Abstract
We report the first case of neonatal Legionnaires' disease associated with water birth in a spa bath at home. Legionella pneumophila serogroup 6 was detected from postmortem lung tissue.
CASE REPORT
In June 1999, a 3.5-kg baby girl was born after a 42-week gestation period in a bathtub that employed the ever-ready system in her home by water birth delivery in the absence of doctors and midwives. The mother had no health problems during her pregnancy. The midwife arrived 15 min after the girl was born. The baby was normal at that time, but she developed a fever and jaundice on day 4. She was admitted to a private obstetrics-gynecology hospital and received phototherapy. The following day the baby left the hospital, because fever had decreased and jaundice had disappeared. On day 7 the baby had recurrent symptoms of fever and vomiting, although she drank milk well that night. At noon on the next day the baby showed sudden apnea and was transported to our emergency room with cardiopulmonary arrest. She could not be resuscitated.
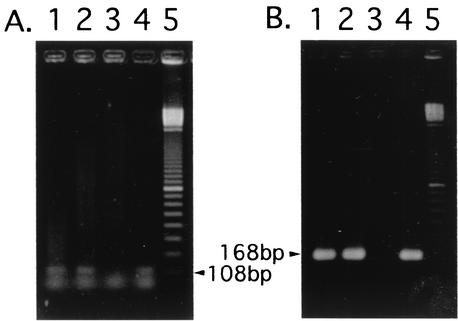
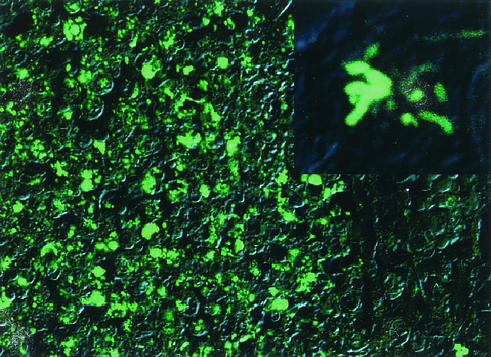
An autopsy was performed 3 h after death. There were numerous yellow nodules, which were less than 5 mm in diameter and which were scattered over the bilateral lung parenchyma. In the pulmonary nodular lesions, the alveoli showed severe infiltration of neutrophils with prominent karyorrhexis and aggregation of macrophages with fibrinous exudates. Many gram-negative but Gimenez-positive bright red bacteria were found in the macrophages. As the baby was suspected to have legionellosis, DNA was extracted from the formalin-fixed postmortem lung tissue homogenate (10) and was tested in a PCR with specific primers for Legionella 5S rRNA DNA and the Legionella pneumophila mip gene (according to the guidelines in the package insert for EnviroAmp Legionella, Perkin Elmer Cetus Corporation, Norwalk, Conn.). Amplified DNA fragments with expected sizes corresponding to each gene of L. pneumophila were detected from the specimen by using both primer sets (Fig. 1). The nucleotide sequence of an amplified mip gene fragment was determined by using the BigDye Terminator Cycle Sequencing Ready Reaction kit V2.0 (Applied Biosystems, Foster City, Calif.) and the ABI PRISM 310 DNA Genetic Analyzer (Applied Biosystems) with forward and reverse primers for the mip gene. The nucleotide sequence was 100% matched with the region between primers of the mip gene in L. pneumophila serogroups 1, 2, and 6 (GenBank/EMBL/DDBJ accession numbers S42595, AF022316, and AF022320, respectively). The lung homogenate was also screened by indirect fluorescent antibody testing (1) with 10 Legionella species-specific monovalent antisera (Denka Seiken, Tokyo, Japan). These Legionella spp. include the individual type strains of L. pneumophila serogroups 1 to 6, Legionella bosemanii, Legionella dumoffii, Legionella gormanii, and Legionella micdadei. The homogenate was added to wells on glass slides, air dried, and processed for immunofluorescence microscopy. The specimen was positive only with an antiserum to L. pneumophila serogroup 6. Numerous rod-shaped bacteria in macrophages of pulmonary nodular lesions were also stained by indirect fluorescent antibody (Fig. 2). Since we could not detect any other abnormal macroscopic or microscopic findings in the autopsy, we consider the lung lesions the main cause of her death. During the course of the environmental investigation, numerous living Legionella organisms (14,640 CFU/100 ml) were detected in the water obtained in the same bathtub a week after the baby's death. Unfortunately, we could not characterize the isolated Legionella organisms further.
FIG. 1.
Results of PCR analysis with AmpliTaq DNA polymerase (Applied Biosystems) and GeneAmp PCR System 2400 (Applied Biosystems). (A) Primers (Perkin Elmer Cetus) were designed to amplify the Legionella genus-specific 5S rRNA region. Forward primer, 5′-GGCGACTATAGCG(A/G)TTTGGAA-3′; reverse primer, 5′-GCGATGACCTACTTTC(A/G)CATGA-3′. (B) Primers were designed to detect only the L. pneumophila mip gene. Forward primer, 5′-GCATTGGTGCCGATTTGG-3′; reverse primer, 5′-G(C/T)TTTGCCATCAAATCTTT(C/T)TGAA-3′. Cycling parameters for PCR were as follows: 94°C for 1 min; 30 cycles of 94°C for 1 min, 55°C for 1 min, and 72°C for 1 min; and finally, 72°C for 7 min and 4°C for storage. As for the detection of the mip gene, secondary PCR was needed with 1/100 volume of the first PCR product as a template. Cycling parameters for secondary PCR were as follows: 94°C for 1 min; 30 cycles of 94°C for 1 min, 60°C for 1 min, and 72°C for 1 min; and finally, 72°C for 7 min and 4°C for storage. Templates used for PCR were as follows. Lanes: 1, the formalin-fixed patient lung tissue; 2, formalin-fixed A/J mouse lung infected by L. pneumophila serogroup 1; 3, uninfected formalin-fixed A/J mouse lung; 4, chromosome of L. pneumophila serogroup 1. Lane 5 indicates DNA size markers of a 100-bp ladder. DNA extraction from lung tissue (10) and bacteria (8) was performed by the method previously described.
FIG. 2.
Microscopic finding in the lung by using immunofluorescence staining. The slides with paraffin sections of lungs were overlaid with rabbit antiserum against each L. pneumophila organism at a dilution of 1:25 and were incubated at 37°C for 30 min in a moisture chamber. After being rinsed in phosphate-buffered saline (pH 7.2) to remove unbound globulin, the slides were incubated at 37°C for 30 min with fluorescein-conjugated goat immunoglobulin G (IgG) F(ab′)2 to rabbit IgG (Organon Teknica Corp., Cappel Product) at a dilution of 1:50, rinsed, mounted in 90% glycerol (pH 9.0) and a coverslip, and examined under a confocal laser scanning microscope (Olympus FLUOVIEW IX70) with a 40× objective lens. Immunofluorescence and corresponding differential interference contrast images were merged. An enlargement of a single mononuclear phagocyte is shown in the upper right corner. The specific immunofluorescence against L. pneumophila serogroup 6 was detected.
Since an outbreak in Philadelphia in 1976 (3), Legionella has been recognized as an important etiological agent of hospital- and community-acquired pneumonia. But only 10 sporadic cases of Legionnaires' disease in neonates have been reported (2, 6). Besides those published cases, in 1996 there was a nosocomial outbreak of Legionnaires' disease in Japan (N. Yamashita, S. Kuida, Y. Morikawa, K. Kawasaki, N. Okazaki, N. Matsuo, and K. Yamaguchi, Abstr. 101st Annu. Meet. Jpn. Pediatr. Soc., abstr. 474, 1998 [in Japanese]) in which L. pneumophila serogroups 1 and 6 caused pneumonia in four neonates and caused one of them to die. The newest case was a newborn infected by L. pneumophila serogroup 1 after water birth in a hospital in Italy in December 1999 (2). In contrast with all these cases of nosocomial infection, our case is a rare example of community-acquired Legionella pneumonia in a neonate.
In 1984, a 24-h (always ready) bathing system appeared on the Japanese market, and by 2001 one and a half million systems had been manufactured and shipped in Japan. Over the years, many incremental improvements have been made in bathing facilities, and the present ever-ready bath that fits into the bathtub is one of the newest. The bath water circulates and is maintained to be clean and warm at about 40°C without changing the water for several days to a month. The bath water cleaning systems vary from filtering, heating, and chemical disinfectant to UV light; but some organisms may survive the killing or removal.
Since a case of Legionnaires' disease due to aspiration of hot spring water was reported in 1993 (7), attention has been drawn to the risk of infection with Legionella species and other microorganisms, because the purification of human byproducts in the bath water is executed mainly by biological cleaning by using microorganisms naturally occurring in the filter equipment. The water cleaned by the ever-ready bath system may be clean enough for several days to several weeks of normal bathing, but it is not clean enough for drinking and certainly not clean enough for use by newborn infants or immunocompromised persons. In fact, microorganisms, such as Legionella species, mycobacteria, Pseudomonas aeruginosa, and free-living amoeba, have been detected in water and in filter systems of 24-h bath spas (12, 14). Furthermore, a familial cluster of cutaneous Mycobacterium avium infection was reported recently in Japan which was associated with a large number of acid-fast bacilli in the fluid wrung from the filter of a bath tub heating unit (11).
The water birth technique has been developed mainly in Europe and the United States, but it has not been popular in Japan (only 0.05% of 44,730 deliveries was reported to be performed by birth underwater in Aichi Prefecture in 2000 [13]). Consequently, there is not enough evidence to evaluate the use of immersion in water during labor (9). The water birth movement has largely been motivated by requests for the comfort and relaxation that water-assisted labor affords for both mother and child. However, we believe that delivering babies under water requires that safety measures be established in order to avoid risk to the baby (5). In addition, spa baths for water birth are recommended with disposable filters for a single use as shown by the Global Maternal/Child Health Association (Wilsonville, Oreg.) (4). Our report is of the first case of community-acquired neonatal Legionnaires' disease and warns that there is a risk of neonatal Legionella infection when home water birth is undertaken with a 24-h bathing system unless there has been extremely careful disinfection immediately beforehand.
REFERENCES
- 1.Brown, S. L., W. F. Bibb, and R. M. McKinney. 1984. Retrospective examination of lung tissue specimens for the presence of Legionella organisms: comparison of an indirect fluorescent-antibody system with direct fluorescent-antibody testing. J. Clin. Microbiol. 19:468-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Franzin, L., C. Scolfaro, D. Cabodi, M. Valera, and P. A. Tovo. 2001. Legionella pneumophila pneumonia in a newborn after water birth: a new mode of transmission. Clin. Infect. Dis. 33:e103-e104. [DOI] [PubMed] [Google Scholar]
- 3.Fraser, D. W., T. R. Tsai, W. Orenstein, W. E. Parkin, H. J. Beecham, R. G. Sharrar, J. Harris, G. F. Mallison, S. M. Martin, J. E. McDade, C. C. Shepard, and P. S. Brachman. 1977. Legionnaires' disease: description of an epidemic of pneumonia. N. Engl. J. Med. 297:1189-1197. [DOI] [PubMed] [Google Scholar]
- 4.Hamper, B. 1994. Gentle birth choices: a guide to making informed decisions about birthing centers, birth attendants, water birth, home birth, hospital birth. Healing Art Press, Rochester, Vt.
- 5.Harmsworth, G. 1994. Waterbirth. Safety first. Nurs. Times 90:30-32. [PubMed] [Google Scholar]
- 6.Levy, I., and L. G. Rubin. 1998. Legionella pneumonia in neonates: a literature review. J. Perinatol. 18:287-290. [PubMed] [Google Scholar]
- 7.Mashiba, K., T. Hamamoto, and K. Torikai. 1993. A case of Legionnaires' disease due to aspiration of hot spring water and isolation of Legionnlla pneumophila from hot spring water. Kansenshogaku Zasshi 67:163-166. [DOI] [PubMed] [Google Scholar]
- 8.Miura, K. 1967. Preparation of bacterial DNA by the pheno-pH9-RNase method. Methods Enzymol. 68:543-545. [Google Scholar]
- 9.Nikodem, V. C. 2002. Immersion in water in pregnancy, labour and birth Cochrane Database Syst. Rev. 2000:CD000111. [Online.] [DOI] [PubMed]
- 10.Shiozawa, A., T. Kubota, S. Akahane, H. Hattori, T. Yagi, T. Miyake, K. Takami, and S. Kaneko. 1995. Detection of Yersinia pseudotuberculosis DNAs in paraffin-embedded tissues from dead chimpanzees by using PCR. Contrib. Microbiol. Immunol. 13:156-157. [PubMed] [Google Scholar]
- 11.Sugita, Y., N. Ishii, M. Katsuno, R. Yamada, and H. Nakajima. 2000. Familial cluster of cutaneous Mycobacterium avium infection resulting from use of a circulating, constantly heated bath water system. Br. J. Dermatol. 142:789-793. [DOI] [PubMed] [Google Scholar]
- 12.Takahashi, T., E. Yabuuchi, T. Endo, and K. Furuhata. 1998. Sanitary problems of 24h-bath and administrative countermeasures for them. Jpn. J. Environ. Infect. 13:129-136. (In Japanese.)
- 13.Togari, H., and K. Ishikawa. 2001. Investigation on diversity of recent pregnancy and delivery method with reference to birth underwater. Investigation and research by the Association of Perinatal Medical Treatment in Aichi prefecture in the 2000 fiscal year. Association of Perinatal Medical Treatment, Aichi, Japan.
- 14.Yabuuchi, E., S. Sakai, K. Fuwa, M. Tamesada, K. Asano, T. Takahashi, and T. Tsujino. 1999. Mycobacterium avium: detection from porous filtrating material, sponge filter, and bath tub water of 24h-home bath systems in Japan. Jpn. J. Environ. Infect. 14:181-188. (In Japanese.)