus detected in Calcutta, India. J. Med. Virol. 64:583-588. [DOI] [PubMed] [Google Scholar]
- 14.Kohli, E., L. Maurice, J. F. Vautherot, C. Bougreois, J. B. Bour, J. Cohen, and P. Pothier. 1992. Localization of group specific epitopes as the major capsid protein of group A rotavirus. J. Gen. Virol. 73:907-914. [DOI] [PubMed] [Google Scholar]
- 15.Krishnan, T., A. Sen, J. S. Choudhury, S. Das, T. N. Naik, and S. K. Bhattacharya. 1999. Emergence of adult diarrhoea rotavirus in Calcutta, India. Lancet 353:380-381. [DOI] [PubMed] [Google Scholar]
- 16.Mackow, E. R., R. Werner-Eckert, M. E. Fay, H. Tao, and G. Chen. 1993. Identification and baculovirus expression of the VP4 protein of the human group B rotavirus ADRV. J. Virol. 67:2730-2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mackow, E. R. 1995. Group B and C rotaviruses, p. 983-1008. In M. J. Blaser, P. D. Smith, J. I. Ravdin, H. Greenberg, and R. L. Guerrant (ed.), Infections of the gastrointestinal tract. Ravdin Press, New York, N.Y.
- 18.Nakata, S., M. K. Estes, D. H. Graham, S. Wang, G. W. Gary, and J. L. Melnik. 1987. Detection of antibody to group B adult diarrhea rotaviruses in humans. J. Clin. Microbiol. 25:812-818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Penaranda, M. E., M. S. Ho, Z. Y. Fang, H. Dong, B. X. Sheng, W. W. Ye, M. K. Estes, P. Echeverria, T. Hung, and R. I. Glass. 1989. Seroepidemiology of adult diarrhea rotavirus in China, 1977 to 1987. J. Clin. Microboiol. 27:2180-2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petric, M., K. Mayur, S. Vonderfecht, and J. J. Eiden. 1991. Comparison of group B rotavirus gene 9 and 11. J. Gen. Virol. 72:2801-2804. [DOI] [PubMed] [Google Scholar]
- 21.Saif, L. J., and B. Jiang. 1994. Non group A rotaviruses of human and animals, p. 339-371. In R. Raming (ed.), Rotaviruses. Springer-Verlag, Berlin, Germany.
- 22.Sanekata, T., Y. Yoshida, and H. Okada. 1981. Detection of rotavirus in faeces by latex agglutination. J. Immunol. Methods. 41:377-385. [DOI] [PubMed] [Google Scholar]
- 23.Sanekata, T., Y. Kuwamoto, S. Akamatsu, N. Sakon, M. Oseto, K. Taniguchi, S. Nakata, and M. K. Estes. 1996. Isolation of group B rotavirus in cell culture. J. Clin. Microbiol. 34:759-761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sen, A., N. Kobayashi, S. Das, T. Krishnan, S. K. Bhattacharya, S. Urasawa, and T. N. Naik. 2001. Amplification of various genes of human group B rotavirus from stool specimens by RT-PCR. J. Clin. Virol. 17:177-181. [DOI] [PubMed] [Google Scholar]
- 25.Taniguchi, K., F. Wakasugi, Y. Pongsuwanna, T. Urasawa, S. Ukae, S. Chiba, and S. Urasawa. 1992. Identification of human and bovine rotavirus serotypes by polymerase chain reaction. Epidemiol. Infect. 109:303-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsunemitsu, H., D. Morita, H. Takaku, T. Nishimori, K. Imai, and L. J. Saif. 1999. First detection of bovine group B rotavirus in Japan and sequence of its VP7 gene. Arch. Virol. 144:805-815. [DOI] [PMC free article] [PubMed] [Google Scholar]