Abstract
During a community echovirus type 33 outbreak, the virus was detected in the feces and cerebrospinal fluid of a 3-year-old boy with right arm weakness that followed a mild nonspecific febrile illness. This is the first time an association between echovirus type 33 infection and acute flaccid paralysis has been reported.
CASE REPORT
In August 2000, a previously healthy 3-year-old boy presented to the Wellington Children's Hospital, Wellington, New Zealand, with acute flaccid paralysis (AFP) of his right arm. Four days earlier he had experienced a febrile illness with a clear nasal discharge, mild vomiting, and diarrhea. On the morning of admission he awoke complaining of a painful right shoulder and had limited movement of this arm. He had received all his routine childhood immunizations, including the oral poliomyelitis vaccine at 6 weeks and 3 and 5 months of age. There was no recent travel history, and other household members were well.
On examination he was afebrile and irritable but nontoxic and had no rash or meningeal signs. Except for the right arm, the neurologic examination was unremarkable. The right arm was hypotonic and areflexic. On the right he had 4+/5 power in his hand, 2/5 flexion and extension at the wrist, 3/5 flexion and 2/5 extension at the elbow, and 1/5 abduction and adduction of the shoulder. There was no sensory loss or bulbar involvement.
Hematological investigations included a hemoglobin level of 11.7g/dl, a platelet count of 355 × 109/liter, a total leukocyte count of 11.7 × 109/liter, and an erythrocyte sedimentation rate of 8 mm/h. Magnetic resonance imaging showed no evidence of cord swelling. In the sagittal T-2 weighted images, a bilateral high signal intensity was seen posteriorly in the pons extending down the brain stem and spinal cord to the low thoracic level. In the axial T-2 weighted images, there was an increased signal localized to the grey matter bilaterally, with greater accentuation on the right side. There was no enhancement with gadlinium. Lumbar puncture produced clear cerebrospinal fluid (CSF) with 53 × 106 white blood cells (91% lymphocytes) per liter, a protein concentration of 27 mg/dl, and a glucose concentration of 50 mg/dl. No organisms were seen on Gram staining, and bacterial pathogens were not detected by culture.
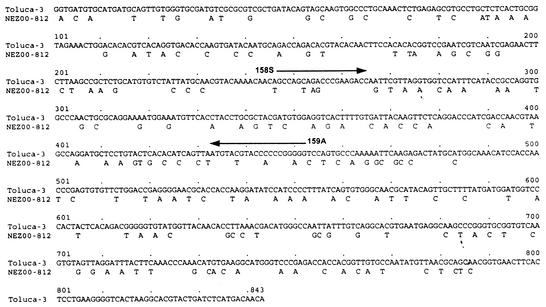
At the time of the patient’s illness there was a community outbreak of echovirus type 33 (E33) infection (8). That outbreak began in March 2000 in the Waikato region of New Zealand's North Island and spread 325 miles south to Wellington, where 17 cases were reported between June and November of that year. The outbreak lasted 9 months and was widespread throughout the North Island. A total of 75 cases were reported, mostly involving children with meningitis. Outbreak isolates were submitted by regional virology laboratories to the National Polio Reference Laboratory at the Institute of Environmental Science and Research (ESR), Porirua, Wellington, for further characterization. Initial typing by antibody neutralization tests with Lim and Benyesh-Melnick (9a) pools had suggested that these isolates were E33 strains. However, confirmatory neutralization tests at ESR with pooled and monospecific antisera (National Institute for Public Health and the Environment, Bilthoven, The Netherlands) against E33 were inconclusive. The outbreak strain's identity as E33 was finally established by molecular serotyping. Generic reverse transcription (RT)-PCR primers that amplify all human enterovirus serotypes were used to amplify a portion of the VP1 capsid gene, the sequence of which has been shown to correlate with the serotype (10). The partial VP1 sequence was 78.4% identical to that of the prototype E33 strain (96.8% amino acid identity) and less than 70% identical to all other enterovirus VP1 sequences, confirming the isolate's identity as E33. An E33-specific RT-PCR test was developed on the basis of the sequences of the outbreak strains and other E33 sequences available at the Centers for Disease Control and Prevention. Primer 158S (5′-GCI AGI ARI CCI GAR GAY C-3′; E33 VP1 nucleotides 247 to 265) anneals to a site that encodes an E33-specific amino acid motif (ASKPEDQ), whereas primer 159A (5′-CCI CCI GGN GGI ACR TAC AT-3′; E33 nucleotides 434 to 452) anneals to a site that encodes a motif that is highly conserved among all enteroviruses (MYVPPGG).
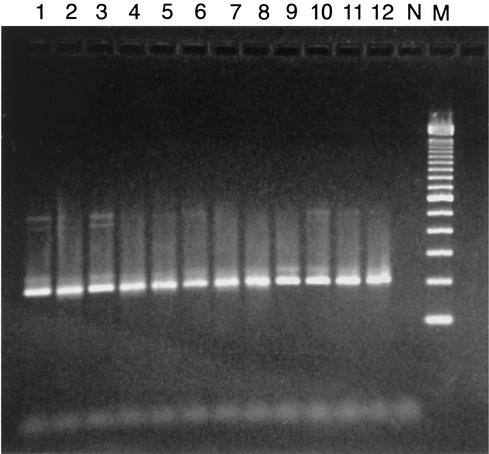
It was during this E33 outbreak, which included 17 cases within the local community, that the patient's pharyngeal secretions, CSF, and three fecal samples collected 3, 6, and 7 days after the onset of paralysis were transported to ESR. These specimens were inoculated into HF, RD, MEK, Hep-2, and L20B cells (12). All cell lines except the poliovirus-specific L20B cells inoculated with the three fecal specimens yielded E33 viruses, with typing confirmed by sequencing. Later, PCR testing of these cell lines established the presence of a 206-bp E33-specific amplicon identical to those of other E33 clinical isolates and the E33 prototype strain (Fig. 1). E33 RNA was also detected in the patient's CSF by the serotype-specific RT-PCR, but the result could not be confirmed by repeat testing because of insufficient sample volume (data not shown). The isolate responsible for AFP was further sequenced, and the resulting VP1 sequence (Fig. 2) differed from that of the prototype E33 strain by 21.6% (3.2% amino acid difference).
FIG. 1.
Specific amplification of E33 outbreak isolates by RT-PCR with the 158S-159A primer set. Lanes 1 to 12, clinical E33 isolates involved in an outbreak; lane N, negative control; lane M, molecular size marker.
FIG. 2.
Pairwise comparison of VP1 nucleotide sequences showing sequence differences between the E33 fecal isolate from the patient with AFP (NEZ00-812) and the E33 prototype strain, Toluca-3. The locations of diagnostic primers 158S and 159A are also indicated.
No other viruses or bacterial pathogens were detected in the fecal or respiratory secretions, and serological evidence for recent mycoplasma or Epstein-Barr virus infection was lacking. Nonpoliovirus enterovirus complement fixation titers of 1:16 were detected in serum collected on the day of admission. No convalescent-phase serum was available for testing.
Meanwhile, the boy initially received methylprednisolone, which was followed by a marked reduction in his irritability. Sixty days later movement had returned to his hand, wrist, and elbow, but shoulder abduction was limited to 30° and deltoid muscle wasting was present. After 1 month his tendon reflexes had returned but his muscle wasting persisted, especially wasting of the right shoulder muscle. At that stage he had almost normal power in his hand, wrist, and elbow but was unable to abduct his arm beyond 60°. Two years later his parents reported further improvement in arm movement and power, although shoulder muscle wasting remains.
The E33 prototype strain, Toluca-3, was isolated from a child in Toluca, Mexico, in 1959 (11). Since then, E33 has been linked to outbreaks and sporadic cases of meningitis, respiratory disease, gastroenteritis, and abortion in several Northern Hemisphere countries (2, 4, 13). However, to our knowledge this is the first time that E33 has been associated with paralytic disease.
Several features of this boy's illness suggest E33 as the possible causative agent. The virus was isolated from several fecal specimens, and serotype-specific PCR testing of the CSF detected the 206-bp E33-specific amplicon. The finding of a nonspecific low titer of enterovirus antibodies in an acute-phase serum sample implies that the fecal shedding of E33 most likely followed recent exposure. Moreover, his presentation occurred in the setting of an E33 outbreak in which several cases were concurrently identified in the local community. None of the patients with these other E33 cases developed AFP, although among those older than 12 months of age, nearly 90% had aseptic meningitis (8). Finally, no other pathogens were detected and no other causes of AFP were identified (5, 9). Unfortunately, convalescent-phase serum was not available to demonstrate type-specific seroconversion.
World Health Organization reports of nonpoliovirus enteroviruses from 1967 to 1970 found that paralysis was present in less than 1% of all patients with echovirus and coxsackievirus type A and B infections reported (1). However, as the global eradication of polioviruses approaches, proportionately more cases of nonpoliovirus enterovirus infections causing AFP and mimicking acute paralytic poliomyelitis will emerge. Of the at least 20 nonpoliovirus enterovirus serotypes associated with AFP, coxsackievirus types A7 and A9, the group B coxsackieviruses, echovirus type 9, and enterovirus type 71 are the most commonly encountered (5-7). Although the majority of cases are mild and transient, persistent muscle weakness, as illustrated by the case of the young child described here, or even death has been reported.
As the spectrum of nonpoliovirus enteroviruses causing AFP continues to expand (3), we report on E33 as a possible agent associated with this illness. E33 and other enteroviruses should be considered in the course of AFP surveillance activities in countries where poliovirus has been eradicated.
REFERENCES
- 1.Assad, F., and W. C. Cockburn. 1972. Four-year study of WHO virus reports on enteroviruses other than poliovirus. Bull. W. H. O. 46:329-336. [PMC free article] [PubMed] [Google Scholar]
- 2.Basso, N. G. S., M. E. F. Fonseca, A. G. P. Garcia, J. A. T. Zuardi, M. R. Silva, and H. Outani. 1990. Enterovirus isolation from foetal and placental tissues. Acta Virol. 34:49-57. [PubMed] [Google Scholar]
- 3.Chaves, S. S., S. Lobo, M. Kennett, and J. Black. 2001. Coxsackie virus A24 infection presenting as acute flaccid paralysis. Lancet 357:605.. [DOI] [PubMed] [Google Scholar]
- 4.Druyts-Voets, E., F. Yane, E. Bosmans, J. Colaert, and J. Desmyter. 1985. Method for selecting optimal cells for enterovirus isolation as determined in an outbreak of echovirus type 33 meningitis. Eur. J. Clin. Microbiol. 4:331-334. [DOI] [PubMed] [Google Scholar]
- 5.Gear, J. H. S. 1984. Nonpolio causes of polio-like paralytic syndromes. Rev. Infect. Dis. 6(Suppl. 2):S379-S384. [DOI] [PubMed] [Google Scholar]
- 6.Grist, N. R., and E. J. Bell. 1984. Paralytic poliomyelitis and nonpolio enteroviruses: studies in Scotland. Rev. Infect. Dis. 6(Suppl. 2):S385-S386. [DOI] [PubMed] [Google Scholar]
- 7.Huang, C.-C., C.-C. Liu, Y.-C. Chang, C.-Y. Chen, S.-T. Wang, and T.-F. Yeh. 1999. Neurologic complications in children with enterovirus 71 infection. N. Engl. J. Med. 341:936-942. [DOI] [PubMed] [Google Scholar]
- 8.Huang, Q. S. 2000. Echovirus type 33 outbreak in 2000. Lablink 7:29-30. [Google Scholar]
- 9.Kidd, D., H. Manji, D. Brown, and R. S. Howard. 1996. Acute paralytic illness. Postgrad. Med. J. 72:699-701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9a.Lim, K. A., and M. Benyesh-Melnick. 1960. Typing of viruses by combinations of antiserum pools: application to typing of enteroviruses (cocksackie and echo). J. Immunol. 84:309-317. [PubMed] [Google Scholar]
- 10.Oberste, M. S., K. Maher, M. R. Flemister, G. Marchetti, D. R. Kilpatrick, and M. A. Pallansch. 2000. Comparison of classic and molecular approaches for the identification of “untypeable”enteroviruses. J. Clin. Microbiol. 38:1170-1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosen, L., and J. Kern. 1965. Toluca-3, a newly recognised enterovirus. Proc. Soc. Exp. Biol. Med. 118:389-391. [DOI] [PubMed] [Google Scholar]
- 12.Rotbart, H. A. 1999. Enteroviruses, p. 990-998. In P. R. Murray, E. J. Baron, M. A. Pfaller, F. C. Tenover, and R. H. Yolken (ed.), Manual of clinical microbiology, 7th ed. American Society for Microbiology, Washington, D.C.
- 13.Sato, K., T. Yamashita, K. Sakae, Y. Suzuki, N. Ishikawa, and Y. Nishimura. 1998. A new-born baby outbreak of echovirus type 33 infection. J. Infect. 37:123-126. [DOI] [PubMed] [Google Scholar]