Abstract
A novel Babesia species, designated Babesia bicornis sp. nov., was identified in three black rhinoceroses (Diceros bicornis) that died in wildlife areas in Tanzania and South Africa. Screening of black rhinoceroses in South Africa revealed, in addition to B. bicornis, a second parasite, designated Theileria bicornis sp. nov.
CASE REPORT
Our investigation started in the Ngorongoro Conservation Area in northern Tanzania, where significant mortality had occurred in 2000 and 2001, particularly among buffaloes, wildebeests, and lions. Among the animals that died, the deaths of two female black, or hook-lipped, rhinoceroses (Diceros bicornis) were of particular concern. The rhinoceroses, named Bahati (case 1) and Maggie (case 2), died after a sudden clinical illness in January 2001. Bahati had displayed general signs of illness and hemoglobinuria before she died. Postmortem findings included icterus, anemia, a yellow-brown liver with whitish spots, approximately 2 liters of red urine in the bladder, bloody pericardial fluid, hemorrhage in the small intestines, and blood oozing from natural orifices. Ticks collected from the perianal region were identified as Amblyomma variegatum and Rhipicephalus compositus. Blood smears and brain crush smears showed a 30% parasitemia caused by Babesia-like piroplasms (Fig. 1). A postmortem report was not available for Maggie (case 2). Tissues from both animals were available for further examination (Table 1).
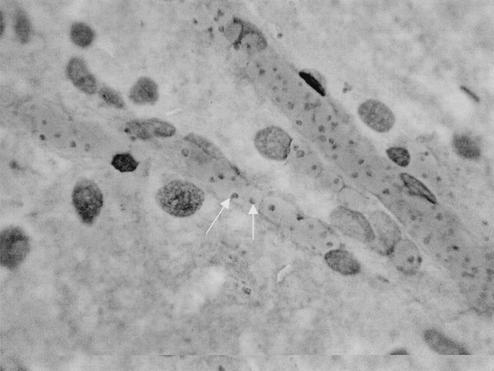
FIG. 1.
Giemsa-stained brain smear of Bahati, a black rhinoceros that died inside the Ngorongoro Conservation Area in Tanzania with numerous intraerythrocytic Babesia parasites inside brain capillaries, indicated by the arrows. Magnification, ×1,000.
TABLE 1.
Biological material of 15 black rhinoceroses examined in this study
| Rhino identificationa | Origin | Date of death | Material examined | RLB result |
|---|---|---|---|---|
| Bahati (case 1) | Ngorongoro Conservation Area, Tanzania | 15 January 2001 | Brain and spleen tissue, blood smears | Positive catchall signal in brain tissue, full sequence determined (B. bicornis) |
| Maggie (case 2) | Ngorongoro Conservation Area, Tanzania | January 2001 | Blood smears, spleen tissue | B. bicornis |
| Benji (case 3) | Addo National Park, South Africa | 1995 | Giemsa-stained blood smear | B. bicornis |
| S1440-96 (case 4) | Hluhluwe Umfolczi South Africa | May 1996 | Spleen and brain tissue | DNA could not be extracted due to prolonged storage in formalin |
| 14 | GFRRC | NAb | Citrated blood | T. bicornis |
| 15 | GFRRC | NA | Citrated blood | Positive catchall signal in blood, full sequence determined (T. bicornis) |
| 22 | GFRRC | NA | Citrated blood | B. bicornis |
| 24 | GFRRC | NA | Citrated blood | T. bicornis |
| 25 | GFRRC | NA | Citrated blood | B. bicornis |
| 41 | GFRRC | NA | Citrated blood | B. bicornis |
| 42 | GFRRC | NA | Citrated blood | T. bicornis |
| 51 | GFRRC | NA | Citrated blood | B. bicornis |
| 52 | GFRRC | NA | Citrated blood | T. bicornis |
| 60 | GFRRC | NA | Citrated blood | T. bicornis |
| 61 | GFRRC | NA | Citrated blood | B. bicornis and T. bicornis |
Eleven black rhinoceroses (rhinoceroses 14, 15, 22, 24, 25, 41, 42, 51, 52, 60, and 61) live in the Great Fish River Reserve Complex in South Africa.
NA, not applicable.
The deaths of these black rhinoceroses motivated us to investigate two additional fatal cases that had occurred in nature reserves in South Africa. The third case concerned the acute death of an injured male black rhinoceros named Benji in the Addo Elephant National Park in South Africa in 1995 after immobilization during a period of unusually cold weather. The kidneys showed diffuse nephrosis with generalized membranous glomerulopathy. The lungs were emphysematous and revealed interstitial and alveolar edema. There was also hepatosis; and the heart showed subendocardial hemorrhage, congestion, and interstitial edema of the myocardium. A high level of parasitemia caused by an unidentified Babesia species was found in blood smears and several organs.
Case 4 (identification no. S1440-96) was a pregnant 25- to 30-year-old black rhinoceros from the Umfolozi section of the Hluhluwe-Umfolozi Park, KwaZulu-Natal, South Africa, that died from translocation-related causes in May 1996. After the animal was darted with etorphine, airlifted to a holding pen, and given the antidote diprenorphine, she did not respond normally and was unable to enter the transport crate unaided. A few minutes later she collapsed on her hind legs and was severely distressed, as shown by her struggling and sweating with increased respiration. After treatment with dexamethasone, azaperone, and naltrexone (intramuscularly), she was unloaded into a holding pen and cooled down with water. She appeared to be normal again within an hour. However, 2 days later she was found to have hindquarter paresis and was treated with dexamethasone, finadyne, naltrexone, vitamins, and antibiotics. She did not improve, and the animal died the next day. Postmortem examination revealed an emaciated carcass; hydropericardium; (sub)acute bronchopneumonia; splenic, hepatic, and kidney congestion; hemoglobinuria; and congestion of the musculature and lymph nodes in the hindquarters. A preliminary diagnosis of capture myopathy aggravated by poor condition, age, pregnancy, and stress during loading and possibly spinal trauma was made (D. Cooper, personal communication). However, when tissue samples in formalin were sent to the Department of Pathology, Faculty of Veterinary Science, University of Pretoria, histopathological examination revealed a severe parasitemia caused by a Babesia-like species parasitizing the red blood cells in all organs; a marked hemoglobinuric nephrosis; myocardial necrosis; and pulmonary hemorrhage, congestion, and edema. A diagnosis of babesiosis was made.
Blood and tissue samples from each of these four animals with fatal cases were subjected to PCR and reverse line blot hybridization analysis (RLB) (4) to identify the causal agents (Table 1). Moreover, blood samples obtained from 11 black rhinoceroses living in South African nature reserves at present were also examined for blood parasites by PCR and RLB.
DNA was extracted by adding 200 μl of EDTA-buffered whole blood to 500 μl of phosphate-buffered saline, mixed, and centrifuged (16,000 × g) for 5 min; and the supernatant was discarded. These steps were repeated two to four times until the pellet was white and the supernatant was clear. A total of 100 μl of a lysis buffer (50 mM KCl, 0.5% Tween 20, 10 mM Tris-HCl [pH 8.0]) and 1 μl of a proteinase K solution (1,000 μg/ml) were mixed with the pellet, and the mixture was incubated overnight at 56°C and heated at 100°C for 10 min to inactivate the proteinase K. Extraction of DNA from tissue samples was performed with the DNeasy tissue kit (QIAGEN, Hilden, Germany) according to the instructions of the manufacturer. DNA was extracted from Giemsa-stained blood smears by using a modified protocol for the QIAamp tissue kit as described previously (13). PCR was conducted as described by Gubbels et al. (4) with the following modifications: amplification was carried out with forward primer RLB-F2 (5′-GAC ACA GGG AGG TAG TGA CAA G-3′) and reverse primer RLB-R2 (biotin-5′-CTA AGA ATT TCA CCT CTG ACA GT-3′). All primers were obtained from Isogen (Maarssen, The Netherlands). A 25-μl reaction volume consisted of 12.5 μl of Platinum Quantitative PCR Supermix-UDG (Invitrogen, Leek, The Netherlands), 25 pmol of each primer, and 2.5 μl of the template DNA. The PCR program used was 3 min at 37°C; 10 min at 94°C; and 10 cycles of 94°C for 20 s, 67°C for 30 s, and 72°C for 30 s, with lowering of the temperature of the annealing step by 2°C after every second cycle (touchdown). Forty cycles of 94°C for 20 s, 57°C for 30 s, and 72°C for 30 s were performed with an automated cycler (I-Cycler; Bio-Rad, Richmond, Calif.). Full-length small-subunit 18S rRNA genes were amplified with forward primer 18SAN (5′-GGT TGA TCC TGC CAG TAG TCA T-3′) and reverse primer 18SBN (5′-CAC CTA CGG AAA CCT TGT TAC G-3′). The resulting products were sequenced by Baseclear, Leiden, The Netherlands.
RLB was performed essentially as described by Gubbels et al. (4), with the following modifications: denatured PCR products (10 μl) were diluted in 2× SSPE (1× SSPE is 0.18 M NaCl, 10 mM NaH2PO4, and 1 mM EDTA [pH 7.7])-0.1% sodium dodecyl sulfate, loaded onto the membrane, and incubated at 42°C for 1 h. Thereafter, the membrane was washed twice at 50°C instead of 42°C.
The Multalin online interface (http://www.toulouse.inra.fr/multalin.html) was used to align the sequences, which were manually edited with GeneDoc software (version 2.6.001). Phylogenetic trees were created by using the Phylip package (Felsenstein, version 3.5c) with full-length sequences including variable regions. For parsimony analysis, the SEQBOOT program created 500 bootstrap data sets, and unrooted parsimony was carried out for these sets with the DNAPARS program. The final consensus tree was made with the CONSENSUS program and was edited with TreeView software (version 1.6.6) (Fig. 2). The same procedure was followed for neighbor-joining analysis with the Kimura two-parameter distance calculation in the PHYLIP programs DNADIST and Neighbor instead of DNAPARS (Fig. 3). When the analysis was repeated on the basis of the nonvariable regions of the 18S rRNA sequences, no significant changes in topology were found (data not shown).
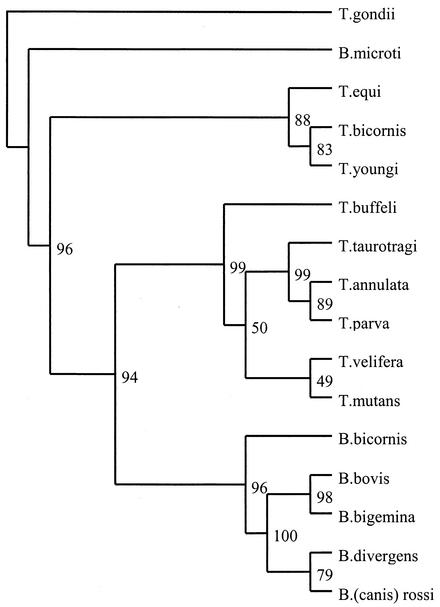
FIG. 2.
Parsimony tree showing the phylogenetic relationship of B. bicornis and T. bicornis with other Babesia and Theileria spp. based on full-length 18S rRNA sequences. The numbers represent the percentage of 500 replications (bootstrap support) for which the same branching patterns were obtained. Toxoplasma gondii was used as an outgroup.
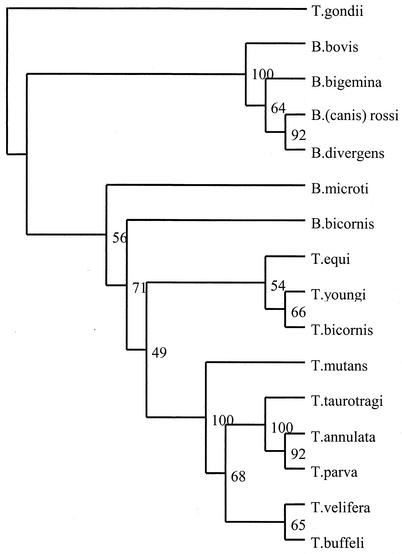
FIG. 3.
Neighbor-joining tree showing the phylogenetic relationship of B. bicornis and T. bicornis with other Babesia and Theileria spp. based on full-length 18S rRNA sequences. The numbers represent the percentage of 500 replications (bootstrap support) for which the same branching patterns were obtained. T. gondii was used as an outgroup.
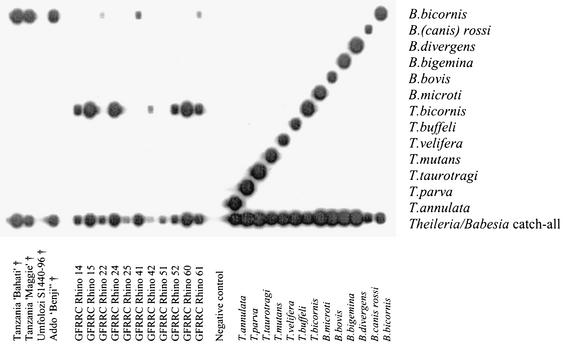
When DNA extracted from the brain of the Tanzanian black rhinoceros Bahati (case 1) (Fig. 1) was subjected to RLB, the amplified product displayed a positive Theileria and Babesia catchall signal without a species-specific signal, indicating that a novel protozoan parasite may be present. Sequence analysis, based on the 18S rRNA gene sequence, confirmed the presence of a new species, designated Babesia bicornis sp. nov. B. bicornis clusters with typical Babesia species such as B. bovis, B. bigemina, B. divergens, and B. rossi (B. canis) in the parsimony tree (Fig. 2) but appears to be more closely related to B. microti in the neighbor-joining tree (Fig. 3). However, separation of B. microti from other Babesia species is not very marked (bootstrap value, 56), confirming the identity of B. bicornis as a separate Babesia species. Subsequently, we developed a B. bicornis-specific RLB probe (Table 2) to examine postmortem material from the rhinoceroses Maggie (case 2), Benji (case 3), and S1440-96 (case 4), as well as blood samples from black rhinoceroses in South African nature reserves. Both Maggie and Benji were positive for B. bicornis (Fig. 4). However, it was impossible to extract DNA from the material of Umfolozi rhinoceros S1440-96 (case 4). The material had been stored for more than 5 years in 4% formaldehyde, which renders retrieval of nucleic acids impossible (6). Nevertheless, the clinical signs, the postmortem findings, and the high level of parasitemia in animal S1440-96 make it likely that B. bicornis was the cause of death. Importantly, B. bicornis was also found in 5 of 11 blood samples from healthy black rhinoceroses in the Great Fish River Reserve Complex in Eastern Cape Province, South Africa, which originated from a population in the KwaZulu-Natal Province of South Africa. A second parasite was identified in these animals, because catchall RLB signals without species-specific signals were found in 6 of 11 samples. The complete 18S rRNA gene sequence from one of these samples (sample 15) was determined (GenBank accession number AF499604), and phylogenetic analysis revealed a novel parasite that clustered with Theileria equi and Theileria youngi in both phylogenetic trees (Fig. 2 and 3). For this reason we propose the name Theileria bicornis sp. nov. Subsequently, we developed a T. bicornis-specific RLB probe (Table 2), and all samples were retested (Fig. 4). A T. bicornis-specific signal was found for the six samples that previously gave only a catchall signal. Furthermore, a T. bicornis-specific signal was also found for a sample in which a B. bicornis-specific signal was also present, indicating that dual infections do occur.
TABLE 2.
RLB probes used in this study
| Oligonucleotide probe specificity | 18S sequence (5′ → 3′) | Reference or GenBank accession no. | Tm (°C)a |
|---|---|---|---|
| Babesia bicornis | TTG GTA AAT CGC CTT GGT | AF419313 (this paper) | 54.5 |
| Babesia rossi (B. canis) | CGG TTT GTT GCC TTT GTG | L19097 | 53.7 |
| Babesia divergens | ACT (AG)AT GTC GAG ATT GCA C | Alignment of the sequences with accession nos. U07885, U16370, and Z48751 | 52.8 |
| Babesia bigemina | CGT TTT TTC CCT TTT GTT GG | Gubbles et al. (4) | 53.2 |
| Babesia bovis | CAG GTT TCG CCT GTA TAA TTG AG | Gubbels et al. (4) | 58.9 |
| Babesia microti | GAC TTG GCA TCT TCT GGA | Alignment of the sequences with accession nos. AF028344, AF02843, AB032434, and U09833 | 53.7 |
| Theileria bicornis | GCG TTG TGG CTT TTT TCT G | AF499604 (this paper) | 54.5 |
| Theileria buffeli | GGC TTA TTT CGG (AT)TT GAT TTT | Gubbels et al. (4) | 52.0 |
| Theileria velifera | CCT ATT CTC CTT TAC GAG T | AF097993 | 52.4 |
| Theileria mutans | CTT GCG TCT CCG AAT GTT | Gubbels et al. (4) | 53.7 |
| Theileria taurotragi | TCT TGG CAC GTG GCT TTT | L19082 | 53.7 |
| Theileria parva | GGA CGG AGT TCG CTT TG | Alignment of the sequences with accession nos. L28999 and AF013418 | 55.2 |
| Theileria annulata | CCT CTG GGG TCT GTG CA | M64243 | 57.6 |
| Theileria and Babesia (catchall) | TAA TGG TTA ATA GGA (AG)C(AG) GTT | Gubbels et al. (4) | 52.0 |
The melting point (Tm) was determined empirically.
FIG. 4.
RLB results showing species-specific oligonucleotides of the 18S rRNA gene in the horizontal lanes and PCR products in the vertical lanes. Fifteen blood or tissue samples from black rhinoceroses from Tanzania and South Africa were analyzed. Positive Theileria and Babesia controls as well as negative (H2O) controls are included. †, deceased animals (death was attributed to babesiosis); GFRRC, Great Fish River Reserve Complex.
There is historical support for both parasite names. Brocklesby (3) already reported on a large Babesia species of the black rhinoceros in Kenya in 1967, whereas a smaller Theileria-like piroplasm was also found in 1989 in blood smears of South African rhinoceroses imported into Dallas, Tex. (1), and also in rhinoceroses in Tanzania in 1964 (7). Hence, both parasites were seen long ago, and they now finally receive proper names. Although Babesia and Theileria species are usually characterized by a restricted host spectrum, similar Babesia and Theileria parasites have also been reported in square-lipped, or white, rhinoceroses (Ceratotherium simum) in South Africa (2). We are screening blood samples to confirm whether B. bicornis and T. bicornis also occur in white rhinoceroses. A limited survey among other wildlife species (e.g., buffaloes, wildebeests, and lions) that had died in the Ngorongoro Conservation Area in Tanzania in 2001 did not reveal the parasites. It is clear that B. bicornis can cause fatal babesiosis, whereas there is no evidence yet to suggest that T. bicornis is also pathogenic for the black rhinoceros. Although dual infections do occur (e.g., rhinoceros 61 in Fig. 4), we can only speculate about the possible effects of such infections. Babesia-related mortalities have been reported in various black rhinoceros populations in Kenya (3, 10) and Tanzania (7), and these have limited successful translocation efforts. Mortality due to babesiosis may be triggered by stress factors, since in most cases animals died soon after capture (such as cases 3 and 4 in South Africa), during periods of nutritional or pregnancy-related stress, or during extreme climatic conditions. Translocation is an ongoing exercise in order to strengthen the various rhinoceros subpopulations in East and Southern Africa. For instance, the Addo Elephant National Park in South Africa was initially stocked with animals from Kenya (whose offspring are destined for repatriation to East Africa) and subsequently from Etosha National Park in Namibia. However, the rhinoceroses Bahati (case 1) and Maggie (case 2) did not die in captivity and had been translocated to the Ngorongoro Conservation Area in Tanzania several years earlier. Other causes of anemia in black rhinoceroses, such as leptospirosis (8), trypanosomiasis (7), and autoimmune disorders (9), have also been described and should therefore be taken into account in any differential diagnosis (11, 12).
As far as other tick-borne diseases are concerned, serological evidence for exposure of black and white rhinoceroses to heartwater disease, transmitted by Amblyomma ticks, has been reported from Zimbabwe (5). A broad spectrum of tick species, including Amblyomma rhinocerotis and Dermacentor rhinocerinus, is known to infest black rhinoceroses, but we do not yet know which tick species are responsible for the transmission of B. bicornis and T. bicornis.
Finally, a more extensive survey among black and white rhinoceroses is under way to map the geographical distributions of both parasites in the rhinoceros population in southern Africa, including a risk analysis related to translocations within this population.
Nucleotide sequence accession numbers.
The complete 18S rRNA gene sequence of the new species designated B. bicornis sp. nov. has been submitted to GenBank and has been given accession number AF419313. The complete 18S rRNA gene sequence of the new species designated T. bicornis has been submitted to GenBank and has been given accession number AF499604.
Acknowledgments
We thank Nick Kriek, Dave Cooper, Giuseppe Di Giulio, and Peter Lent for information on the fatal cases of babesiosis in black rhinoceroses. Koos Coetzer, Ivan Horak, and Nancy Kock are thanked for helpful suggestions on the manuscript.
The research reported here was conducted within the framework of the Memorandum of Understanding between the Faculty of Veterinary Science of the University of Pretoria and the Faculty of Veterinary Medicine of Utrecht University, Utrecht, The Netherlands. Financial support was provided by Senter International of the Ministry of Economic Affairs in The Netherlands under project SA 010111, entitled Development of a Biochip for the Molecular Diagnosis of Pathogens in Wildlife in South Africa.
REFERENCES
- 1. Animal and Plant Health Inspection Service, U.S. Department of Agriculture. 1989. Ectoparasites on imported rhinoceroses. Foreign Anim. Dis. Rep. 17:3. [Google Scholar]
- 2.Bigalke, R. D., M. E. Keep, K. J. Keep, and J. H. Schoeman. 1970. A large Babesia sp. and a Theileria-like piroplasm of the square-lipped rhinoceros. J. S. Afr. Vet. Med. Assoc. 41:292-294. [Google Scholar]
- 3.Brocklesby, D. W. 1967. A Babesia species of the black rhinoceros. Vet. Rec. 80:484. [Google Scholar]
- 4.Gubbels, J. M., A. P. de Vos, M. van der Weide, J. Viseras, L. M. Schouls, E. de Vries, and F. Jongejan. 1999. Simultaneous detection of bovine Theileria and Babesia species by reverse line blot hybridization. J. Clin. Microbiol. 37:1782-1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kock, N. D., F. Jongejan, M. D. Kock, R. A. Kock, and P. Morkel. 1992. Serological evidence for Cowdria ruminantium infection in free-ranging black (Diceros bicornis) and white (Ceratotherium simum) rhinoceroses in Zimbabwe. J. Zoo Wildl. Med. 23:409-413. [Google Scholar]
- 6.Lehmann, U., and H. Kreipe. 2001. Real-time PCR analysis of DNA and RNA extracted from formalin-fixed and paraffin-embedded biopsies. Methods 25:409-418. [DOI] [PubMed] [Google Scholar]
- 7.McCulloch, B., and P. L. Achard. 1969. Mortalities associated with capture, translocation, trade and exhibition of black rhinoceroses. Int. Zoo Yearb. 9:184-195. [Google Scholar]
- 8.Miller, R. E. 1993. Hemolytic anemia in the black rhinoceros, p. 455-458. In M. E. Fowler (ed.), Zoo and wild animal medicine, current therapy. The W. B. Saunders Co., Philadelphia, Pa.
- 9.Miller, R. E., and W. J. Boever. 1982. Fatal hemolytic anemia in the black rhinoceros: case report and a survey. J. Am. Vet. Med. Assoc. 181:1228-1231. [PubMed] [Google Scholar]
- 10.Mugera, G. M., and J. G. Wandera. 1967. Degenerative polymyopathies in East African domestic and wild animals. Vet. Rec. 80:410-413. [DOI] [PubMed] [Google Scholar]
- 11.Murray, S., N. P. Lung, T. P. Alvarado, K. C. Gamble, M. A. Miller, D. E. Paglia, and R. J. Montali. 1999. Idiopathic hemorrhagic vasculopathy syndrome in seven black rhinoceros. J. Am. Vet. Med. Assoc. 216:230-233. [DOI] [PubMed] [Google Scholar]
- 12.Paglia, D. E. 1993. Acute episodic hemolysis in the African black rhinoceros as an analogue of human glucose-6-phosphate dehydrogenase deficiency. Am. J. Hematol. 42:36-45. [DOI] [PubMed] [Google Scholar]
- 13.Vince, A., M. Poljak, and K. Seme. 1998. DNA extraction from archival Giemsa-stained bone-marrow slides: comparison of six rapid methods. Br. J. Haematol. 101:349-351. [DOI] [PubMed] [Google Scholar]