Abstract
The emergence of West Nile (WN) virus in New York and the surrounding area in 1999 prompted an increase in surveillance measures throughout the United States, including the screening of sentinel chicken flocks for antibodies. An enzyme-linked immunosorbent assay (ELISA) for the detection of chicken immunoglobulin M (IgM) to WN virus was developed, standardized, and characterized as a rapid and sensitive means to detect WN viral antibodies in sentinel flocks. Serum specimens from experimentally infected chickens were analyzed by using this assay, and IgM was detected as early as 3 to 7 days postinfection. Persistence of IgM varied from at least 19 to more than 61 days postinfection, which indicates the need to bleed sentinel flocks at least every 2 weeks for optimal results if this method is to be used as a screening tool. The ELISA was compared to hemagglutination-inhibition and plaque reduction neutralization tests and was found to be the method of choice when early detection of WN antibody is required. House sparrows and rock doves are potential free-ranging sentinel species for WN virus, and the chicken WN IgM-capture ELISA was capable of detecting anti-WN IgM in house sparrow serum samples from laboratory-infected birds but not from rock dove serum samples. The chicken WN IgM-capture ELISA detected anti-WN antibodies in serum samples from naturally infected chickens. It also detected IgM in serum samples from two species of geese and from experimentally infected ring-necked pheasants, American crows, common grackles, and redwinged blackbirds. However, the test was determined to be less appropriate than an IgG (IgY)-based assay for use with free-ranging birds. The positive-to-negative ratios in the ELISA were similar regardless of the strain of WN viral antigen used, and only minimal cross-reactivity was observed between the WN and St. Louis encephalitis (SLE) IgM-capture ELISAs. A blind-coded serum panel was tested, and the chicken WN IgM-capture ELISA produced consistent results, with the exception of one borderline result. A preliminary test was done to assess the feasibility of a combined SLE and WN IgM-capture ELISA, and results were promising.
With the introduction of WN virus to the New York area in 1999, a coalition of agencies and experts recommended that increased surveillance be conducted to enable early detection of WN virus in forthcoming transmission seasons (8). Sentinel chicken flocks are part of the traditional arthropod-borne virus (arbovirus) surveillance methodology (9), and recent studies have been conducted to determine the usefulness of chickens as a sentinel species for WN virus (12). It is important that a rapid technique be available with which to screen the flocks for WN viral antibodies and that the method is both sensitive and capable of detecting early antibodies. Detection of antiviral immunoglobulin M (IgM) by enzyme-linked immunosorbent assay (ELISA) has been established as a useful method for detection of other arboviruses in chicken serum (16, 17). The test is capable of detecting viral infections as early or earlier in the course of infection than other methods such as plaque reduction neutralization tests (PRNT), hemagglutination-inhibition (HAI) tests, and IgG ELISA (2). One advantage that the IgM ELISA has over other methods is that IgM is produced earlier in the course of infection than IgY, the avian equivalent of mammalian IgG (IgY is referred to as IgG in the remainder of this study), and persists for a relatively short time, thus allowing a recent infection to be identified (14). With the use of controls, a standardized ELISA is easy to interpret and gives a qualitative but numerical result.
In the present study we developed and standardized a chicken WN IgM-capture ELISA for use in monitoring sentinel birds. The test was formulated to be as similar as possible to the human arboviral IgM-capture ELISAs (14) to allow easy integration of the test into those laboratories that are currently using these assays.
MATERIALS AND METHODS
Antigens and conjugate.
The WN virus antigens were made as β-propiolactone-inactivated, sucrose-acetone-extracted suckling mouse brain preparations (4). Control antigens were similar preparations made from mock-infected suckling mouse brains. The detecting antibody used in the ELISA (St. Louis encephalitis [SLE] virus 6B6C-1 horseradish peroxidase [HRP]) (18) was custom conjugated to HRP by Jackson ImmunoResearch Laboratories, Inc., West Grove, Pa.
Infection of chickens with WN virus.
Twelve Delta Dekalb hens (Hudson Pullet Farms, Fort Lupton, Colo.) aged 20 and 60 weeks old were bled prior to infection via bracheal venipuncture by using a 1-ml tuberculin syringe with a 26-gauge 3/8-in. needle. Six of the hens were inoculated subcutaneously with ca. 10,000 PFU of WN virus (strain NY99-4132, 1 Vero passage). Six uninfected cage mates were included as controls. Blood (0.3 ml) was collected daily up to day 7 postinfection (p.i.), and subsequently (0.6 ml) on days 10, 12, 15, 19, 23, 26, and 61 p.i. Serum was separated from the whole blood and stored at 4°C.
IgM-capture ELISA.
Optimum reagent dilutions were determined via box titration. The serum dilution was arrived at by titrating a panel of samples from chickens infected by injection and comparing the resulting ELISA optical densities (ODs) to those of uninfected chickens. The dilution chosen was that for which the mean difference of ODs between the infected and uninfected chickens was the greatest. The coefficient of variation of the means of the negative control OD means (n = 30) was 21%. The range of mean ODs for the negative controls was 0.121 to 0.248. The dilution of the positive control serum specimen was chosen such that the resulting OD was ca. 1.0. Final working parameters were as follows: Immulon II HB flat-bottomed 96-well plates (Dynatech Laboratories, Inc., Chantilly, Va.) were coated with 75 μl of coating antibody goat anti-chicken IgM (ICN Biomedicals, Irvine, Calif.) per well in coating buffer (0.015 M sodium carbonate, 0.035 M sodium bicarbonate buffer; pH 9.6) at a 1:2,000 dilution. Only the inner 60 wells were coated. Plates were incubated overnight at 4°C, after which the coating solution was removed, and 200 μl per well of blocking buffer (5% milk in phosphate-buffered saline with 0.5% Tween 20) was applied to the plates, followed by incubation at room temperature for 30 min. The plates were washed five times with 250 μl of wash buffer (0.05% Tween 20 in phosphate-buffered saline) by using an automatic plate washer (Skatron Instruments, Sterling, Va.). Fifty microliters per well of the chicken serum test specimens and the negative serum control diluted in wash buffer to 1:400 (screening dilution) were added. The positive control was added at a dilution of 1:800. The diluted test and control sera were added to a block of six wells each. This allowed for eight serum specimens plus a positive and negative control serum to be tested in triplicate against both WN viral and control antigens on each plate. Plates were incubated for 1 h at 37°C in a humidified environment. After five washes, 50 μl per well of WN virus (strain Eg101) antigen, diluted 1:100 in wash buffer, was added to three wells per each specimen and, similarly, control antigen was added to three wells per serum sample, including the control sera. Plates were incubated and humidified overnight at 4°C, after which they were washed five times. Then, 50 μl of the detecting conjugate, flavivirus group-reactive monoclonal antibody SLE 6B6C-1 HRP, was added per well. Plates were incubated humidified at 37°C for 1 h and were then washed 10 times before the addition of 75 μl of substrate (3,3′5,5′-tetramethylbenzidine [TMB-ELISA; Gibco-BRL, Gaithersburg, Md.]). The plates were incubated for 10 min at room temperature in the dark, and the colorimetric reactions stopped by adding 50 μl of 1 N H2SO4 per well to all 96 wells. The ODs were read at A450 with an automated microplate reader (Bio-Rad, Hercules, Calif.). The positive-to-negative (P/N) ratios were determined as follows: the mean OD of the three replicates of the test or positive control serum specimens reacted with viral antigen, divided by the mean OD of the three replicates of the negative control serum reacted with viral antigen. In order for a test to be valid, it was necessary for the P/N ratio of the positive control serum to be ≥2.0. A test chicken sample was considered positive for WN virus IgM when its P/N ratio was ≥2.0, and the mean OD of the test serum reacted with the viral antigen was at least twofold greater than the mean OD of the test serum reacted with control antigen; if the latter criterion was not met, the result was regarded as uninterpretable. This was not found to be a common occurrence with the chicken serum samples, whereas human sera quite commonly react nonspecifically with mouse brain antigen. P/N ratios between 2.0 and 3.0 were considered borderline positive, and experience with other ELISAs has shown that this is where occasional false-positive results occur. The “gold standard” test for seroconversion is the PRNT (13). To confirm that the chickens had seroconverted to WN virus, prebleed and day 15 p.i. serum samples from needle-inoculated chickens were heat inactivated to remove complement and adventitious viruses and then tested by this method. The results were recorded at the 90% plaque reduction level.
Comparison of the IgM-capture ELISA to other serologic tests with serum from chickens infected with WN virus via mosquito bite.
The chickens in the present study were infected with WN virus (NY99) isolated from Corvus brachyrhychos brain 99-41-32 (New York State Wildlife Pathology Unit, 1 Vero passage). Culex tritaeniorhynchus mosquitoes were inoculated intrathoracically with 1.5 × 105 PFU. Two 17-week-old female chickens were infected by exposing five infected mosquitoes to each bird. The mosquitoes were removed when one mosquito was fully engorged. The chickens were bled daily up to day 7 p.i. and then every second day for up to 28 days p.i. All serum specimens were held at −20°C prior to obtaining endpoint antibody titrations in the IgM-capture ELISA, HAI test, and PRNT. Endpoint titrations were performed by using a twofold serum dilution series. For the HAI test, the serum samples were acetone extracted, and the test was performed by the method of Clarke and Casals (4).
Analysis of serum samples from experimentally infected rock doves, house sparrows, and other species.
Rock doves (Columba livia) and house sparrows (Passer domesticus) are suitable free-ranging sentinel birds because they are abundant, peridomestic, and do not require specialized permits for sampling (10). To determine whether the chicken IgM-capture ELISA was useful for the detection of rock dove and house sparrow IgM in birds of known dates of infection, six rock doves were infected by mosquito bite, and seven house sparrows were infected by subcutaneous injection with 103 PFU of WN virus (NY99 or Eg101 strains). On day 14 after infection, the rock doves were bled, and the serum was separated. The same was done with the house sparrows on day 13. Serum was tested at a screening dilution of 1:400 in the chicken IgM-capture ELISA. Four other species of birds were experimentally infected with WN virus in the same manner as the rock doves: ring-necked pheasant (Phasianus colchicus), American crow (Corvus brachyrynchos), common grackle (Quiscalus quiscula), and redwinged blackbird (Agelaius phoeniceus). The birds were bled at 14 days p.i., and the IgM was detected in serum by the chicken IgM-capture ELISA. These species are less appropriate than rock doves or house sparrows as free-ranging sentinels.
Analysis of serum samples from chickens and other birds naturally infected with WN virus.
Twenty-nine serum samples from naturally infected domestic chickens were tested by the IgM-capture ELISA to determine whether this method would be sensitive enough to detect the levels of IgM found in these birds. Similarly, 35 PRNT-positive serum samples were tested to ascertain whether the chicken IgM ELISA would detect WN virus IgM of seven other species: Canada goose (Branta canadensis), rock dove, house sparrow, mourning dove (Zenaida macoura), mallard (Anas platyrynchus), domestic goose (Anser spp.), and turkey (Meleagris gallopavo). The serum specimens from naturally infected chickens and other birds were collected in New York in September 1999 during an epizootic of WN virus (11).
Comparison of the Eg101 and NY99 strains of WN virus antigen in the chicken IgM-capture ELISA.
To determine whether interchanging the Eg101 strain with the NY99 strain of WN virus antigen would alter the sensitivity of the test, the NY99 strain antigen was titrated against the positive control serum. The dilution used in the comparison was 1:50, and 29 serum samples from WN virus-positive and -negative chickens were tested at the 1:400 dilution on both antigens.
Specificity of the WN chicken IgM-capture ELISA.
Eighteen SLE HAI-positive chicken serum samples were tested by IgM-capture ELISA for detection of WN and SLE antibodies. The SLE IgM-capture ELISA was run in the same format as the WN ELISA, but with SLE antigen initially titrated against a known positive serum control, and substituted for the WN antigen in the ELISA at a 1:200 dilution. The positive control serum was used at a 1:400 dilution. Similarly, 29 WN IgM-positive chickens were tested for SLE IgM by using the SLE IgM-capture ELISA.
Analysis of blind-coded serum specimens.
Sixteen serum samples that included both known WN IgM-positive and -negative specimens were blind-coded and tested in the WN chicken IgM-capture ELISA to determine the reproducibility of the test compared to previous results for the same samples.
Combined screening IgM-capture ELISA for both WN and SLE viral antibodies.
A useful tool for identifying sentinel chicken flock serum samples that are negative to both WN and SLE viruses would be a combined IgM-capture ELISA. Since WN and SLE are both flaviviruses and the individual tests use the same reagents (with the exception of the antigens), an ELISA that included both antigens was tested to make an initial feasibility assessment. The antigens were used concurrently in the wells at the dilutions determined for the individual ELISAs. Specimens previously found to be positive for WN virus IgM were tested side by side in the WN IgM-capture ELISA and in the WN-SLE combined IgM-capture ELISA. Similarly, serum samples positive for SLE virus IgM were compared by the SLE IgM-capture ELISA and the WN-SLE combined test. Samples that tested negative previously in both WN and SLE tests were included in the combined ELISA. The positive and normal serum controls were included in the test and were combined in the wells at the concentration used in the individual ELISAs.
RESULTS
IgM-capture ELISA applied to experimentally infected chickens and comparison of the ELISA to other serologic assays.
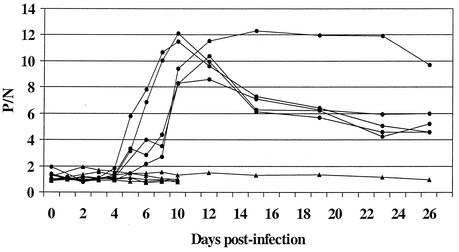
The chickens infected with WN virus by needle developed detectable IgM beginning at 4 days p.i. (Fig. 1). By day 6 p.i., all six infected chickens had detectable IgM. Maximum P/N values were noted between days 10 and 12 p.i., after which the P/N ratios generally diminished. IgM persisted for more than 61 days p.i. (day 61 data is not shown in Fig. 1). None of the six uninfected cage mates developed IgM by day 10 p.i. of the test group. One control chicken was bled through day 61, and no IgM developed in that time. Seroconversion was confirmed in the infected chickens by PRNT. WN viral antibody in chickens infected by mosquito was detected as early as day 3 (chicken 2) and day 7 p.i. (chicken 1) by the ELISA method, and for both birds IgM persisted beyond 28 days. The HAI method gave an initial positive result on day 10 (chicken 2) and day 12 (chicken 1), with antibodies persisting beyond day 28 p.i. Neutralization was seen on day 10 p.i. for both birds (Table 1). Two additional chickens were infected via mosquito bite but were bled on a more widely spaced schedule than the two birds used in the comparison described above. Positive IgM results were obtained up to day 19 p.i. in one bird and up to day 31 p.i. in the other (data not shown).
FIG. 1.
IgM response of chickens needle inoculated with WN virus versus days p.i. Symbols: •, virus-infected birds; ▴, uninfected cage mates. A P/N value of >2.0 was considered a positive result.
TABLE 1.
Comparison of methods used to detect WN virus infection in two chickensa
| Time p.i. (days) | Chicken 1
|
Chicken 2
|
||||
|---|---|---|---|---|---|---|
| IgM-ELISA P/N | HAI titer | PRNT titer | IgM-ELISA P/N | HAI titer | PRNT titer | |
| 0 | 1.51 | <10 | - | 1.39 | <10 | - |
| 1 | -b | - | - | - | - | - |
| 2 | 1.01 | - | - | 1.61 | - | - |
| 3 | 1.30 | <10 | <10 | 2.99 | <10 | <10 |
| 4 | - | - | - | - | - | - |
| 5 | 1.93 | <10 | <10 | 3.46 | <10 | <10 |
| 6 | - | - | - | - | - | - |
| 7 | 6.83 | <10 | <10 | 10.32 | <10 | <10 |
| 10 | 17.94 | <10 | 80 | 23.54 | 40 | 40 |
| 12 | 15.43 | 40 | - | 18.08 | 40 | - |
| 14 | 14.82 | 80 | 40 | 16.88 | 40 | 40 |
| 17 | 12.03 | 80 | 80 | 7.72 | 20 | 40 |
| 19 | 13.09 | 80 | - | 6.83 | 20 | - |
| 21 | 11.22 | 80 | 40 | 5.65 | 20 | 40 |
| 24 | 9.64 | 80 | 80 | 4.63 | 20 | 80 |
| 26 | 10.04 | 80 | - | 4.83 | 20 | - |
| 28 | 10.51 | 80 | 160 | 3.92 | 20 | 40 |
A P/N value of <2.0 is considered a negative result; a HAI titer of <10 is considered a negative result; a PRNT titer of <10 is considered a negative result.
-, not done.
Use of the chicken WN IgM-capture ELISA for detecting IgM in potential sentinel species.
Serum samples collected from experimentally infected rock doves and house sparrows were assayed for WN virus IgM by using the chicken IgM-capture ELISA. All seven experimentally infected house sparrows circulated detectable IgM at 13 days p.i. P/N ratios ranged from 3.15 to 6.01, with a mean P/N of 4.3. The WN chicken IgM-capture ELISA was unable to detect IgM in any of the six rock dove serum specimens collected at 14 days p.i.
WN IgM in naturally infected chickens and other birds.
Of 29 naturally infected chickens, 17 had P/Ns of ≥2.0. The maximum P/N was 4.97. Of 35 other birds (representing seven species), positive results were obtained only for a Canada goose and a domestic goose (Table 2.). Both of these serum samples were retested to confirm the positive results.
TABLE 2.
WN IgM-capture ELISA P/N ratios for chickens and other birdsa from New York City boroughs trapped and surveyed in September 1999
| Species | P/N | |
|---|---|---|
| Chicken | 3.45 | |
| Chicken | 2.87 | |
| Chicken | 3.04 | |
| Chicken | 1.66 | |
| Chicken | 1.52 | |
| Chicken | 1.95 | |
| Chicken | 2.32 | |
| Chicken | 4.34 | |
| Chicken | 3.23 | |
| Chicken | 4.46 | |
| Chicken | 1.22 | |
| Chicken | 2.72 | |
| Chicken | 1.57 | |
| Chicken | 2.27 | |
| Chicken | 1.49 | |
| Chicken | 1.27 | |
| Chicken | 2.48 | |
| Chicken | 2.52 | |
| Chicken | 1.56 | |
| Chicken | 1.78 | |
| Chicken | 4.45 | |
| Chicken | 4.97 | |
| Chicken | 3.15 | |
| Chicken | 1.82 | |
| Chicken | 1.45 | |
| Chicken | 3.02 | |
| Chicken | 2.19 | |
| Chicken | 2.34 | |
| Chicken | 1.5 | |
| Canada goose | 1.05 | |
| Canada goose | 8.01c | |
| Canada goose* | 0.74Canada goose | 0.82 |
| Canada goose | -b | |
| Domestic goose | 0.76 | |
| Domestic goose | 0.81 | |
| Domestic goose | 0.60 | |
| Domestic goose | 0.77 | |
| Domestic goose | 0.70 | |
| Domestic goose | 0.76 | |
| Domestic goose | 5.98c | |
| Mallard duck | 0.86 | |
| Domestic turkey | 0.74 | |
| Wild turkey | 0.65 | |
| Rock dove | 1.04 | |
| Rock dove | 0.71 | |
| Rock dove | 0.71 | |
| Rock dove | 0.84 | |
| Rock dove | 0.78 | |
| Rock dove | 0.89 | |
| Rock dove | 0.70 | |
| Rock dove | 0.48 | |
| Rock dove | 0.56 | |
| Rock dove | 0.65 | |
| Rock dove | 0.60 | |
| Rock dove | 1.02 | |
| Rock dove | 0.94 | |
| Mourning dove | 0.58 | |
| House sparrow | 1.20 | |
| House sparrow | -b | |
| House sparrow | 1.62 | |
| House sparrow | -b | |
| House sparrow* | 1.48 | |
| House sparrow | -b |
These birds had reciprocal 90% PRNT titers of ≥40, with the exception of the birds marked with an asterisk, which had titers of 10. A P/N of 2.0 is considered a positive result.
-, uninterpretable result due to background reaction on normal antigen.
A positive P/N ratio was confirmed by a repeat test.
Comparison of the Eg101 and NY99 strains of WN virus antigen.
Of the 17 naturally infected chicken sera that were IgM positive, 15 samples tested positive by both Eg101 and NY strain antigens. P/N values were generally within 0.5 of each other, with neither antigen giving consistently higher or lower P/N ratios. Two serum specimens that gave borderline positive results with the Eg101 strain gave negative results with the NY99 strain of antigen. Two negative results (P/N of just below 2) with the Eg101 strain gave positive results with the NY99 strain.
Specificity of the chicken WN virus IgM-capture ELISA.
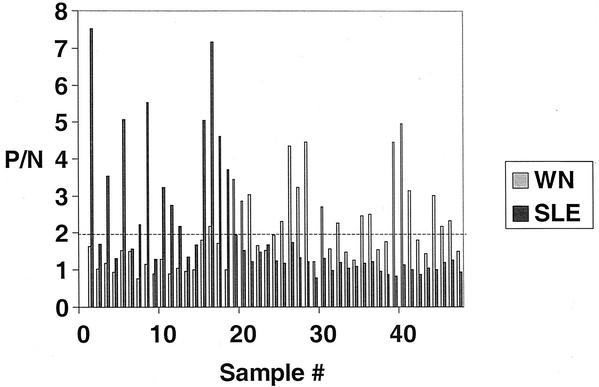
Comparative SLE and WN IgM-capture ELISAs were run for 18 sentinel chicken serum specimens that had previously tested positive for SLE by HAI. The dates of infection were unknown. Twelve specimens tested positive for SLE IgM, and six tested negative, with a maximum P/N ratio of 7.53 (Fig. 2). The discrepancy between test results was probably due to the variable longevity of IgM. Only one serum sample had a positive (borderline) result for WN IgM, and this was a specimen that had a relatively high P/N ratio for SLE (7.17). Of 29 naturally infected, WN virus PRNT-positive chickens, none had positive P/Ns in response to SLE virus, although one sample did have a P/N that approached the cutoff of 2.0 (P/N = 1.94).
FIG. 2.
Specificity of the chicken WN IgM-capture ELISA. Specimens 1 to 18 previously tested positive by HAI for anti-SLE antibodies; specimens 19 to 47 tested positive for anti-WN virus antibodies. The bar graph shows the results of both WN and SLE IgM ELISAs with these specimens, and the dashed line represents the positive cutoff P/N value below which results are considered negative.
Blind-coded specimens.
Sixteen chicken serum specimens were blind coded and tested by the WN IgM-capture ELISA. The results were compared to P/N values obtained from an initial noncoded test. Positive and negative results were found to be consistent between the tests in all but one instance, in which a negative result from the initial test returned a borderline positive result (P/N = 2.33) in the blind-coded test.
Combined WN and SLE chicken IgM-capture ELISA.
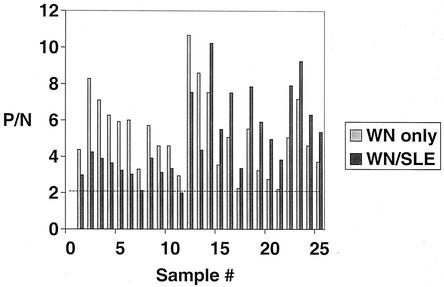
An ELISA combining both WN and SLE antigens was used to test known WN virus-positive and known SLE virus-positive chicken sera and controls. The results are shown in Fig. 3. Of the 13 WN virus-positive samples, 12 tested positive by the combined method. One specimen with a low P/N ratio to WN virus (2.99) tested negative (1.99) by the combined ELISA. In general, the P/N ratios in the combined test were lower than those detected in the WN IgM-capture ELISA. Conversely, all 12 specimens that tested positive for SLE virus tested positive in the combined ELISA, exhibiting higher P/N ratios. All originally negative serum samples gave negative results in the combined test.
FIG. 3.
Combined WN and SLE chicken IgM-capture ELISA results. Specimens were tested both by WN virus-only and a combined WN-SLE ELISA in which titrated antigens were coincubated. The dashed line represents the positive cutoff P/N value below which results are considered negative.
DISCUSSION
Sentinel chicken flocks are tested for antibody weekly or biweekly in most cases, and a quick and convenient serologic test is important to have in place for this testing. The most commonly used techniques for the detection of antiarboviral antibodies are the PRNT, HAI test, IgM/IgG ELISAs, and immunofluorescence assay. The effectiveness of these methods has been investigated for western equine, eastern equine, St. Louis, and Murray Valley encephalitis viruses (1, 2, 16, 17). Chiles and Reisen (3) developed a test to screen for anti-western equine encephalomyelitis and anti-SLE IgG in four orders of bird species concurrently, and Hall et al. (6) used an epitope blocking IgG ELISA to differentiate between infections of the closely related Kunjin and Murray Valley encephalitis viruses in chickens. The ability to use noninfectious viral antigen is an advantage in the HAI test and ELISA, and the use of IgM-capture ELISA is optimal for detecting recent infections. ELISAs also have the benefit of producing numerical results. The P/N values do not relate directly to the antibody levels. It is therefore not advisable to compare the P/N values between specimens except to report an antibody-positive or -negative result. Once a positive specimen has initially been identified, it is useful to titrate the serum by using twofold dilutions beginning at 1:400, as was done for a number of samples in the present study (data not shown). The resulting curve helps in making the determination of whether the specimen was a true positive or a false positive. A specimen that gives diminishing P/N values as the dilution increases most likely has viral antibody present. One that gives a flat or undulating line (especially with P/N values persisting around the 2.0 to 3.0 level, regardless of specimen dilution) is likely a false positive. If a serum sample does not yield a positive P/N at the 1:400 dilution in the titration (i.e., the screening test was not confirmed), then the sample is also regarded as negative for viral antibody. Like most serological techniques, the performance of the chicken WN IgM-capture ELISA is dependent upon the correct standardization of the reagents involved. It is necessary therefore to determine the working dilutions of new lot numbers of reagents, and it should be emphasized that the dilutions used in this publication pertain only to the specific lot numbers used here.
The length of persistence of IgM in the chicken sera was variable among the chickens that were laboratory infected via mosquito bite. In some individual birds in which the IgM levels were relatively low, IgM was no longer detectable after 19 days (data are not shown for this group of chickens). IgM was first detectable at 3 to 7 days p.i.; thus, the window of detection is 11 to 15 days at a minimum. In others birds, the response lasted more than 61 days. This may have been due to a variation in efficiency of virus transmission and probably reflects a natural scenario. Therefore, to efficiently detect WN virus infections in chickens that exhibit a low IgM response, it is optimal to bleed the sentinel flocks on a biweekly basis. To make sure that no chickens that may have briefly seroconverted are overlooked (therefore rendering them redundant in the sentinel flock), it may be prudent to periodically test the birds by a method that also measures the IgG in serum such as PRNT or HAI test.
Sentinel chicken flocks and wild birds are being used to track the spread of WN virus throughout the United States, and areas in which the virus is found to be newly active are being surveyed particularly closely. Serum samples from 35 PRNT-positive nonchicken free-ranging birds were tested by using the chicken WN IgM-capture ELISA. Of these, only two goose serum specimens gave positive results. Because these birds were captured as part of a serosurvey in the fall of the initial U.S. outbreak of WN virus (11), IgM should have been present in at least some of the specimens besides the goose sera. The lack of ability of the chicken IgM-capture ELISA to detect IgM in these samples indicates that the anti-chicken IgM capture antibody may be unable to capture IgM from many birds. Therefore, the test in its current format should not be assumed to be useful for other bird species. Serologic testing of free-ranging birds is best accomplished by using PRNT, the HAI test, or IgG ELISA (5). Regardless of the avian species, a test that detects antiviral IgG is usually more appropriate to use because the dates of infection of free-ranging birds are unknown and because IgG in serum normally persists for a protracted period. Recent infections in free-ranging birds may be determined by sampling “hatch-year” birds for IgG antibodies (10).
It is worth noting that for the HAI, the tests are valid when an acetone extraction method is used for chicken sera in the case of WN virus and for flaviviruses in general (L. Stark, unpublished data). This is not true for chicken sera with antibodies to alphaviruses such as eastern equine encephalitis virus or for wild bird antiserum to any arbovirus. For these sera, it is necessary to add protamine sulfate to the extraction procedure to remove nonspecific hemagglutinin inhibitors (7). Dissolution of the extracted serum tends to be problematic as a result of the protamine sulfate, and extra measures are required to get the serum to dissolve properly in the resuspending buffer. It was observed during the HAI experiments that the use of the homologous antigen strain (i.e., NY99) was important for obtaining optimal results.
WN virus antigen strains Eg101 and NY99 were compared in the chicken WN IgM-capture ELISA and found to perform equally well. This is significant because it is likely that minor mutations in the virus over time will have no effect on whether or not IgM can be detected by the test.
The sera tested by using the individual WN and SLE chicken IgM-capture ELISAs had very low rates of cross-reaction, a finding which differs from results obtained with the human ELISAs for the same viruses (15). The results of a blind-coded panel of serum specimens illustrated that the WN IgM-capture ELISA generates reliable results. Only one borderline specimen was inconsistent between runs.
The combined WN-SLE IgM-capture ELISA gave promising results. A decrease in P/N ratios for the WN-positive sera and an increase in P/N ratios for the SLE-positive serum samples compared to the individual tests was probably due to the mean normal serum control OD for the WN antigen being consistently less than for the SLE antigen. This value was used in all of the P/N calculations. The use of a combined SLE and WN IgM-capture ELISA indicated that, although the antigen levels were not optimized fully in these tests, the approach might be worth considering as a way to screen for both of these flaviviruses in areas of the country where they cocirculate. If a positive result were to occur, it would be important to titrate the serum to verify the presence of antibody, in addition to performing a confirmatory test such as a PRNT.
In summary, the chicken WN IgM-capture ELISA is a suitable screening test for use with sentinel chicken flocks to detect WN viral activity in a timely fashion. For the most meaningful results to be obtained, flocks should be bled and tested for antibody at least every 14 days. The presence of IgM in the serum indicates a recent viral infection, which makes this test advantageous over IgG-detecting methods. The chicken WN IgM-capture ELISA is not an appropriate test for use with free-ranging birds of any species.
Acknowledgments
We thank Lillian Stark of Florida Department of Health for providing SLE-positive chicken sera and Brent Davis, Mike Bunning, and Nick Panella of CDC/DVBID for helping with several aspects of this work.
REFERENCES
- 1.Broom, A. K., J. Charlick, S. J. Richards, and J. S. Mackenzie. 1987. An enzyme-linked immunosorbent assay for detection of flavivirus antibodies in chicken sera. J. Virol. Methods 15:1-9. [DOI] [PubMed] [Google Scholar]
- 2.Calisher, C. H., H. N. Fremont, W. L. Vesely, A. O. el-Kafrawi, and M. I. Mahmud. 1986. Relevance of detection of immunoglobulin M antibody response in birds used for arbovirus surveillance. J. Clin. Microbiol. 24:770-774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chiles, R. E., and W. K. Reisen. 1998. A new enzyme immunoassay to detect antibodies to arboviruses in the blood of wild birds. J. Vector Ecol. 23:123-135. [PubMed] [Google Scholar]
- 4.Clarke, D. H., and J. Casals. 1958. Techniques for hemagglutination and hemagglutination-inhibition with arthropod-borne viruses. Am. J. Trop. Med. Hyg. 7:561-573. [DOI] [PubMed] [Google Scholar]
- 5.Ebel, G. D., A. P. Dupuis, I. I., D. Nicholas, D. Young, J. Maffei, and L. D. Kramer. 2002. Detection by enzyme-linked immunosorbent assay of antibodies to West Nile virus in birds. Emerg. Infect. Dis. 8:979-982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hall, R. A., A. K. Broom, A. C. Hartnett, M. J. Howard, and J. S. Mackenzie. 1995. Immunodominant epitopes on the NS1 protein of MVE and KUN viruses serve as targets for a blocking ELISA to detect virus-specific antibodies in sentinel animal serum. J. Virol. Methods 51:201-210. [DOI] [PubMed] [Google Scholar]
- 7.Holden, P., D. Muth, and R. B. Shriner. 1966. Arbovirus hemagglutinin-inhibition in avian sera: inactivation with protamine sulfate. Am. J. Epidemiol. 84:67-73. [DOI] [PubMed] [Google Scholar]
- 8.Gubler, D. J., G. L. Campbell, R. Nasci, N. Komar, L. Peterson, and J. T. Roehrig. 2000. West Nile virus in the United States: guidelines for detection, prevention, and control. Viral Immunol. 13:469-475. [DOI] [PubMed] [Google Scholar]
- 9.Komar, N. 2000. West Nile viral encephalitis. Rev. Sci. Tech. 19:166-176. [DOI] [PubMed] [Google Scholar]
- 10.Komar, N. 2001. West Nile surveillance using sentinel birds. Ann. N. Y. Acad. Sci. 951:58-73. [DOI] [PubMed] [Google Scholar]
- 11.Komar, N., N. A. Panella, J. Burns, S. Dusza, T. Mascarenhas, and T. Talbot. 2001. Serologic evidence for West Nile virus infection in birds in the New York vicinity during an outbreak in 1999. Emerg. Infect. Dis. 7:621-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Langevin, S. A., M. Bunning, B. Davis, and N. Komar. 2001. Experimental infection of chickens as candidate sentinels for West Nile virus. Emerg. Infect. Dis. 7:726-729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lindsey, H. S., C. H. Calisher, and J. H. Mathews. 1976. Serum dilution neutralization test for California group virus identification and serology. J. Clin. Microbiol. 4:503-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martin, D. M., D. A. Muth, T. Brown, A. J. Johnson, N. Karabatsos, and J. T. Roehrig. 2000. Standardization of immunoglobulin M capture enzyme-linked immunosorbent assays for routine diagnosis of arboviral infections. J. Clin. Microbiol. 38:1823-1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martin, D. A., B. Biggerstaff, R. Allen, A. J. Johnson, Lanciotti, R. S., and J. T. Roehrig. 2002. Use of immunoglobulin M cross-reactions in differential diagnosis of human flaviviral encephalitis infections in the United States. Clin. Diagn. Lab. Immunol. 9:544-549. [DOI] [PMC free article] [PubMed]
- 16.Olson, J. G., T. W. Scott, L. H. Lorenz, and J. L. Hubbard. 1991. Enzyme immunoassay for detection of antibodies against eastern equine encephalomyelitis virus in sentinel chickens. J. Clin. Microbiol. 7:1457-1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reisen, W. K., S. B. Presser, J. Lin, B. Enge, J. L. Hardy, and R. W. Emmons. 1994. Viremia and serological responses in adult chickens infected with western equine encephalomyelitis and St. Louis encephalitis viruses. J. Am. Mosq. Control Assoc. 10:549-555. [PubMed] [Google Scholar]
- 18.Roehrig, J. T., J. H. Mathews, and D. W. Trent. 1983. Identification of epitopes on the E glycoprotein of Saint Louis encephalitis virus using monoclonal antibodies. Virology 128:118-126. [DOI] [PubMed] [Google Scholar]