Abstract
One approach to the accurate determination of the pathogenic potential (pathotype) of isolated Escherichia coli strains would be through a complete assessment of each strain for the presence of all known E. coli virulence factors. To accomplish this, an E. coli virulence factor DNA microarray composed of 105 DNA PCR amplicons printed on glass slides and arranged in eight subarrays corresponding to different E. coli pathotypes was developed. Fluorescently labeled genomic DNAs from E. coli strains representing known pathotypes were initially hybridized to the virulence gene microarrays for both chip optimization and validation. Hybridization pattern analysis with clinical isolates permitted a rapid assessment of their virulence attributes and determination of the pathogenic group to which they belonged. Virulence factors belonging to two different pathotypes were detected in one human E. coli isolate (strain H87-5406). The microarray was also tested for its ability to distinguish among phylogenetic groups of genes by using gene probes derived from the attaching-and-effacing locus (espA, espB, tir). After hybridization with these probes, we were able to distinguish E. coli strains harboring espA, espB, and tir sequences closely related to the gene sequences of an enterohemorrhagic strain (EDL933), a human enteropathogenic strain (E2348/69), or an animal enteropathogenic strain (RDEC-1). Our results show that the virulence factor microarray is a powerful tool for diagnosis-based studies and that the concept is useful for both gene quantitation and subtyping. Additionally, the multitude of virulence genes present on the microarray should greatly facilitate the detection of virulence genes acquired by horizontal transfer and the identification of emerging pathotypes.
Escherichia coli is a normal inhabitant of the intestinal tract of humans and warm-blooded animals. Although usually harmless, various E. coli strains have acquired genetic determinants (virulence genes) rendering them pathogenic for both humans and animals. These pathogens are responsible for three main types of clinical infections: (i) enteric and diarrheal diseases, (ii) urinary tract infections, and (iii) sepsis and meningitis. On the basis of their distinct virulence properties and the clinical symptoms of the host, pathogenic E. coli strains are divided into numerous categories or pathotypes. The diarrheagenic E. coli strains include (i) enterotoxigenic E. coli (ETEC) strains, which are associated with traveler's diarrhea and porcine and bovine diarrhea; (ii) enteropathogenic E. coli (EPEC) strains, which cause diarrhea in children and animals; (iii) enterohemorrhagic E. coli (EHEC) strains, which are associated with hemorrhagic colitis and hemolytic-uremic syndrome in humans; (iv) enteroaggregative E. coli (EAEC) strains, which are associated with persistent diarrhea in humans; and (v) enteroinvasive E. coli (EIEC) strains, which are involved in invasive intestinal infections, watery diarrhea, and dysentery in humans and animals (71). Extraintestinal infections are caused by three separate E. coli pathotypes: (i) uropathogenic (UPEC) strains that cause urinary tract infections in humans, dogs, and cats (8, 36, 87); (ii) strains involved in neonatal meningitis (MENEC) (87); and (iii) strains that cause septicemia in humans and animals (25, 41, 66, 87).
Numerous virulence factors including adhesins, host cell surface-modifying factors, invasins, toxins, and secretion systems are involved in E. coli pathogenic mechanisms. Strains of the same pathotype are genetically similar and carry the same virulence determinants involved in the infection. These virulence genes are ideal targets for the determination of the pathogenic potential of any given E. coli isolate. Numerous molecular methods have been used to detect and identify pathogenic E. coli strains, including DNA-DNA hybridization, PCR, and multiplex PCR. However, these approaches suffer from a variety of limitations, the most serious of which is related to the large variety of virulence factors distributed among the known pathotypes. A thorough assessment of any particular isolate for each known virulence gene by PCR would be very labor intensive. More importantly, this approach can result in numerous false-negative results since the PCR primers may not hybridize to variants of the target genes because of their high degrees of specificity, thus further limiting the use of PCR to the study of only a few virulence factors.
Microarray technology offers the most rapid and practical tool to detect the presence or absence of a large set of virulence genes simultaneously within a given E. coli strain. Although microarray technology theoretically permits a genome-scale analysis for the presence of thousands of genes, only a few studies have reported on the use of microarrays as a diagnostic tool (16, 18, 19, 63, 70).
Here, we describe a new approach for the detection of a large number of virulence factors present in E. coli strains and the subsequent determination of the strain's pathotype. All known virulence factors including associated virulence genes were amplified by PCR and immobilized onto glass slides to create a virulence DNA microarray chip. By probing this virulence gene microarray with labeled genomic E. coli DNA, the virulence pattern of a given strain can be assessed and its pathotype can be determined in a single experiment.
MATERIALS AND METHODS
Strains and media.
The E. coli strains used to produce PCR templates are listed in Table 1. E. coli isolates including characterized strains (nonpathogenic strain K-12-derived E. coli strain DH5α, enterohemorrhagic strain EDL933, uropathogenic strain J96, enterotoxigenic strain H-10407, and enteropathogenic strains E2348/69 and P86-1390) and uncharacterized clinical strains of bovine (B00-4830 and B99-4297), avian (Av01-4156), canine (Ca01-E179), and human (H87-5406) origin were used to assess the detection thresholds and hybridization specificities of the virulence gene microarray. Most of the E. coli strains were obtained from the Escherichia coli Laboratory Collection at the Faculté de Médecine Vétérinaire of the Université de Montréal. E. coli strains A22, AL851, and C248 (21) were kindly provided by Carl Marrs (University of Michigan), and strain IA2 was kindly provided by J. R. Johnson (University of Minnesota), All strains were stored in Luria-Bertani broth plus 25% glycerol at −80°C. E. coli cultures were grown at 37°C in Luria-Bertani broth (6) for genomic DNA extraction and purification.
TABLE 1.
Genes targeted, primer sources, and strains used as PCR amplification templates in this study
| Gene | GenBank accession no. | Size (bp) | Strain | Reference(s) or source for primers |
|---|---|---|---|---|
| afaBC3 | X76688 | 793 | A22 | 22, 62 |
| afaE5 | X91748 | 470 | AL851 | This study |
| afaE7 | AF072901 | 618 | 262-KH 89 | 61 |
| afaD8 | AF072900 | 351 | 2787 | 61 |
| aggA | U12894 | 432 | 17.2 | 78 |
| aggC | U12894 | 528 | 17.2 | 78 |
| aida | X65022 | 644 | 2787 | 5 |
| bfpA | U27184 | 324 | O126:H6 E2348/69 | 39 |
| bmaE | M15677 | 505 | 215 | 54 |
| cdt1 | U03293 | 412 | O15:KRVC383 OvinS5 | Eric Oswalda |
| cdt2 | U04208 | 556 | O15:KRVC383 OvinS5 | This study |
| cdt3 | U89305 | 556 | O15:KRVC383 OvinS5 | This study |
| cfa1 | S73191 | 479 | H-10407 cfaI | This study |
| clpG | M55389 | 403 | 215 | 7 |
| cnf1 | X70670 | 1,112 | J96 O4:K12 | 74 |
| cnf2 | U01097 | 1,240 | O15:KRVC383 OvinS5 | 74 |
| cs1 | M58550 | 321 | PB-176P cfa-II | This study |
| cs3 | M35657 | 401 | PB-176 cfa+II | This study |
| cs31a | M59905 | 710 | 31a | 37 |
| cvaC | X57525 | 680 | 1195 | 54 |
| derb122 | U87541 | 260 | O4:K12 J96 | This study |
| eae | U66102 | 791 | O157:H7 STJ348 | 4 |
| eaf | X76137 | 397 | O126:H6 E2348/69 | 32 |
| eastI | L11241 | 117 | O149:K9 IP97-2554B | 89 |
| ehxA | AF043471 | 158 | O157:H7 STJ348 | 31 |
| espA group I | AF064683 | 478 | P86-1390 | This study |
| espA group II | AF071034 | 523 | O157:H7 EDL933 | This study |
| espA group III | AJ225016 | 481 | O126:H6 E2348/69 | This study |
| espB group I | AF071034 | 502 | O157:H7 EDL933 | This study |
| espB group II | Z21555 | 377 | O126 H6 E2348/69 | This study |
| espB group III | X99670 | 395 | P86-1390 | This study |
| espC | AF297061 | 500 | O126 H6 E2348/69 | This study |
| espP | AF074613 | 1,830 | 215 | 13 |
| etpD | Y09824 | 509 | O157:H7 EDL933 | 82; this study |
| F17A | AF022140 | 441 | O15:KRVC383 OvinS5 | 20 |
| F17G | L33969 | 950 | O15:KRVC383 OvinS5 | 54 |
| F18 | M61713 | 510 | O139:K82 P88-1199 | 45 |
| F4 | M29374 | 601 | O149:K91 P97-2554B | 22 |
| F41 | X14354 | 431 | O9:K30 B44s | 72 |
| F5 | M35282 | 450 | O9:K30 B44s | 72 |
| F6 | M35257 | 566 | O9:K- P81-603A | 72 |
| fimA group I | Z37500 | 331 | 3292 | 65 |
| fimA group II | Z37500 | 331 | O157:H7 EDL933 | 65 |
| fimH | AJ225176 | 508 | O157:H7 EDL933 | 54 |
| fliC | U47614 | 625 | O157:H7 E32511 | 34 |
| focG | S68237 | 359 | O4:K12 J96 | 54 |
| fyuA | Z38064 | 207 | 1195 | 3 |
| hlyA | M10133 | 500 | O4:K12 J96 | 88 |
| hlyC | M10133 | 556 | O4:K12 J96 | 9 |
| ibe10 | AF289032 | 170 | O18 H87-5480 | 44, 54 |
| iha | AF126104 | 827 | O157:H7 E32511 | 53 |
| invX | L18946 | 258 | H84 (EIEC) | This study |
| ipaC | X60777 | 500 | O157:H7 E32511 | This study |
| iroN | AF135597 | 668 | CP9 | 53, 79 |
| irp1 | AF091251 | 1,689 | 1195 | 3 |
| irp2 | L18881 | 1,241 | 1195 | 3 |
| iss | X52665 | 607 | 3292 | Hojabr Dezfuliana |
| iucD | M18968 | 778 | 4787 | 42 |
| iutA | X05874 | 300 | 4787 | 47 |
| katP | X89017 | 2,125 | O157:H7 EDL933 | 14 |
| kfiB | X77617 | 501 | K5(F9) 3669 | This study |
| kpsMTII | X53819 | 270 | K5(F9) 3669 | 54 |
| kpsMTIII | AF007777 | 390 | 215 | 54 |
| 17095 | AF074613 | 659 | O157:H7 EDL933 | 15, 64 |
| leoA | AF170971 | 501 | O149:K91 P97-2554B | This study |
| lngA | AF004308 | 424 | PB-176P cfa-II | This study |
| lt | J01646 | 275 | O149:K91 P97-2554B | 23, 33 |
| neuC | M84026 | 500 | O2:K1 U9/41 | This study/PICK> |
| nfaE | S61970 | 537 | 31a | 50 |
| ompA | V00307 | 1,422 | O4:K12 J96 | 77 |
| ompT | X06903 | 559 | O4:K12 J96 | 51 |
| paa | U82533 | 360 | O157:H7 STJ348 | This study |
| papAH | X61239 | 721 | O4:K12 J96 | 54 |
| papC | X61239 | 318 | 4787 | 62 |
| papEF | X61239 | 336 | O4:K12 J96 | 88 |
| papG group I | M20146 | 461 | O4:K12 J96 | 67 |
| papG group II | M20181 | 190 | IA2 | 48 |
| papG group III | X61238 | 268 | O4:K12 J96 | 48 |
| pai | AF081286 | 922 | h140 8550 | 54 |
| rfbO9 | D43637 | 501 | O9:F6 K P81-603A | This study |
| rfbO101 | X59852 | 500 | O101 h510a | This study |
| rfbO111 | AF078736 | 406 | O111 H87-5457 | 25 |
| rfbE O157 | S83460 | 292 | O157:H7 EDL933 | 43 |
| rfbE O157 H7 | S83460 | 259 | O157:H7 STJ348 | 75 |
| rfcO4 | U39042 | 786 | O4:K12 J96 | 34 |
| rtx | AE005229 | 521 | O157:H7 EDL933 | This study |
| sfaDE | X16664 | 408 | 4787 | 62 |
| sfaA | X16664 | 500 | 4787 | This study |
| stah | M29255 | 201 | H-10407 | This study |
| stap | M58746 | 163 | O149:K91 P97-2554B | 81 |
| stb | M35586 | 368 | O149:K91 P97-2554B | 58 |
| stx1 | L04539 | 583 | O157:H7 EDL933 | 35 |
| stx2 | AF175707 | 779 | O157 KNIH317 | 35 |
| stxA group I | M23980 | 502 | O157:H7 EDL933 | This study |
| stxA group II | Y10775 | 482 | O157:H7 EDL933 | This study |
| stxB group I | M23980 | 151 | O157:H7 EDL933 | This study |
| stxB group II | Y10775 | 211 | O157:H7 EDL933 | This study |
| stxB group III | M36727 | 226 | O101 h510a | This study |
| tir group I | AF045568 | 442 | RDEC-1B | This study |
| tir group II | AF070067 | 479 | O157:H7 EDL933 | This study |
| tir group III | AB036053 | 443 | O126:H6 E2348/69 | This study |
| traT | J01769 | 288 | 3292 | 54 |
| tsh | AF218073 | 640 | O78:K80 Av 89-7098(143) | 26 |
| uidA | S69414 | 250 | O157:H7 EDL933 | 17 |
| uspA | AB027193 | 501 | h140 8550 | This study |
Personal communication.
Selection and sequence analysis of virulence gene probes.
The selection included virulence genes of E. coli pathotypes involved in intestinal and extraintestinal diseases in humans and animals (Table 1). The primers used for probe amplification were either chosen from previous studies on virulence gene detection or designed from available gene sequences (Table 2). Ninety-one E. coli virulence genes were targeted in this study, including genes encoding (i) toxins (heat-labile toxin [LT]; human heat-stable toxin [ST] STaH; porcine ST STaP; Shiga toxins [Stxs] Stx1 and Stx2; hemolysins Hly and Ehx; East1; STb; EspA; EspB; EspC; cytolethal distending toxin Cdt; and cytotoxic necrotizing factors Cnf, Cva, and Leo); (ii) adhesion factors (Cfa, Iha, Pap, Sfa, Tir, Bfp, Eaf, Eae, Agg, Lng, Aida, Foc, Afa, Nfa, Drb, Fim, Bma, ClpG, F4, F5, F6, F17, F18, and F41); (iii) secretion systems (Etp); (iv) capsule antigens (KfiB, KpsMTII, KpsMTIII, and Neu); (v) somatic antigens (RfcO4, RfbO9, RfbO101, RfbO111, and RfbEO157); (vi) flagellar antigen (FliC); (vii) invasins (IbeA, IpaC, and InvX); (viii) autotransporters (Tsh); and (ix) aerobactin systems (IucD, TraT, and IutA). In addition, probes targeting espP (serine protease), katP (catalase), omp (outer membrane proteins A and T), iroN (catechol siderophore receptor), iss (serum survival gene), the putative RTX family exoprotein (rtx), and paa (related attaching-and-effacing gene) were used. The Yersinia high-pathogenicity island (irp1, irp2, and fyuA) present in different E. coli pathotypes and other members of the family Enterobacteriaceae was also targeted (3). An E. coli positive control gene, uidA, which encodes the E. coli-specific β-glucuronidase protein (17, 31), and the uspA gene, which encodes a uropathogenic strain-specific protein (60), were added to this collection.
TABLE 2.
DNA sequences of primers designed for this study
| Gene | Forward sequence | Reverse sequence |
|---|---|---|
| afaE5 | GCGATCATGGCCGCGACCAGCA | CAACTCACCCAGTAGCCCCAGT |
| cdt2 | GAAAGTAAATGGAATATAAATGa | TTTGTGTTGCCGCCGCTGGTGAA |
| cdt3 | GAAAGTAAATGGAATATAAATGa | TTTGTGTCGGTGCAGCAGGGAAA |
| cfaI | GGTGCAATGGCTCTGACCACA | GTCATTACAAGAGATACTACT |
| cs1 | GCTCACACCATCAACACCGTT | CGTTGACTTAGTCAGGATAAT |
| cs3 | GGGCCCACTCTAACCAAAGAA | CGGTAATTACCTGAAACTAAA |
| derb122 | CGTGTGGGAGCCCTGAGCCTT | CCGGCCTGGTTGCTAGTATT |
| espA group I | CATCAGTTGCTAGTGCGAATG | CAGCAAATGTCAAATACGTT |
| espA group II | CGACATCGACGATCTATGACT | CCAAGGGATATTGCTGAAATA |
| espA group III | CATCAGTTGCTAGTGCGAATG | CAGCAAATGTCAAATACGTT |
| espB group I | CGGAGAGTACGACCGGCGCTT | GCACGGCTGGCTGCTTTCGTT |
| espB group II | GCTGCCATTAATAGCGCAACT | TATTGTTGTTACCAGCCTTGC |
| espB group III | GTAATGACGGTTAATTCTGTT | GCCGCATCAATAGCCTTAGAA |
| espC | CCCATAACGGAACAACTCAT | CAGAATAGACCAAACATCTGCA |
| etpD | GGCCACTTTCAATGTTGGTCAb | CGACTGCACCTGTTCCTGATTA |
| invX | TCTGATATAGTTTATATGGGT | TCAAACCCCACTCTTAATTAA |
| ipaC | TTGCAAAAGCAATTTTGCAAC | TGCCGAACAATGTTCTCTGCA |
| kfiB | AATTGTTTTAAAATCTGTTCT | TGAGACTGAAATTACATTTAA |
| leoA | GAACAATTCAAACAGTTCAGT | TTATTCAAATCGCGCAATACC |
| lngA | CAAATACAGTCCGCGTACGA | CCATTGTTACCTAAAGAGCGT |
| neuC | TTGGCAGTTACAGGAATGCAT | AACAGTGAACCATATTTTAGT |
| paa | ATGAGGAACATAATGGCAGG | TCTGGTCAGGTCGTCAATAC |
| rfbO9 | GGTGATCGATTATTCCGCTGA | ACGCCTCATCGGTCAGCGCCT |
| rfbO101 | TCTGCACGTTTAAAATTATTG | GTTTCTCCGTCAGAATCAAGC |
| rtx | CTACCGTAGCGGGCGATGGTA | CAGCGCCTGTCCGTGTTCGGC |
| sfaA | CCCTGACCTTGGGTGTTGCGA | GTACTGAACTTTAAAGGTGG |
| stah | AAGAAATCAATATTATTTAT | AATAGCACCCGGTACAAG |
| stxA group I | GCGAAGGAATTTACCTTAGA | CAGCTGTCACAGTAACAAAC |
| stxA group II | CTTGAACATATATCTCAGGG | ACAGGAGCAGTTTCAGACAGT |
| stxB group I | GGTGGAGTATACAAAATATAA | ATGACAGGCATTAGTTTTAAT |
| stxB group II | TTCTGTTAATGCAATGGCGG | TTCAGCAAATCCGGAGCCTGA |
| stxB group III | GAAGAAGATGTTTATAGCGG | ACTGCAGGTATTAGATATGAT |
| tir group I | ATTGGTGCCGGTGTTACTGCTG | CTCCCATACCTAAACGCAAT |
| tir group II | ATTGGTGTTGCCGTCACCGCT | ACGCCATGACATGGGAGG |
| tir group III | ATTGGTGCTGGTGTAACGACT | ATTGCGTTTAGGTATGGG |
| uspA | CTACTGTTCCCGAGTAGTGTG | GGTGCCGTCCGGAATCGGCGT |
Sequence obtained from Eric Oswald (personal communication).
Published primer sequence (82).
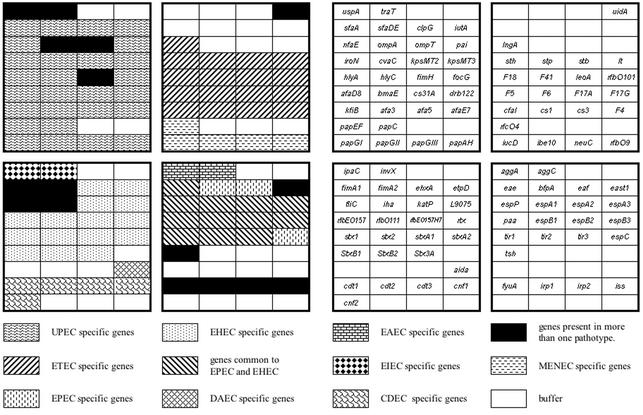
The DNA sequence of each gene was analyzed by BLAST analysis and alignment with the ClustalW program, followed by phylogenetic analysis. When the sequence of the selected gene showed over 10% divergence among different strains, new primers were designed to amplify the probe from each phylogenetic group, as was the case for the espA, espB, and tir genes. The sequences of the new primers were selected from areas with conserved sequences flanking the area of divergence in order to ensure gene discrimination at the hybridization level. Phylogenetic analysis of attaching-and-effacing locus (LEE) genes espA, espB, and tir permitted us to distinguish three phylogenetic groups with regard to the sequence divergence cutoff value (<10%) chosen for this study. Attaching-and-effacing genes from strains EDL933, E2348/69, and RDEC-1 belonging to the different phylogenetic groups have been cloned and sequenced (27, 76, 91). Genomic DNAs from strains EDL933 (EHEC), E2348/69 (human EPEC), and RDEC-1 (rabbit EPEC) were used as templates to amplify by PCR the different probes: espA2-espB1-tir2, espA3-espB2-tir3, and espA1-espB3-tir1, respectively. The amplified probes were sequenced to confirm their identities and printed onto the pathotype microarray as shown in Fig. 1. For some virulence determinants, several genes of the cluster were targeted, such as hly (hlyA and hlyC), pap (papAH, papEF, papC, and papG), sfa (sfaDE and sfaA), and agg (aggA and aggC). The use of several genes per cluster assisted in the confirmation of positive signals, in addition to the assessment of cluster integrity. DNA probes detecting the genetic variants of Shiga toxins (stx1, stx2, stxA1, stxA2, stxB1, and stxB2), cytolethal distending toxin (cdt1, cdt2, and cdt3), cytotoxic necrotizing factor (cnf1 and cnf2), and papG alleles (papGI, papGII, and papGIII) were also included. In total, this gene sequence analysis resulted in the selection of 105 gene probes (Table 1).
FIG. 1.
Print pattern of the E. coli pathotype microarray indicated by grouping of genes by category and locations of the individual genes.
Probe amplification, purification, and sequencing.
E. coli strains were grown overnight at 37°C in Luria-Bertani medium. A 200-μl sample of the culture was centrifuged, and the pellet was washed and resuspended in 200 μl of distilled water. The suspension was boiled for 10 min and centrifuged. A 5-μl aliquot of the supernatant was used as a template for PCR amplification. PCRs were carried out in a total volume of 100 μl containing 50 pmol of each primer, 25 μmol of deoxynucleoside triphosphates, 5 μl of template, 10 μl of 10× Taq buffer (500 mM KCl, 15 mM MgCl2, 100 mM Tris-HCl [pH 9]), and 2.5 U of Taq polymerase (Amersham-Pharmacia). PCR products were analyzed by electrophoresis on 1% agarose gels in TAE (40 mM Tris-acetate, 2 mM disodium EDTA) and then purified with the Qiaquick PCR purification kit (Qiagen, Mississauga, Ontario, Canada) and eluted in distilled water. Since the annealing temperatures of the various PCR primers ranged from 40 to 65°C and genomic DNAs from 36 E. coli strains were used as templates, all the PCR amplifications were done separately. A total of 103 virulence factor probes and 2 positive control probes (uidA and uspA) were successfully amplified, as determined by the amplicon sizes and the DNA sequences. The purity of the amplified DNA was confirmed by agarose gel electrophoresis of 50 to 100 ng of each amplified fragment. The sizes of the PCR products ranged from 117 bp (east1) to 2,121 bp (katP), with an average length of 500 bp for the majority of the DNA probes (Table 1). For quality control purposes, all PCR fragments were partially sequenced (with an Applied Biosystems 377 DNA sequencer with the dRhodamine Terminator Cycle Sequencing Ready Reaction kit) for gene verification.
Genomic DNA extraction and labeling.
Cells were collected by centrifuging 5 ml of an overnight culture and were washed with 4 ml of solution 1 (0.5 M NaCl, 0.01 M EDTA [pH 8]), resuspended in 1.2 ml of buffer 2 (solution 1 containing 1 mg of lysozyme per ml), and then incubated at room temperature for 30 min. After the addition of sodium dodecyl sulfate and phenol-chloroform extraction, total DNA was precipitated by the addition of 1 volume of isopropanol. The harvested pellet was washed with 1 volume of 70% ethanol, dried, and then resuspended in 100 μl of Tris-EDTA buffer. Before labeling, total DNA was reduced in size by digestion with restriction enzymes (New England BioLabs, Mississauga, Ontario, Canada), and following digestion, the enzymes were removed by phenol-chloroform extraction. The cyanine dye Cy3 was covalently attached to DNA by using a commercial chemical labeling method (Mirus Label IT reagent; Mirus), with the extent of labeling depending primarily on the ratio of the amount of reagent to the amount of DNA and the reaction time. These parameters were varied to generate labeled DNAs of different intensities. Two micrograms of the digested DNA was chemically labeled with 4 μl of the Label IT reagent, 3 μl of 10× Mirus labeling buffer A, and distilled water in a total volume of 30 μl. The reactions were carried out at 37°C for 3 h. The labeled DNA was then separated from free dye by washing four times with water and centrifugation through Microcon YM-30 filters (Millipore, Bedford, Mass.). The amount of incorporated fluorescent cyanine dye was quantified by scanning the probe from 200 to 700 nm and subsequently inputting the data into the percent incorporation calculator found at http://www.pangloss.com/seidel/Protocols/percent_inc.html. This method is based on the calculation of the ratio of the number of micrograms of incorporated fluorescent dye to the number of micrograms of labeled DNA.
Optimization of microarray detection threshold with a prototype microarray.
A prototype chip was constructed and used to assess various parameters, namely, fragment length and the extent of fluorescence labeling of the target (test) DNA, to optimize the spot detection threshold of the microarray. DNA amplicons from 34 E. coli virulence genes, including probes for EHEC virulence genes espP (13), EHEC-hlyA (80), stx1 (84), stx2 (90), stxC (90), stxA group II (46), paa (1), and eae (4), were generated by PCR amplification and printed in triplicate. The probe lengths ranged from 125 bp (east1) to 1,280 bp (irp1). HindIII-EcoRI digestion was used to generate large fragments (average size, ∼6 kb) from E. coli O157:H7 strain STJ348 genomic DNA, and Sau3A-AluI digestion was used to produce smaller DNA fragments (average size, ∼0.2 kb) from E. coli O157:H7 strain STJ348 genomic DNA. The restricted DNAs were labeled and used as the target for hybridization with the prototype microarray. In our experiments, the strongest hybridization signal was obtained by using larger fragments labeled at an optimal Cy3 rate in the range of 7.5 to 12.5% (data not shown). An estimate of the microarray's sensitivity was calculated by the following equation, as described by De Boer and Beumer (24): percent sensitivity = [p/(p + number of false-negative spots)] × 100, where p is the number of true-positive spots.
Construction of the E. coli pathotype microarray.
Virulence factor probes were grouped by pathotype, with the resulting array being composed of eight subarrays each corresponding to well-characterized E. coli categories (Fig. 1). The subarray for EHEC included probes for Stx genes (stx1, stx2, stxA1, stxA2, stxB1, stxB2, and stxB3), attaching-and-effacing genes (espA, espB, tir, eae, and paa), EHEC-specific plasmid pO157 genes (etpD, ehxA, L9075, katP, and espP), and O157 and O111 somatic antigen genes (rfbEO157 and rfbO111). EPEC was targeted by spotting LEE-specific gene probes (eae, tir, espA, and espB), espC, and EPEC EAF plasmid probes (bfpA and eaf). The subarray for ETEC included probes for human ST (STaH), porcine ST (STaP), ST type II (STb), LT, and adhesion factors shared by human ETEC strains (CFAI, CS1, CS3, and LngA) or by animal ETEC strains (F4, F5, F6, F18, and F41). DNA probes for O101-specific somatic antigen (rfbO101) and ETEC toxin (leoA) were also included. To identify uropathogenic strains, the subarray for UPEC was composed of 27 probes selected for detection of extraintestinal E. coli adhesins Pap (papGI, papGII, papGIII, papAH, papEF, and papC), Sfa (sfaA and sfaDE), Drb (drb122), and Afa (afa3, afa5, afaE7, and afaD8); F1C (focG); nonfimbrial adhesin type 1 (nfaE); the M-agglutinin subunit (bmaE); CS31A (clpG); toxins including hemolysins (hlyA and hlyC), cytotoxic necrotizing factor (cnf1), and colicin V (cvaC); the aerobactin receptor (iutA); and capsular antigen-specific genes kfiB (K5), kpsMTII (K1, K5, and K12), and kpsMTIII (K10 and K54), in addition to the surface exclusion gene (traT) and uspA probes. The cell-detaching subarray (for detection of the cell-detaching E. coli pathotype) contained probes for toxin-encoding genes cnf1, cnf2, cdt1, cdt2, and cdt3. Probes for the iucD, neuC, ibe10, rfbO9, and rfcO4 genes were designed to detect the meningitis-associated E. coli pathotype (MENEC). Probes for EAEC were derived from fimbrial antigen-specific genes aggA and aggC, whereas the enteroinvasive pathotype (EIEC) was targeted by probes for invasin genes ipaC and invX. AIDA (which encodes an adhesin involved in diffuse adherence) was the unique marker for the diffusely adherent pathotype of E. coli.
It is noteworthy that some virulence genes, such as fimA, fimH, irp1, irp2, iss, fyuA, ompA, east1, iha, fliC, tsh, and ompT, are shared by several E. coli pathotypes. Finally, a positive control, the uidA gene-specific probe (17, 31), as well as a negative control composed of 50% dimethyl sulfoxide solution was added. An estimate of the specificity of the virulence gene microarray was calculated by the following equation (24): percent specificity = [n/(n + number of false-positive spots)] × 100, where n is the number of true-negative spots.
Printing and processing of the microarrays.
Two micrograms of each DNA amplicon was lyophilized in a speed vacuum and resuspended in filtered (pore size, 0.22 μm) 50% dimethyl sulfoxide. The concentrations of the amplified products were adjusted to 200 ng/μl, and 10 μl of each DNA amplicon was transferred to a 384-well microplate and stored at −20°C until the printing step. DNA was then spotted onto CMT-GAPS slides (Corning Co., Corning, N.Y.) with a VIRTEK ChipWriter with Telechem SMP3 microspotting pins. Each DNA probe was printed in triplicate on the microarray. After printing of the probes, the arrays were subjected to UV cross-linking at 1,200 μJ (UV Stratalinker 1800; Stratagene), followed by heating at 80°C for 4 h. The slides were then stored in the dark at room temperature until use.
Microarray hybridization and analysis.
The microarrays were prehybridized at 42°C for 1 h under a coverslip (22 by 22 mm; Sigma) in 20 μl of prewarmed solution A (DIG Easy Hyb buffer [Roche] containing 10 μg of tRNA and 10 μg of denatured salmon sperm DNA). After the coverslip was removed by dipping the slide in 0.1× SSC (1× SSC contained 150 mM NaCl and 15 mM trisodium citrate [pH 7]), the array was rinsed briefly in water and dried by centrifugation at room temperature in 50-ml conical tubes for 5 min at 600 × g. Fluorescently labeled DNA was chemically denatured as described by the manufacturer and added to 20 μl of a fresh solution of prewarmed solution A. Hybridization was carried out overnight at 42°C, as recommended by the manufacturer. After hybridization, the coverslip was then removed and placed in 0.1× SSC, and the microarray was washed three times in prewarmed 0.1× SSC-0.1% sodium dodecyl sulfate solution and once in 0.1× SSC for 10 min at 50°C. After the array was dried by centrifugation (800 rpm, 5 min, room temperature), the array was analyzed with a fluorescence scanner (Canberra-Packard, Mississauga, Ontario, Canada). The slides were scanned at a resolution of 5 μm at 85% laser power, and the fluorescence was quantified after subtraction of the background by use of QuantArray software (Canberra-Packard). All hybridization experiments were replicated between two and five times per genome.
RESULTS
Assessment of the pathotype microarray for virulence pattern analysis.
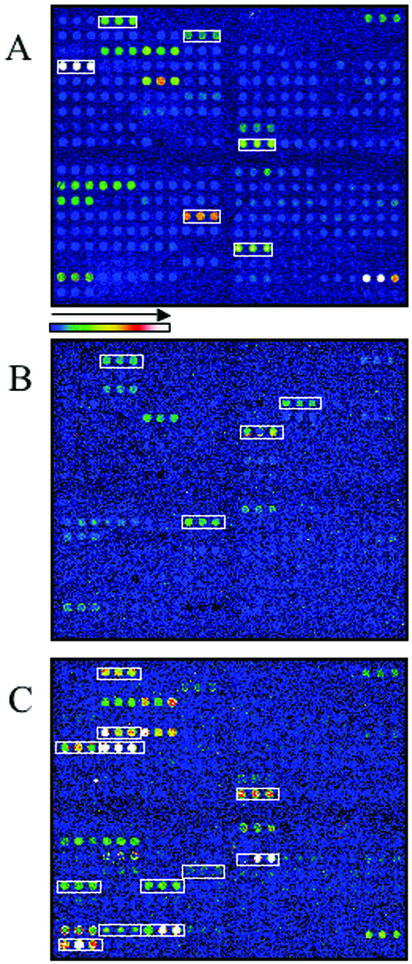
To identify known virulence genes and, consequently, the pathotype of the E. coli strain being examined, genomic DNA from several previously characterized E. coli strains was labeled and hybridized to the pathotype microarray. Strain K-12-derived E. coli strain DH5α was included as a nonpathogenic control. Interestingly, E. coli DH5α produced a fluorescent hybridization signal with the probes for uidA, fimA1, fimA2, fimH, ompA, ompT, traT, fliC, and iss (Fig. 2A). GenBank analysis of the sequenced K-12 strain MG1655 genome revealed the presence of the first seven genes, whereas the sequence of the probe for iss is 90% similar to the sequence of ybcU, a gene encoding a bacteriophage lambda Bor protein homolog (K-12 sequence). Surprisingly, a false-positive signal was obtained with the cdt1 and aggA gene probes. These genes are absent in the E. coli K-12 genome, and their sequences are not homologous to any strain K-12 genes. Moreover, these genes were not positive with K-12 or O157:H7 strain EDL933 in earlier generations of the virulence chip. We postulated that the signal may have been the result of amplicon contamination in the final printing. Therefore, these two probes were not included in all subsequent hybridization analyses.
FIG. 2.
Detection of virulence genes and simultaneous identification of the pathotypes of known E. coli strains after microarray hybridization with genomic DNA from nonpathogenic K-12 strain E. coli DH5α (A), enterohemorrhagic strain EDL933 O157:H7 (B), uropathogenic strain J96 O4:K6 (C), and enterotoxigenic strain H-10407 (D). After HindIII-EcoRI digestion, genomic DNA was labeled with Cy3. Labeled DNA (500 ng) was hybridized to the array overnight at 42°C, washed, dried, and scanned. The boxed spots in panel A represent the virulence genes present in E. coli K-12 strain DH5α (traT, fimA, fimH, ompA, ompT, iss, and fliC). The boxed spots in panels B, C, and D indicate the pathotype-specific genes in the strains tested. Genes present in more than one pathotype (iss, irp2, fliC, and ompT) or present in all pathotypes (fimH, fimA, and ompA) gave positive signals. The horizontal bar indicates the color representation of the fluorescence signal intensity.
Since the genomic sequence of E. coli O157:H7 strain EDL933 is available in the GenBank database (GenBank accession number NC_002655), this strain represented a good choice for use in the assessment of the detection threshold and hybridization specificity of the E. coli virulence factors on the microarray. After hybridization of the pathotype microarray with Cy3-labeled genomic DNA from E. coli O157:H7, the scanned image (Fig. 2B) showed fluorescent signals with the EHEC-specific genes encoding Stxs, the attaching-and-effacing cluster present in EHEC and EPEC strains, the genes carried on EHEC plasmid pO157, and antigen and flagellar antigen-specific genes, as well as iha, an adhesin-encoding gene (GenBank accession number AF401752) found in both the EHEC and the UPEC pathotypes. Therefore, the EHEC pathotype of E. coli O157:H7 was easily confirmed by a rapid visual scan of the virulence gene pattern (Fig. 1) of the scanned image.
UPEC strain J96 (O4:K6) is a prototype E. coli strain from which various extraintestinal E. coli virulence factors have been cloned and characterized (73, 86). This strain possesses two copies of the gene clusters encoding P (pap-encoded) and P-related (prs-encoded) fimbriae, produces F1C (focG), contains two hly gene clusters encoding hemolysin, and produces cytotoxic necrotizing factor type 1 (cnf1). E. coli strain J96 DNA was labeled and hybridized to the pathotype microarray. The scanned array resulted in a UPEC pathotype hybridization pattern (Fig. 2C). All of the UPEC virulence genes cited above were detected, as were other uropathogenic pathotype-specific genes. From a taxonomic perspective, the microarray also permitted the detection of the O4-antigen gene (rfcO4).
An enterotoxin-producing strain of E. coli isolated from a case of cholera-like diarrhea, E. coli strain H-10407 (30), was used as a control strain to assess the ability of the microarray to identify the ETEC pathotype (Fig. 2D). The hybridization results showed the presence of a gene for a heat-stable enterotoxin (stah), antigenic surface-associated colonization factor cfaI, heat-labile enterotoxin LT, and east1 toxin gene; and a weak signal was obtained with the stap gene-specific probe. The hybridization pattern correlated well with the virulence profile and the pathotype group of this strain (28, 29, 68).
Determination of virulence patterns of uncharacterized clinical E. coli strains.
To further validate our pathotype chip, virulence gene detection was assessed by hybridization with genomic DNA from five clinical E. coli strains isolated from a human source (strain H87-5406) and animal sources (Av01-4156, B00-4830, Ca01-E179, and B99-4297). Genomic DNAs from these strains were fragmented and labeled with Cy3, and the microarray hybridization patterns obtained were compared with PCR amplification results.
The virulence gene pattern obtained after microarray hybridization analysis with Cy3-labeled E. coli genomic DNA of avian origin (strain Av01-4156) showed the presence of the extraintestinal E. coli virulence genes (iucD, iroN, traT, and iutA) and genes present in our K-12 strain (fimA1, fimA2, fimH, iss, ompA, and ompT) (Fig. 3A). The temperature-sensitive hemagglutinin gene (tsh) that was often located on the ColV virulence plasmid in avian pathogenic E. coli strains (26) was also detected on the Av01-4156 virulence gene array. A strong hybridization signal was also obtained with the rtx probe whose sequence was derived from a gene located on the O157:H7 chromosome and which encodes a putative RTX family exoprotein. The overall virulence factor detection pattern indicates that this strain is involved in extraintestinal infections.
FIG. 3.
Analysis of virulence potential of E. coli strains isolated from clinical samples by using the E. coli pathotype microarray. (A) Hybridization pattern obtained with genomic DNA from avian E. coli isolate Av01-4156; (B) hybridization pattern obtained with genomic DNA from bovine strain B00-4830; (C) hybridization obtained with genomic DNA from human E. coli isolate H87-540. Labeled DNA (500 ng) was hybridized to the array overnight at 42°C, after which the slide was washed, dried, and scanned. Boxed spots indicate pathotype-specific genes iucD, iron, traT, and iutA in panel A; pathotype-specific genes etpD, F5, stap, and traT in panel B; and pathotype-specific genes stx1, cdt2, cdt3, afaD8, bmaE, iucD, iroN, and iutA in panel C. Positive signals were also obtained with genes present in more than one pathotype (espP, iss, ompT, and fliC) and genes present in all the pathotypes tested (fimA, fimH, and ompA).
Genes encoding ETEC fimbria F5 and ST StaP were detected when the pathotype microarray was hybridized with genomic DNA from strain B00-4830 isolated from bovine ileum (Fig. 3B), indicating that this strain belongs to the animal ETEC pathotype. The hybridization pattern also showed the presence of traT, ompA, fimA1, fimA2, fimH, and fliC genes and EHEC-associated gene etpD.
The virulence pattern obtained after microarray hybridization analysis with Cy3-labeled genomic DNA of E. coli strain H87-5406 of human origin was very complex and did not fall within a single pathotype category. The hybridization pattern revealed the presence of espP, iss, rtx, fimA1, fimA2, fimH, ompA, and ompT genes as well as Stx gene stx1, which was detected in the enterohemorragic pathotype (Fig. 3C). Moreover, virulence genes involved in extraintestinal infections (cdt2, cdt3, afaD8, bmaE, iucD, iroN, traT, and iutA) were also observed. Strain H87-5406 was also positive for the type 2 cytotoxic necrotizing factor, encoded by the cnf2 gene.
The virulence patterns of two other isolates, pulmonary strain Ca01-E179 and bovine strain B99-4297 (used elsewhere in this study), were clearly identified as being of the UPEC pathotype and Stx-positive E. coli, respectively (data not shown). The presence of all the pathotype-specific virulence factors that were positively identified by the microarray data for the animal and human isolates described above was further confirmed by PCR amplification of each positive signal (data not shown).
Discrimination between homologous genes belonging to different subclasses.
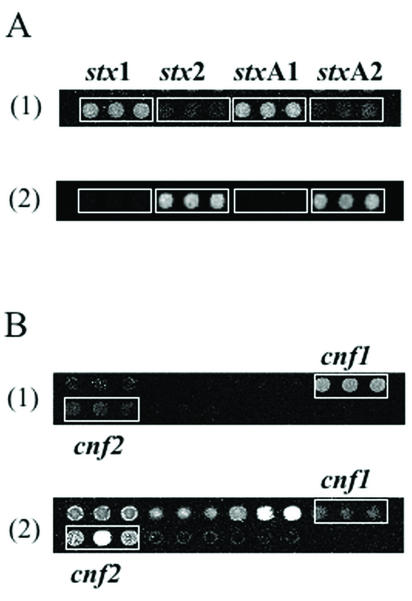
Given the importance of the stx gene family, we designed amplicons stxA1 and stxA2 specific for the A subunits of the stx1 and stx2 families, respectively (Table 2), in addition to using the published amplicons stx1 and stx2 (Table 1), which overlap the A and B subunits of the genes. The sequence similarity between the published stx1 and stx2 amplicons is of the order of 57%; the similarity between our stxA1 and stxA2 amplicons was slightly higher at 61%. As shown in Fig. 4A, the DNA probes used in this study for detection of stx1 and stx2 gene variants were successful in distinguishing stx1 from stx2 when either the previously published amplicons or the stxA subunit probes were used.
FIG. 4.
Detection of stx and cnf variant genes in clinical isolates of E. coli by use of the pathotype microarray. The white boxes in panel A outline the stx genes hybridized with human strain H87-5406 (row 1) and bovine strain B99-4297 (row 2). The white boxes in panel B outline the cnf genes hybridized with strain Ca01-E179 (row 1) and strain H87-5406 (row 2). Labeled DNA (500 ng) was hybridized to an array overnight at 42°C, after which the slide was washed, dried, and scanned.
To further explore the potential of microarrays to distinguish gene variants within homologous gene families, the primers used for cnf1 and cnf2 probe amplification were derived from studies on the detection of cnf gene variants by PCR amplification. The resulting amplicons had 85% sequence similarity. The hybridization results obtained with genomic DNA from cnf-positive strains H87-5406 and Ca01-E1799 (Fig. 4B) showed a clear distinction on the microarray between cnf1 and cnf2 gene variants, an encouraging result given the high degree of similarity and the size (over 1 kb) of the amplicons used.
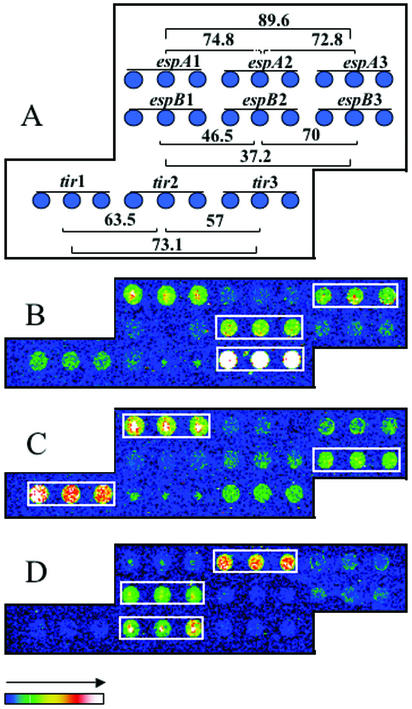
Since the DNA microarray showed initial promise in discriminating between the known variants of the stx and cnf genes, a more defined group of genes was selected in order to test the ability of the pathotype microarray to differentiate between different phylogenetic groups of genes with a sequence divergence cutoff value of >10%. The DNA sequence similarity values of the espA, espB, and tir probes from the three different groups are summarized in Fig. 5A. The microarray was hybridized with labeled genomic DNA from strains EDL933 (EHEC) and E2348/69 (EPEC1). Labeled DNA from another strain, strain P86-1390, which belongs to the same phylogenetic group as RDEC-1, was used to validate the hybridization specificity of the arrayed virulence genes. Hybridizations with the pathotype microarray were performed at 42 and 50°C, and as shown in Fig. 5B to D, the labeled DNA hybridized as expected to probes specific for each phylogenetic group. Genomic DNA from strain P86-1390 hybridized with the espA1-espB3-tir1 probe, indicating that this strain belongs to the same group as RDEC-1, which correlates well with the phylogenetic analysis. A strong cross-hybridization signal was obtained between the espA1 and espA3 probes due to their high DNA similarity score (89.6%). These hybridization patterns were obtained at 42°C as well as at 50°C, suggesting that DNA sequence divergences of 25% can be resolved under standard hybridization conditions. These results demonstrated that the pathotype microarray can be a useful tool for strain genotyping.
FIG. 5.
Use of the E. coli pathotype microarray to identify the phylogenetic groups of E. coli strains on the basis of their hybridization patterns with the attaching-and-effacing gene probes. (A) Print patterns obtained with the espA, espB, and tir probes on the pathotype microarray, with the homology percentages between each immobilized probe indicated; (B) detection of espA3, espB2, and tir3 in human EPEC strain E2348/69; (C) hybridization pattern obtained with genomic DNA from animal EPEC strain P86-1390 (espA1, espB3, and tir1); (D) detection of espA2, espB1, and tir2 in EHEC strain EDL933. The positive hybridization results obtained with the espA, espB, and tir probes are outlined in white boxes.
DISCUSSION
Numerous bioassays and molecular methods have been developed for the detection of genes involved in pathogenic E. coli virulence mechanisms. However, the sheer numbers of known virulence factors have made this a daunting task. The purpose of this study was to provide a rapid and comprehensive tool for the simultaneous detection of all E. coli virulence factor genes and the subsequent identification of the strain's pathotype. We designed a DNA microarray containing 103 gene probes distributed into eight subarrays corresponding to various E. coli pathotypes. To evaluate our chip regarding the specificity of the amplified virulence factor gene fragments, genomic DNAs from different E. coli strains were labeled and hybridized to the virulence factor microarray. For this purpose, we developed a simple protocol for probe and target preparation, labeling, and hybridization. The use of PCR amplification for probe generation and fragmented genomic DNA as the labeled target allowed the detection of all known virulence factors within the E. coli strains characterized. Direct chemical labeling of genomic DNA with a single fluorescent dye (Cy3) facilitated the work.
Because our fluorescence assay was based on direct detection (with a single dye, Cy3) rather than differential hybridization (with multiple dyes), it was necessary at the outset to optimize the signal detection threshold. We found that the signal intensity, apart from DNA homology and DNA labeling efficiency, depended on (i) the size of the immobilized amplicon, (ii) the gene copy number in the target genomic DNA, and (iii) the size of the labeled target DNA. In studies with the large range of probe sizes (between 117 and 2,121 bp) tested, it was clear that the hybridization signal intensity was affected by probe length when homologous DNA was used. Quality control analysis of the printed microarray with terminal transferase showed heterogeneity in the spotted amplicons. Because this enzyme attaches Cy3 to the 3′ end of the fixed DNA amplicon, we expected that the quality control signal would be stronger with smaller amplicons due to an increased number of free ends. Unexpectedly, however, small fragments (less than 200 bp) produced poorer hybridization signals than larger amplicons did, suggesting that it was the actual immobilization of small DNA fragments that is less efficient. Since the immobilized probe size is inherently constrained by gene length, immobilization of small genes and fragments needs further improvement. Despite the aforementioned limitations, by using two strains whose genome sequences are known (strains K-12 and EDL933), we can estimate the level of accuracy (sensitivity and specificity) of the present virulence chip, as outlined in Materials and Methods. The average sensitivity or accuracy in discriminating among the different virulence genes approached 97%. These estimates take into account a shared total of 3 false-negative virulence gene spots among the total of 210 (i.e., 2 × 105) virulence gene spots for both strains. It is important to stress here that the sensitivity and specificity estimations are based on only a few strains and that use of a larger number of isolates will be required to ensure increased accuracy.
Gene location is another factor to be considered when designing gene detection microarrays. After hybridization with genomic DNA from E. coli O157:H7 strain EDL933, we found strong signals for hybridization to etpD, ehxA, L7590, katP, and espP. Since these genes are located on plasmid pO157 (GenBank accession number AF074613) (15), the stronger signal can be attributed to a higher copy number or a higher gene dose. Moreover, many virulence genes are located on mobile elements like plasmids, phages, or transposons (69) and are encoded by foreign DNA acquired via horizontal gene transfer and inserted in the genome. These pathogenicity islands (PAIs) are highly unstable and are constantly shuttled between strains. However, in addition to their total horizontal transfer (12, 38) or deletion (10, 11, 40), several studies suggested that PAIs are subject to continuous modifications in their virulence factor composition (52). In earlier work, the detection of a gene for a single PAI reflected the presumed presence of all the additional virulence genes encoded by the PAI (59), but due to the potential for genetic rearrangements described above, this assumption is risky. Microarray technology represents an excellent tool that can be used to circumvent this PAI plasticity and identify genetic rearrangements by gene deletion or insertion on PAI clusters.
The large number of virulence genes identified makes the microarray a powerful tool for investigation of the pathogenicity potentials of E. coli strains. As shown in this study, more than 100 PCR amplifications could be replaced with one microarray hybridization experiment. In order to minimize the number of PCRs, the choice of PCR primers for detection of specific virulence genes is influenced by two major factors: (i) the need to use conserved primer sets for the specific strain under investigation in order to avoid false-negative results and (ii) the clinical symptoms of the infected host. With regard to the use of primers whose sequences are from conserved regions, the sequence variabilities of virulence genes within these so-called conserved regions could create false-negative results. Indeed, Johnson and Stell (54) found E. coli strains that were PCR negative but probe positive for some virulence genes tested (fimA, kpsmTII, papAH, and cnf1). The use of PCR primer sets chosen on the basis of clinical symptoms gives only a narrow, defined perspective of a strain's virulence gene content. Consequently, this approach will overlook genes not normally expected to be present in the pathotype. Recent investigations of E. coli virulence have revealed new information regarding the prevalence of virulence genes within a specific E. coli pathotype. For example, the cytolethal distending factor (cdt) was first described as a virulence factor associated with EPEC and other diarrhea-associated pathotypes (2, 56, 57). Later, this gene was detected in strains involved in extraintestinal infections in humans and dogs (49-51, 54, 55). More recently, cdt and the urinary tract infection-associated gene (ompT) have been found to be as prevalent as or more prevalent than traditional neonatal bacterial meningitis-associated traits, such as ibeA, sfaS, and the K1 capsule (52). Due to the parallel processing power of DNA microarray technology, the inclusion of all known E. coli virulence genes as well as newly discovered virulence genes would provide a more comprehensive analysis of genes not prevalent in the traditional pathotype pattern. The usefulness of the virulence gene microarray concept for exploring the global virulence pattern of strains and the potential detection of unexpected virulence genes was revealed by total genomic hybridizations with uncharacterized clinical strains. The probe for rtx (which encodes a putative RTX family exoprotein; GenBank accession number AE005229), whose sequence is located on the O157:H7 chromosome, was amplified with genomic DNA from strain EDL933. BLAST analysis did not reveal significant similarities with any available sequences. Analysis of the hybridization patterns of extraintestinal strain Av01-4156 and strain H87-5406 revealed strong signals with the rtx probe, indicating the presence of a gene homologous to the rtx probe (Fig. 3). This gene was successfully amplified in both strains by using the rtx-specific primers. To our knowledge, this is the first report of the presence of this gene in non-O157 strains.
The potential for a given E. coli strain to possess different combinations or sets of virulence genes could lead to the emergence of new pathotypes. Consistent with this hypothesis, we found that a combination of virulence factors from different pathotypes was observed in clinical strain H87-5406. Moreover, microarray hybridization permitted detection of Stx gene stx1, which is associated with EHEC strains, in addition to virulence genes involved in extraintestinal infections (cdt2, cdt3, afaD8, bmaE, iucD, iroN, traT, and iutA). Starcic et al. (83) recently reported on the isolation of a bifunctional E. coli strain from dogs with diarrhea. When that strain was analyzed, only a few strains were positive for ST and none of them produced diarrhea-associated fimbriae K88 and K99, which is in contrast to the results of previous studies (85). However, most of these strains were positive for cytotoxic necrotizing toxin (cnf1), as well as P fimbriae and hemolysin (hly), which are involved in extraintestinal infections in humans and animals. The investigators concluded that hemolytic E. coli strains from dogs with diarrhea have characteristics of both uropathogenic and necrotoxigenic strains.
The present study provides another example illustrating the ability of the virulence gene microarray to provide a more thorough analysis of virulence genes and, consequently, allow the detection of potentially new pathotypes, in that it confirmed the ETEC pathotype of bovine clinical strain B00-4830 (data not shown). In addition to detecting the ETEC-associated virulence genes encoding StaP and F5 in the hybridization pattern, the etpD gene, described by Schmidt et al. (82) as an EHEC type II secretion pathway, was unexpectedly found. In their study, Schmidt et al. (82) reported that they detected the etp gene cluster in all 30 EHEC strains tested by hybridization (with the 11.9-kb etp cluster from EDL933 used as a probe) and by PCR with etpD-specific primers. However, none of the other E. coli pathotypes tested (EPEC, EAEC, EIEC, and ETEC) were positive for the etp gene cluster. As our results are contrary to those of that study, we assayed strain B00-4830 for the etpD gene by PCR with the reverse primer described by Schmidt et al. (82) and a forward primer designed in our study. Amplification of the expected 509-bp fragment was consistent with the microarray results, confirming that the etpD gene can be found in ETEC strains.
Another unexpected finding of our study was the prevalence of the fimH and ompT genes, which have been epidemiologically associated with extraintestinal infections (51, 54). BLAST analysis of the ompT and fimH genes indicated the presence of both genes in E. coli K-12 strain MG1655 and enterohemorrhagic E. coli strains O157:H7 EDL933 and RIMD 0509952. In addition, our hybridization results revealed the presence of the fimH gene in all strains tested in this study, including nonpathogenic E. coli, EPEC, ETEC, and UPEC strains. The ompT gene was less prevalent but was present in Stx-producing strain H87-5406. It was also found in another Stx-producing strain, strain B99-4297, as well as in EPEC strains P86-1390 and E2348/69 (data not shown). The use of these genes as indicators of the UPEC pathotype should be reconsidered.
In summary, we have created an E. coli virulence factor chip by targeting all of the presently known virulence genes found in E. coli including various somatic antigen genes and capsular genes. This study shows that DNA microarray technology can be a valuable tool for pathotype identification, assessment of the virulence potential of E. coli strains, and detection of the emergence of new pathotypes. Our DNA chip design should facilitate epidemiological and phylogenetic studies since the prevalence of each virulence gene can be determined for different pathotypes (and strains) and the phylogenetic associations between the virulence pattern and serotypes of a given strain can be elucidated. In addition, unlike traditional hybridization formats, microchip technology is compatible with the increasing number of newly recognized virulence genes since thousands of individual probes can be immobilized on one glass slide. Although the microarray is able to test more clinical isolates, reducing the number of false-positive and false-negative results will be the goal of future optimizations of our array through the elimination of probe cross-hybridizations (e.g., iss with ybcU) or adjustment of the uniformity of immobilized amplicon lengths.
The proposed DNA labeling methodology for hybridization and pathotype assessment is both rapid and sensitive. The applications of such microarrays extend broadly from the medical field to the testing of drinking water, food quality control, and environmental research and can easily be expanded to the detection of virulence genes in other pathogenic bacteria.
Acknowledgments
This work was supported by Fonds pour la Formation des Chercheurs et l'Aide à la Recherche (FCAR) grant 0214 and Natural Sciences Engineering Research Council of Canada (NSERC) grant 225155 (Canadian Research Network on Bacterial Pathogens of Swine).
We are very grateful to Clarisse Désautels and Mélanie Arbour for technical assistance during this study and Jason Dubois for critical reading of the manuscript. The use of the computer facilities provided by the Canadian Bioinformatics Resource (http://www.cbr.nrc.ca) is also gratefully acknowledged.
REFERENCES
- 1.An, H., J. M. Fairbrother, C. Desautels, and J. Harel. 1999. Distribution of a novel locus called Paa (porcine attaching and effacing associated) among enteric Escherichia coli. Adv. Exp. Med. Biol. 473:179-184. [DOI] [PubMed] [Google Scholar]
- 2.Anderson, J. D., A. J. MacNab, W. R. Gransden, S. M. Damm, W. M. Johnson, and H. Lior. 1987. Gastroenteritis and encephalopathy associated with a strain of Escherichia coli O55:K59:H4 that produced a cytolethal distending toxin. Pediatr. Infect. Dis. J. 6:1135-1136. [PubMed] [Google Scholar]
- 3.Bach, S., A. de Almeida, and E. Carniel. 2000. The Yersinia high-pathogenicity island is present in different members of the family Enterobacteriaceae. FEMS Microbiol. Lett. 183:289-294. [DOI] [PubMed] [Google Scholar]
- 4.Beaudry, M., C. Zhu, J. M. Fairbrother, and J. Harel. 1996. Genotypic and phenotypic characterization of Escherichia coli isolates from dogs manifesting attaching and effacing lesions. J. Clin. Microbiol. 34:144-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benz, I., and M. A. Schmidt. 1992. AIDA-I, the adhesin involved in diffuse adherence of the diarrhoeagenic Escherichia coli strain 2787 (O126:H27), is synthesized via a precursor molecule. Mol. Microbiol. 6:1539-1546. [DOI] [PubMed] [Google Scholar]
- 6.Bertani, G. 1951. Studies on lysogenesis. I. The mode of phage liberation by lysogenic Escherichia coli. J. Bacteriol. 62:293-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bertin, Y., C. Martin, J. P. Girardeau, P. Pohl, and M. Contrepois. 1998. Association of genes encoding P fimbriae, CS31A antigen and EAST 1 toxin among CNF1-producing Escherichia coli strains from cattle with septicemia and diarrhea. FEMS Microbiol. Lett. 162:235-239. [DOI] [PubMed] [Google Scholar]
- 8.Beutin, L. 1999. Escherichia coli as a pathogen in dogs and cats. Vet. Res. 30:285-298. [PubMed] [Google Scholar]
- 9.Bingen, E., B. Picard, N. Brahimi, S. Mathy, P. Desjardins, J. Elion, and E. Denamur. 1998. Phylogenetic analysis of Escherichia coli strains causing neonatal meningitis suggests horizontal gene transfer from a predominant pool of highly virulent B2 group strains. J. Infect. Dis. 177:642-650. [DOI] [PubMed] [Google Scholar]
- 10.Bloch, C. A., and C. K. Rode. 1996. Pathogenicity island evaluation in Escherichia coli K1 by crossing with laboratory strain K-12. Infect. Immun. 64:3218-3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blum, G., M. Ott, A. Lischewski, A. Ritter, H. Imrich, H. Tschape, and J. Hacker. 1994. Excision of large DNA regions termed pathogenicity islands from tRNA-specific loci in the chromosome of an Escherichia coli wild-type pathogen. Infect. Immun. 62:606-614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boyd, E. F., and D. L. Hartl. 1998. Chromosomal regions specific to pathogenic isolates of Escherichia coli have a phylogenetically clustered distribution. J. Bacteriol. 180:1159-1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brunder, W., H. Schmidt, M. Frosch, and H. Karch. 1999. The large plasmids of Shiga-toxin-producing Escherichia coli (STEC) are highly variable genetic elements. Microbiology 145:1005-1014. [DOI] [PubMed] [Google Scholar]
- 14.Brunder, W., H. Schmidt, and H. Karch. 1996. KatP, a novel catalase-peroxidase encoded by the large plasmid of enterohaemorrhagic Escherichia coli O157:H7. Microbiology 142:3305-3315. [DOI] [PubMed] [Google Scholar]
- 15.Burland, V., Y. Shao, N. T. Perna, G. Plunkett, H. J. Sofia, and F. R. Blattner. 1998. The complete DNA sequence and analysis of the large virulence plasmid of Escherichia coli O157:H7. Nucleic Acids Res. 26:4196-4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Call, D. R., F. J. Brockman, and D. P. Chandler. 2001. Detecting and genotyping Escherichia coli O157:H7 using multiplexed PCR and nucleic acid microarrays. Int. J. Food Microbiol. 67:71-80. [DOI] [PubMed] [Google Scholar]
- 17.Cebula, T. A., W. L. Payne, and P. Feng. 1995. Simultaneous identification of strains of Escherichia coli serotype O157:H7 and their Shiga-like toxin type by mismatch amplification mutation assay-multiplex PCR. J. Clin. Microbiol. 33:248-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chizhikov, V., A. Rasooly, K. Chumakov, and D. D. Levy. 2001. Microarray analysis of microbial virulence factors. Appl. Environ. Microbiol. 67:3258-3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cho, J. C., and J. M. Tiedje. 2001. Bacterial species determination from DNA-DNA hybridization by using genome fragments and DNA microarrays. Appl. Environ. Microbiol. 67:3677-3682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cid, D., R. Sanz, I. Marin, H. de Greve, J. A. Ruiz-Santa-Quiteria, R. Amils, and R. de la Fuente. 1999. Characterization of nonenterotoxigenic Escherichia coli strains producing F17 fimbriae isolated from diarrheic lambs and goat kids. J. Clin. Microbiol. 37:1370-1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clegg, S. 1982. Cloning of genes determining the production of mannose-resistant fimbriae in a uropathogenic strain of Escherichia coli belonging to serogroup O6. Infect. Immun. 38:739-744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Daigle, F., J. Harel, J. M. Fairbrother, and P. Lebel. 1994. Expression and detection of pap-, sfa-, and afa-encoded fimbrial adhesin systems among uropathogenic Escherichia coli. Can. J. Microbiol. 40:286-291. [DOI] [PubMed] [Google Scholar]
- 23.Dallas, W. S., D. M. Gill, and S. Falkow. 1979. Cistrons encoding Escherichia coli heat-labile toxin. J. Bacteriol. 139:850-858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Boer, E., and R. R. Beumer. 1999. Methodology for detection and typing of foodborne microorganisms. Int. J. Food Microbiol. 50:119-130. [DOI] [PubMed] [Google Scholar]
- 25.Dozois, C. M., S. Clement, C. Desautels, E. Oswald, and J. M. Fairbrother. 1997. Expression of P, S, and F1C adhesins by cytotoxic necrotizing factor 1-producing Escherichia coli from septicemic and diarrheic pigs. FEMS Microbiol. Lett. 152:307-312. [DOI] [PubMed] [Google Scholar]
- 26.Dozois, C. M., M. Dho-Moulin, A. Bree, J. M. Fairbrother, C. Desautels, and R. Curtiss III. 2000. Relationship between the Tsh autotransporter and pathogenicity of avian Escherichia coli and localization and analysis of the Tsh genetic region. Infect. Immun. 68:4145-4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elliott, S. J., L. A. Wainwright, T. K. McDaniel, K. G. Jarvis, Y. K. Deng, L. C. Lai, B. P. McNamara, M. S. Donnenberg, and J. B. Kaper. 1998. The complete sequence of the locus of enterocyte effacement (LEE) from enteropathogenic Escherichia coli E2348/69. Mol. Microbiol. 28:1-4. [DOI] [PubMed] [Google Scholar]
- 28.Evans, D. G., and D. J. Evans, Jr. 1978. New surface-associated heat-labile colonization factor antigen (CFA/II) produced by enterotoxigenic Escherichia coli of serogroups O6 and O8. Infect. Immun. 21:638-647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Evans, D. G., D. J. Evans, Jr., and H. L. DuPont. 1977. Virulence factors of enterotoxigenic Escherichia coli. J. Infect. Dis. 136(Suppl.):S118-S123. [DOI] [PubMed] [Google Scholar]
- 30.Evans, D. J., Jr., D. G. Evans, S. H. Richardson, and S. L. Gorbach. 1976. Purification of the polymyxin-released, heat-labile enterotoxin of Escherichia coli. J. Infect. Dis. 133(Suppl.):97-102. [DOI] [PubMed] [Google Scholar]
- 31.Feng, P., and S. R. Monday. 2000. Multiplex PCR for detection of trait and virulence factors in enterohemorrhagic Escherichia coli serotypes. Mol. Cell. Probes 14:333-337. [DOI] [PubMed] [Google Scholar]
- 32.Franke, J., S. Franke, H. Schmidt, A. Schwarzkopf, L. H. Wieler, G. Baljer, L. Beutin, and H. Karch. 1994. Nucleotide sequence analysis of enteropathogenic Escherichia coli (EPEC) adherence factor probe and development of PCR for rapid detection of EPEC harboring virulence plasmids. J. Clin. Microbiol. 32:2460-2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Furrer, B., U. Candrian, and J. Luthy. 1990. Detection and identification of E. coli producing heat-labile enterotoxin type I by enzymatic amplification of a specific DNA fragment. Lett. Appl. Microbiol. 10:31-34. [DOI] [PubMed] [Google Scholar]
- 34.Gannon, V. P., S. D'Souza, T. Graham, R. K. King, K. Rahn, and S. Read. 1997. Use of the flagellar H7 gene as a target in multiplex PCR assays and improved specificity in identification of enterohemorrhagic Escherichia coli strains. J. Clin. Microbiol. 35:656-662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gannon, V. P., R. K. King, J. Y. Kim, and E. J. Thomas. 1992. Rapid and sensitive method for detection of Shiga-like toxin-producing Escherichia coli in ground beef using the polymerase chain reaction. Appl. Environ. Microbiol. 58:3809-3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garcia, E., H. E. Bergmans, J. F. Van den Bosch, I. Orskov, B. A. Van der Zeijst, and W. Gaastra. 1988. Isolation and characterisation of dog uropathogenic Escherichia coli strains and their fimbriae. Antonie Leeuwenhoek 54:149-163. [DOI] [PubMed] [Google Scholar]
- 37.Girardeau, J. P., Y. Bertin, C. Martin, M. Der Vartanian, and C. Boeuf. 1991. Sequence analysis of the clpG gene, which codes for surface antigen CS31A subunit: evidence of an evolutionary relationship between CS31A, K88, and F41 subunit genes. J. Bacteriol. 173:7673-7683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Groisman, E. A., and H. Ochman. 1996. Pathogenicity islands: bacterial evolution in quantum leaps. Cell 87:791-794. [DOI] [PubMed] [Google Scholar]
- 39.Gunzburg, S. T., N. G. Tornieporth, and L. W. Riley. 1995. Identification of enteropathogenic Escherichia coli by PCR-based detection of the bundle-forming pilus gene. J. Clin. Microbiol. 33:1375-1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hacker, J., L. Bender, M. Ott, J. Wingender, B. Lund, R. Marre, and W. Goebel. 1990. Deletions of chromosomal regions coding for fimbriae and hemolysins occur in vitro and in vivo in various extraintestinal Escherichia coli isolates. Microb. Pathog. 8:213-225. [DOI] [PubMed] [Google Scholar]
- 41.Harel, J., J. Fairbrother, C. Forget, C. Desautels, and J. Moore. 1993. Virulence factors associated with F165-positive Escherichia coli strains isolated from piglets and calves. Vet. Microbiol. 38:139-155. [DOI] [PubMed] [Google Scholar]
- 42.Herrero, M., V. de Lorenzo, and J. B. Neilands. 1988. Nucleotide sequence of the iucD gene of the pColV-K30 aerobactin operon and topology of its product studied with phoA and lacZ gene fusions. J. Bacteriol. 170:56-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hu, Y., Q. Zhang, and J. C. Meitzler. 1999. Rapid and sensitive detection of Escherichia coli O157:H7 in bovine faeces by a multiplex PCR. J. Appl. Microbiol. 87:867-876. [DOI] [PubMed] [Google Scholar]
- 44.Huang, S. H., C. Wass, Q. Fu, N. V. Prasadarao, M. Stins, and K. S. Kim. 1995. Escherichia coli invasion of brain microvascular endothelial cells in vitro and in vivo: molecular cloning and characterization of invasion gene ibe10. Infect. Immun. 63:4470-4475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Imberechts, H., H. De Greve, C. Schlicker, H. Bouchet, P. Pohl, G. Charlier, H. Bertschinger, P. Wild, J. Vandekerckhove, J. Van Damme, et al. 1992. Characterization of F107 fimbriae of Escherichia coli 107/86, which causes edema disease in pigs, and nucleotide sequence of the F107 major fimbrial subunit gene, fedA. Infect. Immun. 60:1963-1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ito, H., A. Terai, H. Kurazono, Y. Takeda, and M. Nishibuchi. 1990. Cloning and nucleotide sequencing of Vero toxin 2 variant genes from Escherichia coli O91:H21 isolated from a patient with the hemolytic uremic syndrome. Microb. Pathog. 8:47-60. [DOI] [PubMed] [Google Scholar]
- 47.Johnson, J. R., J. J. Brown, U. B. Carlino, and T. A. Russo. 1998. Colonization with and acquisition of uropathogenic Escherichia coli as revealed by polymerase chain reaction-based detection. J. Infect. Dis. 177:1120-1124. [DOI] [PubMed] [Google Scholar]
- 48.Johnson, J. R., and J. J. Brown. 1996. A novel multiply-primed polymerase chain reaction assay for identification of variant papG genes encoding the Gal(α1-4)Gal-binding PapG adhesins of Escherichia coli. J. Infect. Dis. 173:920-926. [DOI] [PubMed] [Google Scholar]
- 49.Johnson, J. R., P. Delavari, M. Kuskowski, and A. L. Stell. 2001. Phylogenetic distribution of extraintestinal virulence-associated traits in Escherichia coli. J. Infect. Dis. 183:78-88. [DOI] [PubMed] [Google Scholar]
- 50.Johnson, J. R., T. T. O'Bryan, P. Delavari, M. Kuskowski, A. Stapleton, U. Carlino, and T. A. Russo. 2001. Clonal relationships and extended virulence genotypes among Escherichia coli isolates from women with a first or recurrent episode of cystitis. J. Infect. Dis. 183:1508-1517. [DOI] [PubMed] [Google Scholar]
- 51.Johnson, J. R., T. T. O'Bryan, D. A. Low, G. Ling, P. Delavari, C. Fasching, T. A. Russo, U. Carlino, and A. L. Stell. 2000. Evidence of commonality between canine and human extraintestinal pathogenic Escherichia coli strains that express papG allele III. Infect. Immun. 68:3327-3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Johnson, J. R., E. Oswald, T. T. O'Bryan, M. A. Kuskowski, and L. Spanjaard. 2002. Phylogenetic distribution of virulence-associated genes among Escherichia coli isolates associated with neonatal bacterial meningitis in The Netherlands. J. Infect. Dis. 185:774-784. [DOI] [PubMed] [Google Scholar]
- 53.Johnson, J. R., T. A. Russo, P. I. Tarr, U. Carlino, S. S. Bilge, J. C. Vary, Jr., and A. L. Stell. 2000. Molecular epidemiological and phylogenetic associations of two novel putative virulence genes, iha and iroNE. coli, among Escherichia coli isolates from patients with urosepsis. Infect. Immun. 68:3040-3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Johnson, J. R., and A. L. Stell. 2000. Extended virulence genotypes of Escherichia coli strains from patients with urosepsis in relation to phylogeny and host compromise. J. Infect. Dis. 181:261-272. [DOI] [PubMed] [Google Scholar]
- 55.Johnson, J. R., A. L. Stell, P. Delavari, A. C. Murray, M. Kuskowski, and W. Gaastra. 2001. Phylogenetic and pathotypic similarities between Escherichia coli isolates from urinary tract infections in dogs and extraintestinal infections in humans. J. Infect. Dis. 183:897-906. [DOI] [PubMed] [Google Scholar]
- 56.Johnson, W. M., and H. Lior. 1988. A new heat-labile cytolethal distending toxin (CLDT) produced by Escherichia coli isolates from clinical material. Microb. Pathog. 4:103-113. [DOI] [PubMed] [Google Scholar]
- 57.Johnson, W. M., and H. A. Lior. 1987. Response of Chinese hamster ovary cells to a cytolethal distending toxin (CLDT) of Escherichia coli and possible misinterpretation as heat-labile (LT) enterotoxin. FEMS Microbiol. Lett. 43:19-23. [Google Scholar]
- 58.Knutton, S., D. R. Lloyd, and A. S. McNeish. 1987. Adhesion of enteropathogenic Escherichia coli to human intestinal enterocytes and cultured human intestinal mucosa. Infect. Immun. 55:69-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kuhnert, P., P. Boerlin, and J. Frey. 2000. Target genes for virulence assessment of Escherichia coli isolates from water, food and the environment. FEMS Microbiol. Rev. 24:107-117. [DOI] [PubMed] [Google Scholar]
- 60.Kurazono, H., S. Yamamoto, M. Nakano, G. B. Nair, A. Terai, W. Chaicumpa, and H. Hayashi. 2000. Characterization of a putative virulence island in the chromosome of uropathogenic Escherichia coli possessing a gene encoding a uropathogenic-specific protein. Microb. Pathog. 28:183-189. [DOI] [PubMed] [Google Scholar]
- 61.Lalioui, L., M. Jouve, P. Gounon, and C. Le Bouguenec. 1999. Molecular cloning and characterization of the afa-7 and afa-8 gene clusters encoding afimbrial adhesins in Escherichia coli strains associated with diarrhea or septicemia in calves. Infect. Immun. 67:5048-5059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Le Bouguenec, C., M. Archambaud, and A. Labigne. 1992. Rapid and specific detection of the pap, afa, and sfa adhesin-encoding operons in uropathogenic Escherichia coli strains by polymerase chain reaction. J. Clin. Microbiol. 30:1189-1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li, J., S. Chen, and D. H. Evans. 2001. Typing and subtyping influenza virus using DNA microarrays and multiplex reverse transcriptase PCR. J. Clin. Microbiol. 39:696-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Makino, S., H. Asakura, T. Obayashi, T. Shirahata, T. Ikeda, and K. Takeshi. 1999. Molecular epidemiological study on tetracycline resistance R plasmids in enterohaemorrhagic Escherichia coli O157:H7. Epidemiol. Infect. 123:25-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Marc, D., and M. Dho-Moulin. 1996. Analysis of the fim cluster of an avian O2 strain of Escherichia coli: serogroup-specific sites within fimA and nucleotide sequence of fimI. J. Med. Microbiol. 44:444-452. [DOI] [PubMed] [Google Scholar]
- 66.Martin, C., E. Rousset, and H. De Greve. 1997. Human uropathogenic and bovine septicaemic Escherichia coli strains carry an identical F17-related adhesin. Res. Microbiol. 148:55-64. [DOI] [PubMed] [Google Scholar]
- 67.Mitsumori, K., A. Terai, S. Yamamoto, and O. Yoshida. 1998. Identification of S, F1C and three PapG fimbrial adhesins in uropathogenic Escherichia coli by polymerase chain reaction. FEMS Immunol. Med. Microbiol. 21:261-268. [DOI] [PubMed] [Google Scholar]
- 68.Moseley, S. L., M. Samadpour-Motalebi, and S. Falkow. 1983. Plasmid association and nucleotide sequence relationships of two genes encoding heat-stable enterotoxin production in Escherichia coli H-10407. J. Bacteriol. 156:441-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Muhldorfer, I., and J. Hacker. 1994. Genetic aspects of Escherichia coli virulence. Microb. Pathog. 16:171-181. [DOI] [PubMed] [Google Scholar]
- 70.Murray, A. E., D. Lies, G. Li, K. Nealson, J. Zhou, and J. M. Tiedje. 2001. DNA/DNA hybridization to microarrays reveals gene-specific differences between closely related microbial genomes. Proc. Natl. Acad. Sci. USA 98:9853-9858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nataro, J. P., and J. B. Kaper. 1998. Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 11:142-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ojeniyi, B., P. Ahrens, and A. Meyling. 1994. Detection of fimbrial and toxin genes in Escherichia coli and their prevalence in piglets with diarrhoea. The application of colony hybridization assay, polymerase chain reaction and phenotypic assays. Zentbl. Veterinarmed. Reihe B 41:49-59. [DOI] [PubMed] [Google Scholar]
- 73.Orndorff, P. E., and S. Falkow. 1984. Organization and expression of genes responsible for type 1 piliation in Escherichia coli. J. Bacteriol. 159:736-744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Oswald, E., P. Pohl, E. Jacquemin, P. Lintermans, K. Van Muylem, A. D. O'Brien, and J. Mainil. 1994. Specific DNA probes to detect Escherichia coli strains producing cytotoxic necrotising factor type 1 or type 2. J. Med. Microbiol. 40:428-434. [DOI] [PubMed] [Google Scholar]
- 75.Paton, A. W., and J. C. Paton. 1998. Detection and characterization of Shiga toxigenic Escherichia coli by using multiplex PCR assays for stx1, stx2, eaeA, enterohemorrhagic E. coli hlyA, rfbO111, and rfbO157. J. Clin. Microbiol. 36:598-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Perna, N. T., G. Plunkett III, V. Burland, B. Mau, J. D. Glasner, D. J. Rose, G. F. Mayhew, P. S. Evans, J. Gregor, H. A. Kirkpatrick, G. Posfai, J. Hackett, S. Klink, A. Boutin, Y. Shao, L. Miller, E. J. Grotbeck, N. W. Davis, A. Lim, E. T. Dimalanta, K. D. Potamousis, J. Apodaca, T. S. Anantharaman, J. Lin, G. Yen, D. C. Schwartz, R. A. Welch, and F. R. Blattner. 2001. Genome sequence of enterohaemorrhagic Escherichia coli O157:H7. Nature 409:529-533. [DOI] [PubMed] [Google Scholar]
- 77.Pfaff-McDonough, S. J., S. M. Horne, C. W. Giddings, J. O. Ebert, C. Doetkott, M. H. Smith, and L. K. Nolan. 2000. Complement resistance-related traits among Escherichia coli isolates from apparently healthy birds and birds with colibacillosis. Avian Dis. 44:23-33. [PubMed] [Google Scholar]
- 78.Rich, C., S. Favre-Bonte, F. Sapena, B. Joly, and C. Forestier. 1999. Characterization of enteroaggregative Escherichia coli isolates. FEMS Microbiol. Lett. 173:55-61. [DOI] [PubMed] [Google Scholar]
- 79.Russo, T. A., U. B. Carlino, A. Mong, and S. T. Jodush. 1999. Identification of genes in an extraintestinal isolate of Escherichia coli with increased expression after exposure to human urine. Infect. Immun. 67:5306-5314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sandhu, K. S., R. C. Clarke, and C. L. Gyles. 1997. Hemolysin phenotypes and genotypes of eaeA-positive and eaeA-negative bovine verotoxigenic Escherichia coli. Adv. Exp. Med. Biol. 412:295-302. [DOI] [PubMed] [Google Scholar]
- 81.Savarino, S. J., A. Fasano, J. Watson, B. M. Martin, M. M. Levine, S. Guandalini, and P. Guerry. 1993. Enteroaggregative Escherichia coli heat-stable enterotoxin 1 represents another subfamily of E. coli heat-stable toxin. Proc. Natl. Acad. Sci. USA 90:3093-3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schmidt, H., B. Henkel, and H. Karch. 1997. A gene cluster closely related to type II secretion pathway operons of gram-negative bacteria is located on the large plasmid of enterohemorrhagic Escherichia coli O157 strains. FEMS Microbiol. Lett. 148:265-272. [DOI] [PubMed] [Google Scholar]
- 83.Starcic, M., J. R. Johnson, A. L. Stell, J. van der Goot, H. G. Hendriks, C. van Vorstenbosch, L. van Dijk, and W. Gaastra. 2002. Haemolytic Escherichia coli isolated from dogs with diarrhea have characteristics of both uropathogenic and necrotoxigenic strains. Vet. Microbiol. 85:361-377. [DOI] [PubMed] [Google Scholar]
- 84.Strockbine, N. A., M. P. Jackson, L. M. Sung, R. K. Holmes, and A. D. O'Brien. 1988. Cloning and sequencing of the genes for Shiga toxin from Shigella dysenteriae type 1. J. Bacteriol. 170:1116-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wasteson, Y., O. Olsvik, E. Skancke, C. A. Bopp, and K. Fossum. 1988. Heat-stable-enterotoxin-producing Escherichia coli strains isolated from dogs. J. Clin. Microbiol. 26:2564-2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Welch, R. A., R. Hull, and S. Falkow. 1983. Molecular cloning and physical characterization of a chromosomal hemolysin from Escherichia coli. Infect. Immun. 42:178-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wilfert, C. M. 1978. E. coli meningitis: K1 antigen and virulence. Annu. Rev. Med. 29:129-136. [DOI] [PubMed] [Google Scholar]
- 88.Yamamoto, S., A. Terai, K. Yuri, H. Kurazono, Y. Takeda, and O. Yoshida. 1995. Detection of urovirulence factors in Escherichia coli by multiplex polymerase chain reaction. FEMS Immunol. Med. Microbiol. 12:85-90. [DOI] [PubMed] [Google Scholar]
- 89.Yamamoto, T., and P. Echeverria. 1996. Detection of the enteroaggregative Escherichia coli heat-stable enterotoxin 1 gene sequences in enterotoxigenic E. coli strains pathogenic for humans. Infect. Immun. 64:1441-1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yamasaki, S., Z. Lin, H. Shirai, A. Terai, Y. Oku, H. Ito, M. Ohmura, T. Karasawa, T. Tsukamoto, H. Kurazono, and Y. Takeda. 1996. Typing of verotoxins by DNA colony hybridization with poly- and oligonucleotide probes, a bead-enzyme-linked immunosorbent assay, and polymerase chain reaction. Microbiol. Immunol. 40:345-352. [DOI] [PubMed] [Google Scholar]
- 91.Zhu, C., T. S. Agin, S. J. Elliott, L. A. Johnson, T. E. Thate, J. B. Kaper, and E. C. Boedeker. 2001. Complete nucleotide sequence and analysis of the locus of enterocyte effacement from rabbit diarrheagenic Escherichia coli RDEC-1. Infect. Immun. 69:2107-2115. [DOI] [PMC free article] [PubMed] [Google Scholar]