Abstract
The fourth transmembrane segment (S4) has been shown to function as a voltage sensor in voltage-gated channels. On membrane depolarization, a stretch of S4 moves outward and initiates a number of conformational changes that ultimately lead to channel opening. Conserved proline residues are in the middle of the S4 of motifs I and III in voltage-dependent Ca2+ channels. Because proline often introduces a “kink” into a helical structure of proteins, these residues might have an intrinsic function in the voltage sensor. Here, we report that the removal of S4 prolines results in a dramatic shortening of channel open time whereas the introduction of extra prolines to the corresponding positions in motif IIS4 and IVS4 lengthens channel open time. The number of S4s with a proline residue showed a clear positive correlation with the mean open time of the channel. The mean open time was >11-fold longer for a channel mutagenized to have prolines in all four S4s compared with a channel that had no prolines in the S4 region. Additionally, prolines in the S4s slowed activation kinetics and shifted the voltage dependence of activation and inactivation in a hyperpolarized direction. Our results strongly suggest that proline residues in the S4s are critical for stabilizing the open state of the channel. Moreover, it is suggested that motif IS4 and IIIS4 contribute to the channel opening more efficiently than motif IIS4 and IVS4.
Voltage-dependent ion channels are thought to go through a series of conformational changes from nonconducting to conducting state on depolarization. Cloning and analysis of the predicted secondary structure of the Na+ channel (1), Ca2+ channel (2), and the K+ channel (3) revealed a sharing of a common feature of positively charged transmembrane segments termed transmembrane segment 4 (S4). The S4 is likely to form an α-helix in which every third or fourth residue is basic (arginine or lysine) and carries positive charges. It is thought that the S4 α-helices traverse the membrane electric field and that, during depolarization, they move outward into the extracellular space and initiate conformational changes of the channel. This concept is supported by mutagenesis studies in which the mutations in charged residues (4–6) or uncharged residues (7–10) in S4 resulted in a shifted voltage dependence of activation. Several models have been proposed to account for the outward movement of S4, including a “sliding helix” (11) and a “propagating helix” (12). Direct proof for the outward movement of S4 has been shown in the Shaker K+ channel (13–15) as well as in the Na+ channel (16).
Proline residues naturally occur in the middle of the S4 of motifs I and III in all known voltage-dependent Ca2+ channel α1 subunits, including α1A, α1B, α1C, α1D, α1E, and α1S (17) and α1G and α1H (18, 19). In the normal helical structure of proteins, proline often causes a turn in the backbone. As an imino acid, it limits the rotation around the N-Cα bond, resulting in a 15–20° kink in the helical structure (20, 21). Thus, it is likely that the proline residues in the S4 putative α-helices of Ca2+ channels introduce kinks to these “moving α-helices.” The high conservation of these prolines among different Ca2+ channels implies a critical importance in channel function. To clarify the role of these evolutionarily conserved prolines, we engineered and characterized mutant channels in which the prolines either were removed from motif IS4 and/or motif IIIS4 or were added to equivalent positions in motif IIS4 and/or IVS4 of the human-heart L-type Ca2+ channel. Here, we describe crucial roles that S4 prolines play not only in voltage sensing but also in open–close transition of the channel.
MATERIALS AND METHODS
Site-Directed Mutagenesis and in Vitro Transcription.
The human heart L-type Ca2+ channel α1C subunit (22) cDNA was constructed in the plasmid pBluescript SK(+) (Stratagene). Mutations were introduced by the PCR method by using primers containing desired mismatches. The full-length mutant Ca2+ channel α1C subunit cDNAs were produced by subcloning mutant cassettes into the full-length wild-type α1C subunit cDNA. The whole region replaced by the PCR was sequenced, and the presence of the desired mutation(s) was confirmed. Capped cRNAs specific for the wild-type α1C subunit, mutant α1C subunits, α2/δa subunit (23), and β3 subunit (24) were synthesized by in vitro transcription.
Expression of Channels and Electrophysiological Measurements.
Xenopus laevis oocytes were defolliculated by incubating with 2 mg/ml collagenase (Type IA; Sigma) in a solution containing (in mM): 82.5 NaCl, 1 KCl, 1 MgCl2, and 5 Hepes (pH 7.5). Stage V and VI oocytes were injected with 5–25 ng of α1 subunit cRNA in combination with α2/δa subunit and β3 subunit cRNA in 1:1:1 molar ratio. Injected oocytes were maintained at 19°C in a solution containing (in mM): 96 NaCl, 2 KCl, 1 MgCl2, 1.8 CaCl2, 5 Hepes, 2.5 sodium pyruvate, and 0.5 theophylline (pH 7.5), supplemented with 100 units/ml penicillin and 100 μg/ml streptomycin for 2–4 days before electrophysiological recording.
Single-channel currents were recorded by using a patch-clamp amplifier (Axopatch 200A, Axon Instruments, Foster City, CA). After mechanically removing the vitelline membrane, the membrane potential of oocytes was zeroed by a high K+ solution containing (in mM): 140 KCl, 2 MgCl2, 5 EGTA, and 5 Hepes (pH 7.4). The patch electrodes were coated with Sylgard and had resistances of 5 to 20 megaohms. The pipette solution contained (in mM): 110 BaCl2 and 10 Hepes (pH 7.4). Only well resolved openings were measured; therefore, all single-channel experiments were conducted in the presence of 3 μM Bay K 8644. Usually, 500 depolarizing pulses were applied from a holding potential of −80 mV to each different test potential. The currents were low-pass filtered at 2 kHz, were digitized at 10 kHz, and were stored for off-line analysis. The leak and capacitive currents were canceled by subtracting the averaged traces without channel activity. Open and closed transitions were detected by using the half-amplitude threshold method. Open time histograms were fitted to multiple exponential functions by using the maximum likelihood estimate.
Whole-cell currents were recorded by using a two-electrode voltage-clamp amplifier (Axoclamp 2A, Axon Instruments). The external solution contained (in mM): 40 Ba(OH)2, 50 N-methyl-d-glucamine, 2 KOH, 5 Hepes, and 0.5 niflumic acid, adjusted to pH 7.4 with methanesulfonic acid. Ca2+-activated Cl− currents were minimized by the use of Cl−-free solution, Ba2+ as a charge carrier, and the inclusion of a Cl− current inhibitor, niflumic acid (25). The voltage recording electrode and the current injection electrode were filled with 3 M KCl and had a resistance of 0.5–1 megaohms. Pulses were applied from a holding potential of −80 mV to potentials between −40 and +50 mV in 10 mV increments. Whole cell leakage and capacitive transients were subtracted by using the P/4 protocol (26). Data were sampled at 1–10 kHz after being filtered at 1 kHz. To minimize an error in the voltage control, currents larger than 2 μA or smaller than 200 nA were excluded from analyses. All measurements were made at room temperature (22–23°C). The PClamp system (Axon Instruments) was used for data acquisition and analysis. Values are presented as means ± standard error. P < 0.05 was considered as statistically significant difference.
RESULTS
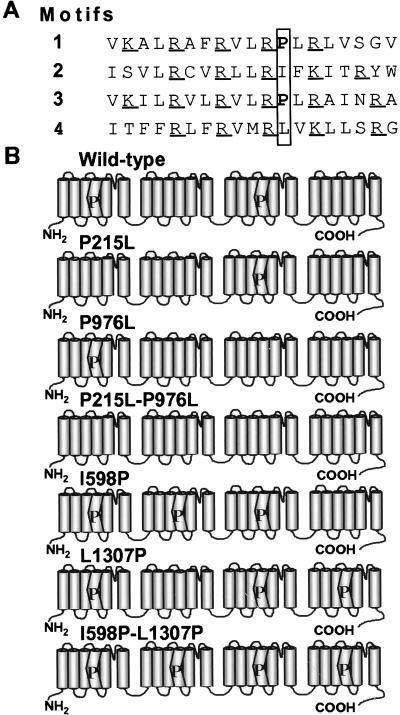
The wild-type calcium channels have two S4 voltage sensors with a proline in the middle of the α-helix (Fig. 1) that might influence the physical movement of these domains. To test the hypothesis as to whether the S4 prolines set the gating properties of the channel, we conducted mutagenesis and functional studies on mutant channels. Two naturally occurring prolines in motif IS4 and motif IIIS4 α-helices, pro215 and pro976 were changed individually into leucines (P215L and P976L), resulting in channels that have only one S4 with a proline. The double mutant P215L-P976L had no proline in the S4. Next, we substituted additional prolines at the equivalent positions of motif IIS4 and motif IVS4 (I598P and L1307P), making channels that have three S4s with a proline. The double mutant I598P-L1307P had prolines in all four S4s.
Figure 1.
The amino acid sequence of S4 in each motif of the wild-type channel and schematic representation of the wild-type and mutant Ca2+ channel α1C subunits. (A) Amino acid sequence of the human heart L-type Ca2+ channel α1 subunit S4. Conserved proline residues and equivalent positions are enclosed in a vertical box. Positively charged residues are underlined. (B) Diagrams describing the wild-type and mutant channels. Cylinders indicate transmembrane segments. S4s containing a proline are depicted as kinked cylinders.
Single-Channel Measurements.
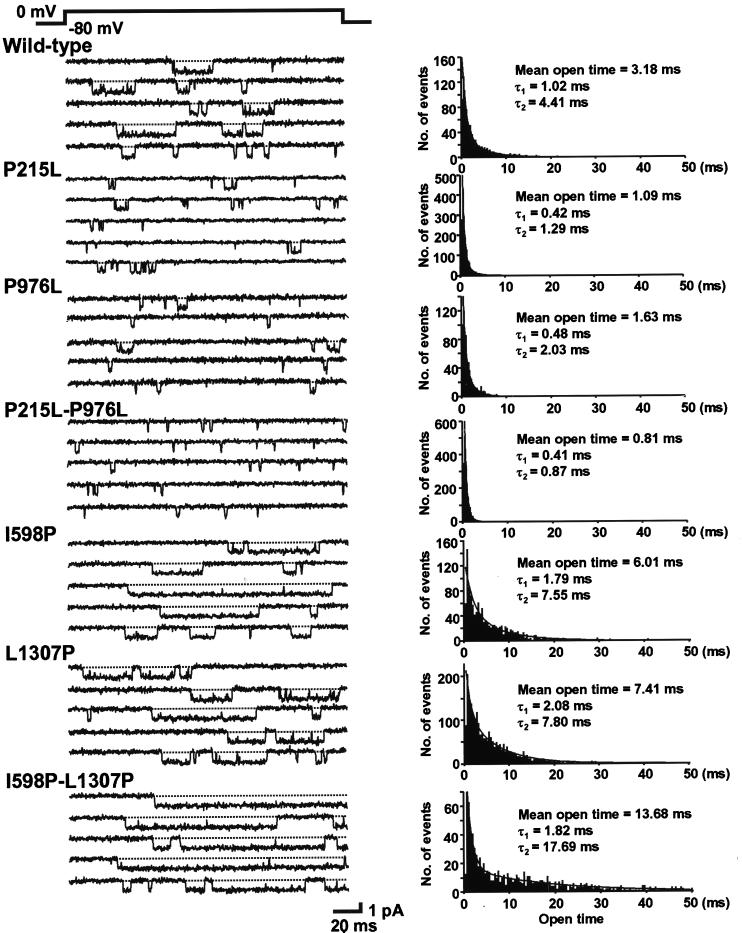
Representative current traces recorded from wild-type and mutant channels are shown in Fig. 2 Left. Channel openings are depicted as downward deflections. As reported (27–29), open time distribution was fitted best to a sum of two exponentials when modified with a dihydropyridine agonist, Bay K 8644 (Fig. 2 Right). This was the case not only for the wild type but also for all six mutant channels. Removal of a proline from motif IS4 or IIIS4 caused a dramatic effect on the channel open time. On average, the mean open time was 1.21 ± 0.08 ms (n = 11) for P215L and 1.38 ± 0.10 ms (n = 7) for P976L, 37 and 42% of the wild type (3.25 ± 0.26 ms, n = 6), respectively. The double mutant P215L-P976L showed an even shorter mean open time (0.82 ± 0.02 ms, n = 7), which was 25% of the wild type.
Figure 2.
Single-channel currents recorded from oocytes expressing the wild-type and mutant Ca2+ channels. Currents were elicited by 180-ms depolarization pulses to 0 mV from a holding potential of −80 mV. Representative current traces are shown in Left. Traces with no channel activity are not shown. Corresponding open time histograms are shown in Right. The smooth curves represent best fits with double exponentials. The values of two time constants, together with the mean open times, are shown in the insets.
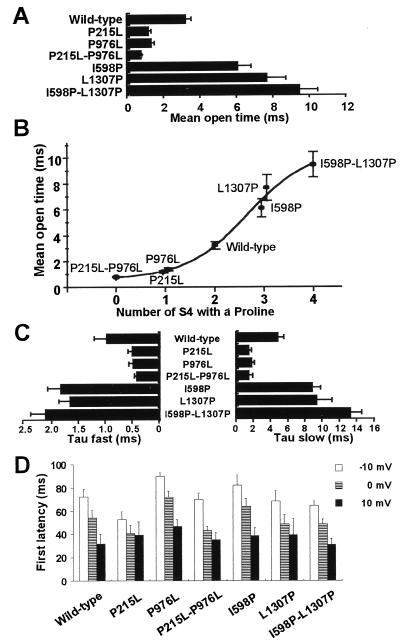
Of interest, introducing additional proline(s) to the S4 produced exactly the opposite effect of the removal of proline(s). The mean open time was 6.11 ± 0.68 ms (n = 8) for I598P and 7.69 ± 0.99 ms (n = 8) for L1307P, 188 and 237% of the wild type, respectively. The longest mean open time was observed at the double mutant I598P-L1307P (9.49 ± 0.94 ms), 292% of the wild type (n = 6). This was 11.6-fold longer than P215L-P976L. These results are summarized in Fig. 3A. When the mean open time was plotted against the number of the S4 with a proline (Fig. 3B), it was clear that the prolines in the S4s prolong the channel open time in a number-dependent manner. The EC50 value of the number of the S4 with a proline is between 2 and 3 (2.67).
Figure 3.
Parameters for single channel recordings. Data are represented as means ± standard error of 6–11 different patches. (A–C) Parameters for the channel open time tested with 500 depolarizing pulses to 0 mV from a holding potential of −80 mV. (A) Mean open time for each clone. (B) Mean open time plotted against the number of the S4 with a proline. (C) Fast and slow time constants for double exponential fitting of open time histograms. (D) Averaged latency to first openings measured at test potentials indicated.
Both fast and slow time constants of the open time distribution were shortened by removal of the proline(s) and were lengthened by the addition of the proline(s) to the S4s (Fig. 3C). This observation suggests that two different open states of the channel are both stabilized by the existence of proline(s) in the S4 voltage sensors. In addition, mutants P215L and P215L-P976L displayed a significantly higher fraction of short openings (Table 1). The same tendency—that is, the more the number of S4 with a proline, the higher the fraction of longer openings—was observed in other mutants, although this effect did not reach a statistical significance. This behavior implies that the S4 prolines make the channel favor to stay in the long-opening state. In contrast to the effect on the channel open time, mutations had little effect on single channel conductance (Table 1). The average latency to the first opening showed only small difference and a random tendency among the wild-type and mutant channels (Fig. 3D). Thus, there is no direct correlation between the number of S4 prolines and the first latency.
Table 1.
Parameters of single channel recordings for wild-type and mutant channels
| τ1, ms | f1, % | τ2, ms | f2, % | Mean open time, ms | Slope conductance, pS | |
|---|---|---|---|---|---|---|
| Wild type | 0.99 ± 0.22 | 45.7 ± 10.9 | 4.90 ± 0.51 | 54.3 ± 10.9 | 3.25 ± 0.26 | 20.2 |
| P215L | 0.51 ± 0.06 | 69.4 ± 3.4 | 1.54 ± 0.17 | 30.6 ± 3.4 | 1.21 ± 0.08 | 19.6 |
| P976L | 0.49 ± 0.08 | 52.3 ± 6.1 | 1.83 ± 0.20 | 47.7 ± 6.1 | 1.38 ± 0.10 | 18.7 |
| P215L-P976L | 0.43 ± 0.03 | 83.1 ± 3.9 | 1.54 ± 0.25 | 16.9 ± 3.9 | 0.82 ± 0.02 | 19.5 |
| I598P | 1.84 ± 0.24 | 38.7 ± 5.0 | 8.88 ± 0.85 | 61.3 ± 5.0 | 6.11 ± 0.68 | 22.5 |
| L1307P | 1.67 ± 0.19 | 31.6 ± 9.4 | 9.44 ± 1.63 | 68.4 ± 9.4 | 7.69 ± 0.99 | 20.6 |
| I598P-L1307P | 2.14 ± 0.26 | 32.6 ± 2.6 | 13.37 ± 1.19 | 67.4 ± 2.5 | 9.49 ± 0.94 | 22.7 |
Open time histograms were fitted with a sum of two exponentials. τ1 and τ2 are the fast and slow time constants.
f1 and f2 are the fraction of open time that are contributed by openings corresponding to τ1 and τ2 respectively.
Data are means ± standard error obtained from 6–11 patches. Underlining indicates statistically significant differences from values for the wild type, with P < 0.05.
Whole-Cell Measurements.
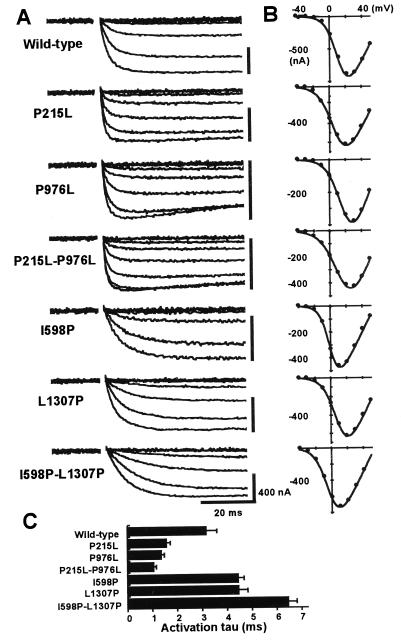
Representative whole-cell Ba2+ currents are illustrated in Fig. 4A. Corresponding I–V relationships are shown in Fig. 4B. There is a clear difference in the current activation kinetics among mutants—that is, faster activation for less proline mutants (P215L, P976L, P215L-P976L) and slower activation for the mutants with extra S4 prolines (I598P, L1307P, I598P-L1307P). The time constants of current activation measured at the peak current potential are summarized in Fig. 4C. The time constants were 1.59 ± 0.10 ms (n = 7), 1.38 ± 0.06 ms (n = 13), and 1.08 ± 0.07 ms (n = 10) for P215L, P976L, and P215L-P976L, respectively, 50, 43, and 34% of the wild type (3.18 ± 0.40 ms, n = 9). In contrast, the time constants were larger for I598P, L1307P, and I598P-L1307P, compared with the wild type: 4.45 ± 0.21 ms (140%, n = 15), 4.49 ± 0.21 ms (141%, n = 10), and 6.49 ± 0.27 ms (204%, n = 9), respectively.
Figure 4.
Summary of the whole-cell recordings. (A) Representative current traces elicited by voltage steps between −40 mV and each peak current potential in 10-mV increments from a holding potential of −80 mV. (B) Corresponding I–V relationships. (C) Time constants of the current activation at the peak current potential. Activation of peak current was fitted to a single exponential. Data represent means ± standard error of 7–15 experiments.
Parameters for the voltage dependence of activation and inactivation for wild-type and mutant channels are summarized in Table 2. The P215L mutation did not shift the midpoint potential (Vmid) for activation but did decrease the slope by 36% whereas P976L significantly shifted the Vmid for activation to the right (9.4 mV), with little change in the slope. These effects were observed in an additive manner in the double mutant P215L-P976L, in which the Vmid for activation was shifted by 9.6 mV to the right, and the slope was decreased by 58%. In contrast, the Vmid for activation was shifted to the left for I598P, L1307P, and I598P-L1307P by 7.9, 2.8, and 10.4 mV, respectively. The slope was decreased slightly in L1307P and I598P-L1307P.
Table 2.
Voltage-dependence of wild-type and mutant channels
| Activation
|
Inactivation
|
|||
|---|---|---|---|---|
| Vmid, mV | Slope, mV | Vmid, mV | Slope, mV | |
| Wild type | 8.4 ± 0.7 | 6.7 ± 0.3 | −1.8 ± 1.6 | 12.0 ± 0.5 |
| P215L | 7.1 ± 2.0 | 9.1 ± 0.2 | −7.7 ± 3.5 | 13.5 ± 0.3 |
| P976L | 17.8 ± 0.8 | 7.4 ± 0.6 | 0.0 ± 1.5 | 11.9 ± 0.9 |
| P215L-P976L | 18.0 ± 0.8 | 10.6 ± 0.2 | 1.2 ± 3.0 | 14.4 ± 0.3 |
| I598P | 0.5 ± 1.1 | 6.3 ± 0.2 | −13.4 ± 2.4 | 9.1 ± 0.9 |
| L1306P | 5.6 ± 1.0 | 8.5 ± 0.4 | −2.0 ± 1.1 | 7.0 ± 0.6 |
| I598P-L1307P | −2.0 ± 0.8 | 8.3 ± 0.1 | −25.1 ± 0.8 | 9.8 ± 0.7 |
Current-voltage relationships or current-prepulse voltage relationships were fitted to Boltzmann distributions to derive values for the midpoint (Vmid) and the slope (mV/e-fold change in G/Gmax) of the curves for steady-state activation and steady-state inactivation. The prepulses were 5,000 ms long. Data represent means ± standard error obtained from 7–14 (activation) and 4–9 (inactivation) measurements. Underlining indicates statistically significant differences from values for the wild type, with P < 0.05.
The removal of prolines from the S4 had little effect on the voltage dependence of inactivation (Table 2). There was no significant shift in the Vmid for inactivation in P215L, P976L, and P215L-P976L. Extra added prolines to the motif IIS4 showed a profound effect on the voltage dependence of inactivation, with the Vmid for inactivation shifted to the left by 11.6 and 23.3 mV for I598P and I598P-L1307P, respectively.
DISCUSSION
It is now well established that the positively charged S4s act as voltage sensors in voltage gated K+, Na+, and Ca2+ channels, initiating a series of conformational changes by an outward movement on depolarization. The present experiments were designed to elucidate roles of evolutionarily conserved proline residues in the S4 voltage sensors of voltage-gated Ca2+ channels. The prolines are conserved even between the high-voltage activated (L, N, P, Q, R type) and the low-voltage activated (T type) Ca2+ channels, which share <30% homology (18, 19, 30), implying critical roles for these residues. Our data demonstrate that the removal of the S4 prolines results in shortening of the channel open time whereas addition of extra prolines to the S4 prolongs it. This strongly suggests that the prolines are acting as a stabilizer of the open state of the channel. Of interest, the “number of S4s with a proline” clearly showed a sigmoidal positive correlation to the mean open time of the channel, with the wild type being close to the EC50, or half-maximal effect.
Numerous studies have shown that mutations in S4 voltage sensors result in altered voltage dependence of activation of K+, Na+, and Ca2+ channels (4–10, 31). In these studies, when the positive charges in S4s were neutralized or reversed, or even when noncharged residues were mutated, the steady-state activation curves were shifted significantly. Experiments clearly show that S4s play an important role in voltage sensing that occurs before the actual channel opening. However, the molecular mechanisms for voltage sensing and channel openings are distinct (28). The molecular site for the activation gates are not known so far, and mechanisms for the coupling between the movement of the voltage sensor and open–close transition of the gate remains to be clarified. One possible mechanism implies the notion that S4 movement “pulls” or distorts the S4-S5 linker, thus contributing to the inner pore of the channel and enabling the activation gate to open. Alternatively, movements of S4 may displace other parts of the channel and may lead to the gate opening (32). Although our results do not prove or disprove either model, it is clear that the proline residues in S4s play a key role in the open–close transition of the gate. Moreover, prolines in four different motifs are likely to act cooperatively; that is, addition or removal of two prolines showed a greater effect than a single mutation.
A direct comparison of whole-cell with single-channel data is not possible because of a voltage shift in channel gating that arises from surface charge effects at different Ba2+ concentrations. However, quantitative comparison among the clones in each condition would be rationalized. Moreover, because the channel open time was only weakly voltage-dependent, even if we compare it at different test potentials that reflect different Vmid for activation, still the tendency is the same (data not shown). At the macroscopic current level, mutations in the S4 proline altered the activation kinetics; that is, removal of prolines accelerated the activation whereas additional prolines slowed it. The effect of S4 mutation on the macroscopic activation kinetics looks almost like the effect on the channel open time at the single channel level: namely, the shorter the mean open time, the faster the current activation. Difference in the activation kinetics is not likely caused by the different speed of the movement of voltage sensors. We observed only small difference in the latency to the first opening among wild-type and mutant channels. This indicates that the time necessary for the channels to undergo multiple closed states, including the outward movement of S4s on depolarization, has not been altered significantly by mutations. These small changes very likely cannot account for the different activation kinetics. Difference in activation kinetics would more likely be attributable to the channel open time itself. According to theoretical considerations based on a simple model (33), when the mean channel open time is 1/α and the mean first latency is 1/β, the time to maximum amplitude tmax is given by an equation:
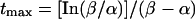 |
Consequently, if the mean first latency is the same (constant β), longer channel open time (smaller α) correlates to larger tmax, or slower activation. In this respect, effects of prolines in the S4s are analogous to the effects of dihydropyridine agonists on the Ca2+ channel, increasing the open time and slowing the activation rate (28). There is, however, an essential difference between these phenomena: S4 prolines increase both short and long time constants for open time histogram; dihydropyridine agonists modulate the channels to favor a long open state.
Significant but less dramatic effects were observed in the voltage dependence of activation by mutations in the S4 prolines. With an exception of P215L, there is a constant tendency for the prolines in S4s to shift the steady-state activation curves to the left. This suggests that the existence of prolines in the S4s decreases the energy barrier for the channel opening. The unique aspect of motif IS4 having only one charged residue downstream (intracellular side) to the proline or its equivalent position (compare to the other three S4s, which have two charged residues) may contribute to the lack of effect of the mutation in IS4 on the voltage dependence. In addition, all mutants except for I598P showed decreased voltage sensitivity (slope) of activation. This is in agreement with the study by Hurst et al. (10) in which an introduction of a proline to the S4 of concatenated potassium channel subunit KV1.1 resulted in a decreased slope of the G-V curve. The shift in the Vmid for activation by proline mutation was relatively small (≈10 mV in both directions), compared to the charge-neutralizing or charge-reversing mutations in the S4 (4–6). Additionally, the fact that these prolines are conserved even in the low voltage-activated Ca2+ channels (18, 19) suggests that the S4 prolines are not an essential determinant of the voltage dependence of activation.
I598P and I598P-L1307P showed a significant difference in Vmid for the steady-state inactivation compared with the wild type. For these two mutants, both the steady-state activation curve and the steady-state inactivation curve shifted to a hyperpolarized direction. These results suggest that activation and inactivation are strongly coupled, similarly to that found for the Shaker K+ channel (5); that is, because, at less positive potentials, more channels are activated for these mutants, higher fraction of channels have a chance to inactivate at less positive potentials. The reason why this was not the case for other mutants is not clear from our data.
From our present results, we suggest that S4s with a proline (motifs IS4 and IIIS4 in the wild-type channel) promote channel opening to a greater extent than the ones without a proline (motifs IIS4 and IVS4). In the S4s of an L-type Ca2+ channel, García et al. (31) neutralized or reversed positively charged residues. They found that significant effects on the Vmid for activation and time constant of activation were produced by mutations in motif IS4 and IIIS4 but not in motif IIS4 and IVS4. These results are nicely consistent with our findings; namely, mutations in IS4 and IIIS4 result in greater effects because of their greater contribution to the open state of the channel. On the other hand, in our work, mutations in S4s in four different motifs all resulted in significant changes in channel characteristics, suggesting that all S4s contribute to the gating of the channel, although not equally.
The principal subunit for the voltage-gated Ca2+ channels and Na+ channels—that is, the α1 subunit of the Ca2+ channels and the α subunit of the Na+ channels—consist of four homologous repeats that resemble a single α subunit of the voltage-gated K+ channels, containing six transmembrane segments. Therefore, it has been theorized that Ca2+ channels and Na+ channels are homologous products of the K+ channel gene duplications that occur early in the evolution of eukaryotes (32, 34). Considering the analogy between the effects of S4 prolines and dihydropyridine agonists—that is, stabilizing the channel open state, shifting the voltage dependence to the hyperpolarizing direction, and slowing the activation rate—it is tempting to speculate that the proline residues were introduced to the S4 voltage sensors of the Ca2+ channels during evolution as an “intrinsic channel agonist” to set the open time of the channel, thus improving the efficiency of Ca2+ ion permeation.
Acknowledgments
We are indebted to Dr. Udo Klöckner for critically reading the manuscript and Drs. Gabor Mikala and Mitsuyoshi Hara for helpful discussions. This work was supported in part by American Heart Association Ohio–West Virginia Affiliate Postdoctoral Fellowship SW-97-35-F (to H.Y.), by a grant from The Naito Foundation (to H.Y.), and by National Institutes of Health Grant PO1 HL22619-19 (to A.S).
ABBREVIATIONS
- S4
transmembrane segment 4
- Vmid, midpoint potential.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Noda M, Shimizu S, Tanabe T, Takai T, Kayano T, Ikeda T, Takahashi H, Nakayama H, Kanaoka Y, Minimino N, et al. Nature (London) 1984;312:121–127. doi: 10.1038/312121a0. [DOI] [PubMed] [Google Scholar]
- 2.Tanabe T, Takeshima H, Mikami A, Flockerzi V, Takahashi H, Kangawa K, Kojima M, Matsuo H, Hirose T, Numa S. Nature (London) 1987;328:313–318. doi: 10.1038/328313a0. [DOI] [PubMed] [Google Scholar]
- 3.Tempel B L, Papazian D M, Schwarz T L, Jan Y N, Jan L Y. Science. 1987;237:770–775. doi: 10.1126/science.2441471. [DOI] [PubMed] [Google Scholar]
- 4.Stühmer W, Conti F, Suzuki H, Wang X, Noda M, Yahagi N, Kubo H, Numa S. Nature (London) 1989;339:597–603. doi: 10.1038/339597a0. [DOI] [PubMed] [Google Scholar]
- 5.Papazian D M, Timpe L C, Jan Y N, Jan L Y. Nature (London) 1991;349:305–310. doi: 10.1038/349305a0. [DOI] [PubMed] [Google Scholar]
- 6.Perozo E, Santacruz-Toloza L, Stefani E, Bezanilla F, Papazian D M. Biophys J. 1994;66:345–354. doi: 10.1016/s0006-3495(94)80783-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Auld V J, Goldin A L, Krafte D S, Catterall W A, Lester H A, Davidson N, Dunn R J. Proc Natl Acad Sci USA. 1990;87:323–327. doi: 10.1073/pnas.87.1.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lopez G A, Jan Y N, Jan L Y. Neuron. 1991;7:327–336. doi: 10.1016/0896-6273(91)90271-z. [DOI] [PubMed] [Google Scholar]
- 9.McCormack K, Tanouye M A, Iverson L E, Lin J-W, Ramaswami M, McCormack T, Campanelli J T, Mathew M K, Rudy B. Proc Natl Acad Sci USA. 1991;88:2931–2935. doi: 10.1073/pnas.88.7.2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hurst R S, North R A, Adelman J P. Receptors Channels. 1995;3:263–272. [PubMed] [Google Scholar]
- 11.Catterall W A. Trends Neurosci. 1986;9:7–10. doi: 10.1016/0166-2236(93)90193-p. [DOI] [PubMed] [Google Scholar]
- 12.Guy H R, Conti F. Trends Neurosci. 1990;13:201–206. doi: 10.1016/0166-2236(90)90160-c. [DOI] [PubMed] [Google Scholar]
- 13.Mannuzzu L M, Moronne M M, Isacoff E Y. Science. 1996;271:213–216. doi: 10.1126/science.271.5246.213. [DOI] [PubMed] [Google Scholar]
- 14.Larsson H P, Baker O S, Dhillon D S, Isacoff E Y. Neuron. 1996;16:387–397. doi: 10.1016/s0896-6273(00)80056-2. [DOI] [PubMed] [Google Scholar]
- 15.Baker O S, Larsson H P, Mannuzzu L M, Isacoff E Y. Neuron. 1998;20:1283–1294. doi: 10.1016/s0896-6273(00)80507-3. [DOI] [PubMed] [Google Scholar]
- 16.Yang N, Horn R. Neuron. 1995;15:213–218. doi: 10.1016/0896-6273(95)90078-0. [DOI] [PubMed] [Google Scholar]
- 17.Varadi G, Mori Y, Mikala G, Schwartz A. Trends Pharmacol Sci. 1995;16:43–49. doi: 10.1016/s0165-6147(00)88977-4. [DOI] [PubMed] [Google Scholar]
- 18.Perez-Reyes E, Cribbs L L, Daud A, Lacerda A E, Barclay J, Williamson M P, Fox M, Rees M, Lee J-H. Nature (London) 1998;391:896–900. doi: 10.1038/36110. [DOI] [PubMed] [Google Scholar]
- 19.Cribbs L L, Lee J-H, Yang J, Satin J, Zhang Y, Daud A, Barclay J, Williamson M P, Fox M, Rees M, et al. Circ Res. 1998;83:103–109. doi: 10.1161/01.res.83.1.103. [DOI] [PubMed] [Google Scholar]
- 20.Suchyna T M, Xu L X, Gao F, Fourtner C R, Nicholson B J. Nature (London) 1993;365:847–849. doi: 10.1038/365847a0. [DOI] [PubMed] [Google Scholar]
- 21.Boncheva M, Vogel H. Biophys J. 1997;73:1056–1072. doi: 10.1016/S0006-3495(97)78138-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schultz D, Mikala G, Yatani A, Engle D B, Iles D E, Segers B, Sinke R J, Weghuis D O, Klöckner U, Wakamori M, et al. Proc Natl Acad Sci USA. 1993;90:6228–6232. doi: 10.1073/pnas.90.13.6228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ellis S B, Williams M E, Ways N R, Brenner R, Sharp A, Leung A T, Campbell K P, McKenna E, Koch W J, Hui A, et al. Science. 1988;241:1661–1664. doi: 10.1126/science.2458626. [DOI] [PubMed] [Google Scholar]
- 24.Klöckner U, Mikala G, Varadi M, Varadi G, Schwartz A. J Biol Chem. 1995;270:17306–17310. doi: 10.1074/jbc.270.29.17306. [DOI] [PubMed] [Google Scholar]
- 25.Wei X, Perez-Reyes E, Lacerda A E, Schuster G, Brown A M, Birnbaumer L. J Biol Chem. 1991;266:21943–21947. [PubMed] [Google Scholar]
- 26.Bezanilla F, Armstrong C M. J Gen Physiol. 1977;70:549–566. doi: 10.1085/jgp.70.5.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hess P, Lansman J B, Tsien R W. Nature (London) 1984;311:538–544. doi: 10.1038/311538a0. [DOI] [PubMed] [Google Scholar]
- 28.Marks T N, Jones S W. J Gen Physiol. 1992;99:367–390. doi: 10.1085/jgp.99.3.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wakamori M, Mikala G, Schwartz A, Yatani A. Biochem Biopys Res Commun. 1993;196:1170–1176. doi: 10.1006/bbrc.1993.2374. [DOI] [PubMed] [Google Scholar]
- 30.Tsien R W. Nature (London) 1998;391:839–841. doi: 10.1038/35981. [DOI] [PubMed] [Google Scholar]
- 31.García J, Nakai J, Imoto K, Beam K G. Biophys J. 1997;72:2515–2523. doi: 10.1016/S0006-3495(97)78896-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marban E, Yamagishi T, Tomaselli G F. J Physiol. 1998;508:647–657. doi: 10.1111/j.1469-7793.1998.647bp.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Colquhoun D, Hawkes A G. In: Single-Channel Recording. Sakmann B, Neher E, editors. New York: Plenum; 1995. pp. 449–452. [Google Scholar]
- 34.Hille B. Ionic Channels of Excitable Membranes. Sunderland, MA: Sinauer; 1992. pp. 525–544. [Google Scholar]