Abstract
Sequence analysis of a specific region of the mycobacterium rpoB gene in 35 mycobacterial strains representing 26 different mycobacterial species of clinical importance showed that there exists a highly polymorphic region. Based on the sequences of the polymorphic region, the oligonucleotide probes of 14 mycobacterial species with relatively high clinical importance were designed and shown to be specific to their corresponding mycobacterial species by dot blot hybridization. The results showed that the probes designed in this study are highly specific to each mycobacterial species, which suggests that these sequences may be useful for the species identification of mycobacteria.
Tuberculosis (TB) is still a major public health problem in the world, with about 8 million new cases and over 2 million deaths reported annually. With the recent dissemination of human immunodeficiency virus infection throughout the world, infections with nontuberculous mycobacteria as well as TB have increased in many parts of the world over the last decade. For example, Mycobacterium avium infections accounted for almost 50% of mycobacterial infections among AIDS patients in certain geographical areas (4, 7). In order to provide proper drug regimens to patients with mycobacterial infections, it is important that species be identified correctly and rapidly, because drug regimens for TB may differ from those for other mycobacterial infections. In addition, with the wide use of liquid culture systems such as the BACTEC system (Becton Dickinson Diagnostic Instrument Systems, Sparks, Md.) and the MB/BacT system (Organon Teknika Corp., Boxtel, The Netherlands), the rapid identification of mycobacterial species has become even more important (3, 17).
Although biochemical tests are available for Mycobacterium species identification, it has proven to be difficult to use these tests because of time-consuming and often incorrect identification. In order to overcome such difficulties, high-performance liquid chromatography has been widely used for species identification based on mycolic acid analysis (1, 14, 25). In addition, with recent developments in molecular techniques and the availability of genome sequencing data, several molecular tests have been developed and are used in clinical mycobacterial laboratories. rRNA sequences, notably that of 16S rRNA, have been most widely used for mycobacterium species identification (18), and commercial kits based on such sequences are available (AccuProbe; Gen-Probe Inc., LiPA, Innogenetics N. V., Zwijnaarde, Szinjdrecht, Belgium). In addition, the hsp65 gene (8, 15, 16, 23), the intergenic region between 16S and 23S rRNA (19), and the rpoB gene (9, 10, 13) are among the targets for molecular technique-based species identification. Sequencing (11, 15, 18, 20-22), DNA hybridization (6, 12), PCR-restriction fragment length polymorphism analysis (RFLP) (8, 9, 13, 16, 23), and microarray technology have also been employed to differentiate Mycobacterium species (5, 24).
Among the target genes, we were particularly interested in the rpoB gene. PCR sequence analysis of a region of the rpoB gene was suggested as a possible means of differentiating 44 species (9, 10, 13). We have also reported a new RFLP method (13) based on a different region of the rpoB gene, which is located between the first variable region (V1) and the second conserved region (C2), as determined using the genetic information of the Escherichia coli rpoB gene. The 360-bp region of the rpoB gene (bases 902 to 1261 and codons 302 to 420 of the rpoB gene of M. tuberculosis; GenBank accession number P47766) was found to be useful in the differentiation of more than 50 Mycobacterium species by a simple RFLP using two restriction enzymes. This clearly indicates that this 360-bp region of the rpoB gene contains highly informative sequences. In the present study, we analyzed sequences of this rpoB region of 35 mycobacterial strains representing 26 different mycobacterial species and prepared DNA probes that can be used in simple DNA hybridization tests for the identification of Mycobacterium species.
A total of 48 mycobacterial reference strains representing 39 Mycobacterium species were used for the PCR amplification of the 360-bp region of the rpoB gene in the present study (Table 1). Among them, 39 mycobacterial strains were obtained from the Korean Institute of Tuberculosis (KIT), Seoul, Korea, and three species were obtained from the Korean Collection for Type Cultures (KCTC) at the Korean Research Institute of Bioscience and Biotechnology (KRIBB). M. abscessus, which was recently separated from M. chelonae as an independent new species, was obtained from Department of Clinical Pathology at Yonsei University Medical Center (YUMC). Finally, five subtypes of M. kansasii were generously provided by V. Vincent at the Laboratoire de Référence des Mycobactéries, Institut Pasteur, Paris, France. Clinical isolates that were subjected to dot blot hybridization to evaluate the specificity of each mycobacterial species-specific probes were obtained from the KIT. All clinical isolates used in this study were identified on the basis of conventional tests that included microbiological characterization and biochemical tests and an rpoB-based RFLP method (13) to precisely identify the clinical isolates.
TABLE 1.
Bacterial strains used in this study
| Species | Strain | Sourcea |
|---|---|---|
| M. africanum | ATCC 25420 | KIT |
| M. scrofulaceum | ATCC 19981 | KIT |
| M. gilvum | ATCC 43909 | KIT |
| M. gastri | ATCC 15754 | KIT |
| M. asiaticum | ATCC 25276 | KIT |
| M. aurum | ATCC 23366 | KIT |
| M. avium | ATCC 25291 | KIT |
| M. moriokaense | ATCC 43059 | KRIBB |
| M. abscessus | Pettenkofer Institute | YUMC |
| M. celatum type I | ATCC 51130 | KIT |
| M. celatum type II | ATCC 51131 | KIT |
| M. chelonae | ATCC 35749 | KIT |
| M. bovis | ATCC 19210 | KIT |
| M. flavescens | ATCC 14474 | KIT |
| M. fortuitum type I | ATCC 6841 | KIT |
| M. fortuitum type II | ATCC 49404 | KIT |
| M. gallinarum | ATCC 19710 | KRIBB |
| M. genavense | ATCC 51233 | KIT |
| M. microti | ATCC 19422 | KIT |
| M. gordonae type I | ATCC 14470 | KIT |
| M. gordonae type II | KIT | |
| M. gordonae type III | KIT | |
| M. gordonae type IV | KIT | |
| M. haemophilum | ATCC 29548 | KIT |
| M. intracellulare | ATCC 13950 | KIT |
| M. interjectum | ATCC 51457 | KIT |
| M. intermedium | ATCC 51848 | KIT |
| M. kansasii type I | Pasteur Institute | |
| M. kansasii type II | Pasteur Institute | |
| M. kansasii type III | Pasteur Institute | |
| M. kansasii type IV | Pasteur Institute | |
| M. kansasii type V | Pasteur Institute | |
| M. mucogenicum | ATCC 49650 | KIT |
| M. neoaurum | ATCC 25795 | KIT |
| M. nonchromogenicum | ATCC 19530 | KIT |
| M. parafortuitum | ATCC 19686 | KIT |
| M. peregrinum | ATCC 14467 | KIT |
| M. phlei | ATCC 11758 | KIT |
| M. pulveris | ATCC 35154 | KRIBB |
| M. malmoense | ATCC 29571 | KIT |
| M. marinum | ATCC 927 | KIT |
| M. szulgai | ATCC 35799 | KIT |
| M. terrae | ATCC 15755 | KIT |
| M. thermoresistibile | ATCC 19527 | KIT |
| M. triviale | ATCC 23292 | KIT |
| M. ulcerans | ATCC 19423 | KIT |
| M. vaccae | ATCC 15483 | KIT |
| M. xenopi | ATCC 19250 | KIT |
| M. tuberculosis H37Rv | ATCC 27294 | KIT |
The primer sets used to amplify the target rpoB gene were 5′-TCAAGGAGAAGCGCTACGA-3′ (RPO5′) and 5′-GGATGTTGATCAGGGTCTGC-3′ (RPO3′), which resulted in a 360-bp PCR product (13). PCR was carried out in a final volume of 50 μl with 10 μl of DNA supernatant containing approximately 10 ng of genomic DNA, 10 pmol of each primer, 2 mM MgCl2, 200 μM concentrations of deoxynucleoside triphosphates, and 1 U of DyNAzymeII DNA polymerase (Finnzymes, Espoo, Finland). DNA samples were first denatured completely by incubation at 94°C for 5 min and then amplified using 35 cycles of (i) denaturation at 94°C for 1 min, (ii) primer annealing at 58°C for 1 min, and (iii) elongation at 72°C in a thermocycler (Perkin-Elmer model 9600; Applied Biosystems, Foster City, Calif.). After the last amplification cycle, the samples were incubated further at 72°C for 7 min to obtain complete elongation of the final PCR products. Positive and negative controls were always included in each PCR. The positive control was the PCR mix with the DNA of the reference strain, M. bovis, and the negative control was a PCR mix without any DNA. After the PCR, the amplification results were visualized using 1.5% agarose gel electrophoresis and ethidium bromide staining.
For sequencing, PCR products were purified using the GeneClean III kit (Bio 101, Vista, Calif.) and cloned into a PCR-TOPO vector in the TOPO TA cloning kit (Invitrogen Co., Carlsbad, Calif.). The TOPO vectors containing PCR products were used for transformation of TOP10 competent cells (Invitrogen Co.). Plasmids containing inserts were purified from broth cultures with a Qiagen (Valencia, Calif.) plasmid kit and sequenced with the AutoRead sequencing kit and ALF DNA sequencer (Pharmacia Biotech, Uppsala, Sweden). Sequences were aligned using the Multialign program developed by F. Corpet (2).
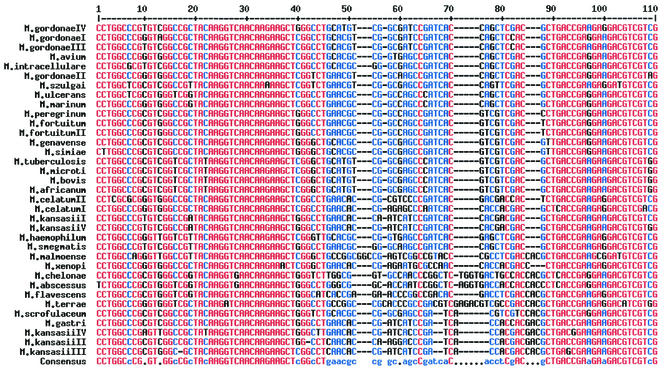
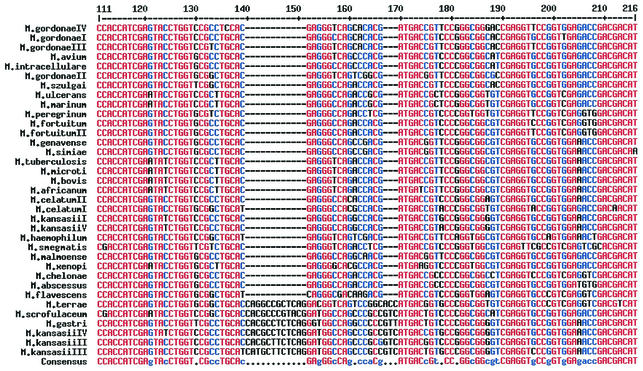
In order to characterize the genetic nature of the 360-bp region of the rpoB gene, a total of 35 reference strains representing 26 different mycobacterial species were sequenced. Some species such as M. gordonae, M. kansasii, M. celatum, and M. fortuitum are known to have several subspecies, and thus these subspecies were also included in the sequence analysis. Among the 360-bp region sequenced in this study, sequences of 216 bp that have not been reported elsewhere are shown in Fig. 1, which shows that there exists a highly polymorphic region (black letters) flanked by highly conserved regions (red letters). Interestingly, the highly polymorphic region seemed to be suitable for the differentiation of mycobacterial species. For example, species differentiation between M. kansasii and M. gastri was possible since the sequences of M. kansasii are different from those of M. gastri, whose differentiation is not possible by 16S rRNA sequence analysis (18). Moreover, these polymorphic sequences were different even between highly closely related species, such as M. abscessus and M. chelonae or M. fortuitum and M. peregrinum, whose exact species identification has been extremely difficult by conventional culture-based microbiological and biochemical tests. In addition, the sequences of this polymorphic region in subspecies of M. kansasii, M. fortuitum, and M. gordonae were also differentiable, suggesting that this region of the rpoB gene may be used as a molecular signature for the differentiation of mycobacteria to the species or even to the subspecies level. However, there was no sequence difference in this region among species of the M. tuberculosis complex, including M. tuberculosis, M. bovis, M. microti, and M. africanum.
FIG. 1.
Multialignment sequences identified in the rpoB region of 35 strains of 26 mycobacterial species of clinical importance. The software used for the alignment of multiple sequences was obtained from reference 2. Sequences with high consensus are shown as red letters, low consensus as blue letters, and neutral consensus as black letters. Among the 360-bp sequences identified in this study, only the 216-bp region of the upstream sequences is shown, since the downstream sequences have been reported elsewhere. The sequences shown here have been submitted to GenBank.
Based on the sequence information, we designed 15- to 20-mer oligonucleotide molecules as species- and subtype-specific probes for 16 strains of 14 mycobacterial species of clinical importance and one for all the mycobacterial species (Table 2). Subsequently, in order to determine the specificity of the probes for the targeted mycobacterial species, dot blot hybridization was carried out using each probe molecule. For these experiments, the target region of the rpoB gene from 48 different mycobacterial strains, representing 39 species listed in Table 1, was amplified by PCR, and the PCR products were then dot blotted on membranes.
TABLE 2.
Mycobacterial species-specific oligonucleotide probes designed and confirmed for their specificity by dot blot hybridization in this study
| Name of oligonucleotide | Sequence | Target organism(s) |
|---|---|---|
| MYC | GACGTCGTCGCCACCATCGA | All mycobacterial species |
| MTB | CATGTCGGCGAGCCC | M. tuberculosis complex |
| AVI | CGGTGAGCCGATCACC | M. avium |
| INT | CCTGCACGCGGGCGA | M. intracellulare |
| SCR | CGTACGGATGGCCAGC | M. scrofulaceum |
| KAN-I | GGCCACGATGACCGTG | M. kansasii types I and V |
| KAN-II | TCTCAGGATGGCCAGC | M. kansasii types II, III, and IV |
| GAS | TCTCAGGGTGGCCAGG | M. gastri |
| FOR-C | CCTGAACGCCGGCCAG | M. fortuitum complex |
| PER | GTTCCGGTCGAGGTGG | M. peregrinum |
| CHE | TGGTGACTGCCACCACG | M. chelonae |
| ABS | GGTGACCACCACCACC | M. abscessus |
| ULC | GGCCAGCCCATCACC | M. ulcerans |
| GEN/SIM | CCAGCCGACGATGACG | M. genavense-M. simiae |
| GOR-I | GTCGGCGATCCGATCA | M. gordonae types I, III, and IV |
| GOR-II | CGTCGGCAAGCCGA | M. gordonae type II |
| SZU | TCTGAACGTCGGCGAG | M. szulgai |
To prepare the DNA dot blot, precut membrane (Hybond-N+ [10 by 10 cm]; Amersham Pharmacia Biotech Korea Ltd., Seoul, Korea) was immersed into the denaturing solution (0.4 N NaOH, 25 mM EDTA; pH 8.0) for 1 min. After excess denaturing solution was allowed to drip from the membrane, it was placed on Whatman 3MM filter paper, and 1 to 2 μl of the PCR product was blotted onto the membrane. The membrane was then air dried for 5 min, rinsed with denaturing solution for another 1 min, placed between two sheets of 3MM filter paper, and baked for 2 h at 80°C. Oligonucleotide probes were labeled using an enhanced chemiluminescence kit for 3′ oligolabeling and detection (Amersham Pharmacia Biotech Korea Ltd.). Subsequent processing, including hybridizing the oligonucleotide probes to the membrane (42°C for 1 h), membrane washing (52°C for 15 min), and signal detection, was carried out using the method recommended by the manufacturer. Each species-specific probe was tested separately using the membrane onto which all PCR products were blotted, and there was no difference in dot blot hybridization conditions for all 16 probes analyzed in this study.
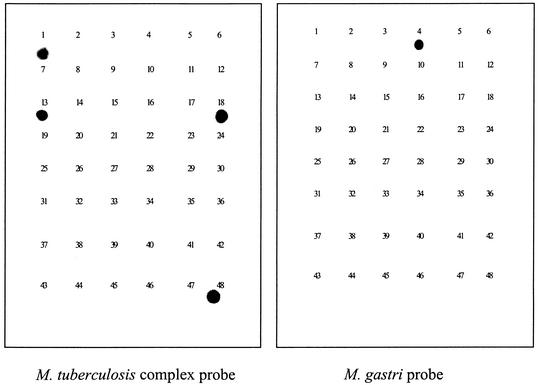
Figure 2 showed dot blot hybridization results using the M. tuberculosis complex probe and M. gastri probe as examples. Each probe hybridized only to the corresponding mycobacterial species, indicating the specificity of molecular probes to each mycobacterial species. The rest of the probes in Table 2 also showed species-specific hybridization to the corresponding species (data not shown).
FIG. 2.
The results of the dot blot hybridizations using each mycobacterial species-specific oligomer probe derived from the novel region of the rpoB genes of species. Dot blot hybridizations were conducted using probes specific for M. tuberculosis complex and M. gastri. The PCR-amplified products from 48 mycobacterial species were blotted on the membrane, and this was followed by hybridization with probes. The identification numbers in the membranes are matched with numbers and species names in Table 1.
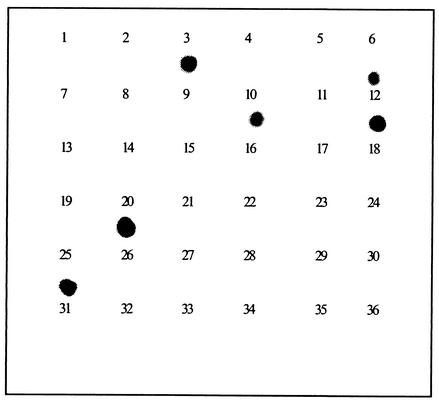
Finally, in order to determine if all clinical isolates that belong to each mycobacterial species can be detected using its specific probe, dot blot hybridization using the M. avium-probe molecule was carried out using 36 clinical isolates, including 6 isolates of M. avium. As shown in Fig. 3, all six M. avium isolates were identified correctly by the M. avium-specific probe in a dot blot hybridization assay, while the probe did not bind to any clinical isolates of other mycobacterial species.
FIG. 3.
Dot blot hybridizations using the M. avium specific oligomer probe. The membrane contained PCR products amplified from 36 clinical isolates of mycobacteria, including six isolates of M. avium species. The clinical isolates were identified by conventional culture and biochemical tests at the KIT.
This study demonstrates that the rpoB gene of Mycobacterium species contains a highly polymorphic region whose DNA sequences can be used for species identification. Sequence analysis of the region showed clearly the difference in nucleotide sequences among 26 Mycobacterium species and subtypes of four species examined in this study. The results also supported clearly our previous report on species identification of mycobacteria by RFLP of the polymorphic region (13). In addition, we showed that the oligonucleotide probes based on the sequences of the region were specific to each Mycobacterium species and useful for species identification of mycobacteria in a dot blot hybridization.
The rpoB gene encodes the β subunit of RNA polymerase, which produces RNA molecules in cells. Thus, rpoB is one of the very critical housekeeping genes that are closely related to cellular vitality and thus becomes the target for rifampin, the major bactericidal drug for M. tuberculosis and M. leprae. It is, therefore, reasonable to assume that the genetic structure of the rpoB gene is highly conserved within the same species. However, unlike 16S rRNA or any other rRNA whose primary structure is functionally critical, the rpoB gene seems to tolerate a more diverse sequence alteration without causing any changes in protein function. In particular, the DNA region that is not involved in the active site of the protein seems to be more polymorphic and does not cause major functional defects. Based on these relationships, it is easily understood that there exist highly conserved DNA regions and relatively variable DNA regions in the rpoB gene. The tolerable sequence variation in the rpoB gene becomes a useful clue for species identification of mycobacteria as reported previously (9, 10, 13). However, sequence analysis of the 360-bp region of the rpoB that we reported herein clearly revealed more extensive variation than expected, leading to develop mycobacterial species-specific probe molecules.
The oligonucleotide probes designed on the basis of this polymorphic region were useful in Mycobacterium species identification by dot blot hybridization assay. No cross-reactive hybridization was found between the 16 mycobacterial species-specific probes (Table 2) and 48 strains of 39 Mycobacterium species (Fig. 2). For example, the M. gastri-specific probe did not hybridize with five subtypes of M. kansasii, although the two species could not be differentiated by 16 rRNA sequence analysis (18). One of our concerns was sequence variation in the 360-bp region among clinical isolates of each Mycobacterium species. In this study, however, there seems to be no variation in the nucleotides of the region among M. avium clinical isolates, because the probe hybridized with all M. avium clinical isolates, as shown in Fig. 3. This was also supported by our previous study in which no variation was found in RFLP enzyme restriction sites among 40 clinical isolates of M. tuberculosis, 40 clinical isolates of M. avium, 50 clinical isolates of M. intracellulare, and 25 clinical isolates of M. gordonae, etc. (13). Although other probes still need to be confirmed for their sensitivity and specificity using multiple clinical isolates of each Mycobacterium species, the probes will be useful in developing a reverse blot hybridization assay by which many isolates can be analyzed for their species identification at the same time. In addition, since the 360-bp region is located near the rpoB mutation sites, which are associated with resistance to rifampin, one can develop an assay in the future which can simultaneously provide information about mycobacterial species identity and rifampin resistance.
Nucleotide sequence accession numbers. The nucleotide sequences listed in Table 2 have been submitted to the EMBL database and have been given the following accession numbers: AY271315 for M. microti, AY271316 for M. terrae, AY271317 for M. scrofulaceum, AY271318 for M. marinum, AY271319 for M. szulgai, AY271320 for M. gastri, AY271321 for M. malmoense, AY271322 for M. avium, AY271323 for M. bovis, AY271324 for M. peregrinum, AY271325 for M. fortuitumI, AY271326 for M. celatum type II, AY271327 for M. flavescens, AY271328 for M. intracellulare, AY271329 for M. abscessus, AY271330 for M. africanum, AY271331 for M. haemophilum, AY271332 for M. xenopi, AY271333 for M. kansasii type I, AY271334 for M. kasasii type II, AY271335 for M. kansaii type IV, AY271336 for M. kansasii type IV, AY271336 for M kansaii AY271337 M. celatum type I, AY271338 for M. genavense, AY271339 for M. simiae, AY271340 for M. fortuitum type II, AY271341 for M. gordonae type IV, AY271342 for M. gordonae type I, AY271343 for M. gordonae type II, AY271344 for M. gordonae type III, and AY271345 for M. smegmatis.
Acknowledgments
This work was supported in part by National Research Laboratory Program M1-0001-00-0089, by the Ministry of Science and Technology National Research and Development Program, and by Brain Korea 21 Project for Medical Sciences in Yonsei University.
REFERENCES
- 1.Butler, W. R., K. C. Jost, Jr., and J. O. Kilburn. 1991. Identification of mycobacteria by high-performance liquid chromatography. J. Clin. Microbiol. 29:2468-2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Corpet, F. 1988. Multiple sequence alignment with hierarchical clustering. Nucleic Acids Res. 16:10881-10890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Evans, K. D., A. S. Nakasone, P. A. Sutherland, L. M. de la Maza, and E. M. Peterson. 1992. Identification of Mycobacterium tuberculosis and Mycobacterium avium-M. intracellulare directly from primary BACTEC cultures by using acridinium-ester-labeled DNA probes. J. Clin. Microbiol. 30:2427-2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.French, A. L., D. A. Benator, and F. M. Gordin. 1997. Nontuberculous mycobacterial infections. Med. Clin. N. Am. 81:361-379. [DOI] [PubMed] [Google Scholar]
- 5.Gingeras, T. R., G. Ghandour, E. Wang, A. Berno, P. M. Small, F. Drobniewski, et al. 1998. Simultaneous genotyping and species identification using hybridization pattern recognition analysis of generic Mycobacterium DNA arrays. Genome Res. 8:435-448. [DOI] [PubMed] [Google Scholar]
- 6.Goto, M., S. Oka, K. Okuzumi, S. Kimura, and K. Shimada. 1991. Evaluation of acridinium-ester-labeled DNA probes for identification of Mycobacterium tuberculosis and Mycobacterium avium-Mycobacterium intracellulare complex in culture. J. Clin. Microbiol. 29:2473-2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Horsburgh, C. R. 1991. Mycobacterium avium complex infection in the acquired immunodeficiency syndrome. N. Engl. J. Med. 324:1332-1338. [DOI] [PubMed] [Google Scholar]
- 8.Hughes, M. S., R. A. Skuce, L.-A. Beck, and S. D. Neill. 1993. Identification of mycobacteria from animals by restriction enzyme analysis and direct DNA cycle sequencing of polymerase chain reaction-amplified 16S rRNA gene sequences. J. Clin. Microbiol. 31:3216-3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim, B.-J., K.-H. Lee, B.-N. Park, S.-J. Kim, G.-H. Bai, S.-J. Kim, and Y.-H. Kook. 2001. Differentiation of mycobacterial species by PCR-restriction analysis of DNA (342 base pairs) of the RNA polymerase gene (rpoB). J. Clin. Microbiol. 39:2102-2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim, B.-J., S.-H. Lee, M.-A. Lyu, S.-J. Kim, G.-H. Bai, S.-J. Kim, G.-T. Chae, E.-C. Kim, C.-Y. Cha, and Y.-H. Kook. 1999. Identification of mycobacterial species by comparative sequence analysis of the RNA polymerase gene (rpoB). J. Clin. Microbiol. 37:1714-1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kirschner, P., B. Springer, U. Vogel, A. Meier, A. Wrede, M. Kiekenbeck, F.-C. Bange, and E. C. Böttger. 1993. Genotypic identification of mycobacteria by nucleic acid sequence determination: report of a 2-year experience in a clinical laboratory. J. Clin. Microbiol. 31:2882-2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lebrun, L., F. Espinasse, J. D. Poveda, and V. Vincent-Levy-Frebault. 1992. Evaluation of nonradioactive DNA probes for identification of mycobacteria. J. Clin. Microbiol. 30:2476-2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee, H., H.-J. Park, S.-N. Cho, G.-H. Bai, and S.-J. Kim. 2000. Species identification of mycobacteria by PCR-restriction fragment length polymorphism of the rpoB gene. J. Clin. Microbiol. 38:2966-2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marks, J., and T. Szulga. 1965. Thin-layer chromatography of mycobacterial lipids as an aid to classification; technical procedures; Mycobacterium fortuitum. Tubercle 46:400-411. [DOI] [PubMed] [Google Scholar]
- 15.Pai, S., N. Esen, X. Pan, and J. M. Musser. 1997. Routine rapid Mycobacterium species assignment based on species-specific allelic variation in the 65-kilodalton heat shock protein gene (hsp65). Arch. Pathol. Lab. Med. 121:859-864. [PubMed] [Google Scholar]
- 16.Plikaytis, B. B., B. D. Plikaytis, M. A. Yakrus, W. R. Butler, C. L. Woodley, V. A. Silcox, and T. M. Shinnick. 1992. Differentiation of slowly growing Mycobacterium species, including Mycobacterium tuberculosis, by gene amplification and restriction fragment length polymorphism analysis. J. Clin. Microbiol. 30:1815-1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reisner, B. S., A. M. Gatson, and G. L. Woods. 1994. Use of Gen-Probe AccuProbes to identify Mycobacterium avium complex, Mycobacterium tuberculosis complex, Mycobacterium kansasii, and Mycobacterium gordonae directly from BACTEC TB broth cultures. J. Clin. Microbiol. 32:2995-2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rogall, T., T. Flohr, and E. Bottger. 1990. Differentiation of mycobacterial species by direct sequencing of amplified DNA. J. Gen. Microbiol. 136:1915-1920. [DOI] [PubMed] [Google Scholar]
- 19.Roth, A., M. Fischer, M. E. Hamid, S. Michalke, W. Ludwig, and H. Mauch. 1998. Differentiation of phylogenetically related slowly growing mycobacteria based on 16S-23S rRNA gene internal transcribed spacer sequences. J. Clin. Microbiol. 36:139-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soini, H., E. C. Böttger, and M. K. Viljanen. 1994. Identification of mycobacteria by PCR-based sequence determination of the 32-kilodalton protein gene. J. Clin. Microbiol. 32:2944-2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Springer, B., P. Kirschner, G. Rost-Mayer, K.-H. Schröder, R. M. Kroppenstedt, and E. C. Böttger. 1993. Mycobacterium interjectum, a new species isolated from a patient with chronic lymphadenitis. J. Clin. Microbiol. 31:3083-3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Springer, B., E. Tortoli, I. Richter, R. Grünewald, S. Rüsch-Gerdes, K. Uschmann, F. Suter, M. D. Collins, R. M. Kroppenstedt, and E. C. Böttger. 1995. Mycobacterium conspicuum sp. nov., a new species isolated from patients with disseminated infections. J. Clin. Microbiol. 33:2805-2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Telenti, A., F. Marchesi, M. Balz, F. Baly, E. C. Böttger, and T. Bodmer. 1993. Rapid identification of mycobacteria to the species level by polymerase chain reaction and restriction enzyme analysis. J. Clin. Microbiol. 31:175-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Troesch, A., H. Nguyen, C. G. Miyada, S. Desvarenne, T. R. Gingeras, P. M. Kaplan, P. Cros, and C. Mabilat. 1999. Mycobacterium species identification and rifampin resistance testing with high-density DNA probe arrays. J. Clin. Microbiol. 37:49-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsang, A. Y., I. Drupa, M. Goldberg, J. K. McClatchy, and P. J. Brennan. 1983. Use of serology and thin-layer chromatography for the assembly of an authenticated collection of serovars within the Mycobacterium avium-Mycobacterium intracellulare-Mycobacterium scrofulaceum complex. Int. J. Syst. Bacteriol. 33:285-292. [Google Scholar]