Abstract
VP6, VP7, VP9, VP10, VP11, and VP12 of Colorado tick fever virus (CTF virus), a virus member of the genus Coltivirus, family Reoviridae, were expressed in bacteria with the pGEX-4T-2 vector. A partial sequence of VP7 (designated pVP7) was chosen to elaborate an enzyme-linked immunosorbent assay (ELISA) for detecting anti-CTF virus immunoglobulin G (IgG) antibodies in humans. This was based on two observations: (i) among all expressed proteins, pVP7 showed the highest immunoreactivity to an anti-CTF virus hyperimmune ascitic fluid; (ii) to provide the highest selectivity of antibody detection, the expressed sequence was chosen within a region which is highly divergent (49% amino acid identity) from the homologous sequence of another coltivirus, the Eyach virus. The pVP7 ELISA was evaluated with 368 serum samples from French blood donors and found to provide 98.1% specificity. Assays with the Calisher set of human serum samples, positive for anti-CTF virus antibodies (C. H. Calisher, J. D. Poland, S. B. Calisher, and L. A Warmoth, J. Clin. Microbiol. 22:84-88, 1985), showed that the pVP7 ELISA provided 100% sensitivity for the tested population. After elaboration of recombinant-protein-based ELISAs for diagnosis of infections with members of the viral genera Orbivirus, Orthoreovirus, and Rotavirus, it was shown that a recombinant protein could be used to detect antibodies to the human pathogen Colorado tick fever virus.
Colorado tick fever (CTF) is a tick-borne viral disease which is usually self-limiting (18). It affects 11 western states in the United States (it was first described in the Rocky Mountain region) and was also reported in Canada (11, 18). Two to three hundred cases are reported annually in the United States. The onset of the disease is abrupt, with flu-like manifestations. The clinical triad (saddleback fever, myalgia, and headache) appears following the bite of an infected adult Dermacentor andersoni tick. Severe complications have been reported, particularly in children, such as encephalitis, meningitis, hepatitis, intravascular coagulopathy, and orchitis (6, 10, 12, 18).
Besides CTF virus, the genus Coltivirus encompasses the European Eyach virus reported earlier in Germany and France (9, 23). This virus is related to CTF virus but shows significant genome divergence, especially in segments 6, 7, and 12 (4). It cannot be propagated in cell culture, and little is known about its epidemiological distribution. However, the Eyach virus has been suspected to be a possible human pathogen because specific antibodies have been detected in serum samples from patients suffering from polyradiculoneuritis (20).
The possible propagation of CTF virus in the usual cell lines has made the elaboration of diagnostic procedures easier. In particular, virus isolation and serological diagnosis with lysates of infected cells (including enzyme-linked immunosorbent assay [ELISA] and immunocapture and Western blot assays) have been applied successfully (2, 7). More recently, PCR detection procedures for the CTF virus genome have been developed (2, 17), taking advantage of the complete characterization of the virus genome (1).
In this study, we designed the first serological assay based on a recombinant CTF virus protein. The recombinant CTF virus VP7 protein was selected for its high immunoreactivity and used for diagnosis in an immunoglobulin G (IgG) ELISA format. This ELISA was evaluated with serum samples from volunteer blood donors and CTF virus-infected humans. This test could be used whenever cell culture facilities for virus propagation are not available or safety restrictions should be considered. It should therefore be helpful for future epidemiological and/or diagnostic studies of CTF virus infection.
MATERIALS AND METHODS
Virus propagation and cloning.
The Florio strain of CTF virus (1943, human isolate) (13, 14) was propagated in BHK-21 cells as described previously (3). The virus genome was cloned and sequenced in a previous study (3).
Production of recombinant proteins: assay of immunoreactivity.
We expressed the VP6, VP7, VP9, VP10, VP11, and VP12 proteins of CTF virus. The immunoreactivity of these proteins was assayed with an anti-CTF virus mouse hyperimmune ascitic fluid sample and a positive convalescent-phase human serum sample by slot blot methodology. All except VP10 were immunoreactive, with VP7 showing the highest reactivity. A partial sequence of VP7 between amino acids 144 and 540 (397 amino acids, designated pVP7) was chosen as the substrate for expression and antibody detection. In this region, VP7 of CTF virus and VP6 of Eyach virus (4) are homologous but show considerable divergence (>50%).
Production of CTF virus recombinant pVP7: construction of vector expressing pVP7.
Segment 7 was amplified under standard conditions with specific primers (underlined) tailed with a restriction enzyme site (bold): VP7expS, GGATCCCCAGGAATTCCCTGTCAAGCTGTTGGTTTGAATC, containing a cleavage site for EcoRI, and VP7expR, GAGCTCGTGAGCGGCCGCTCACTAATGGTGATGGTGATGATGCTCATACATCACCTTCGCTCTG, containing a cleavage site for NotI. The reverse primer also contains a six-His 6xHis tag sequence (shown in italics) followed by two successive stop codons.
The pGEX-4T-2 vector and PCR products were double-digested separately with EcoRI and NotI enzymes (Invitrogen), gel purified, and ligated overnight at 16°C with T4 DNA ligase (Roche).
The recombinant vector was transfected into Escherichia coli BL-21 bacteria. Clones were recovered and grown in Trypticase-soy-casein (TSC) medium containing 100 μg of ampicillin per ml.
Expression and purification of GST-pVP7-6xHis.
Bacteria were grown in TSC-ampicillin to an optical density at 600 nm of 0.5, and then 0.5 mM isopropylthiogalactopyranoside (IPTG) was added for induction during 4 h at 37°C.
Bacteria were pelleted and processed with Bugbuster protein purification (Novagen). Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) showed that the fusion protein was found in the inclusion bodies. It was solubilized in 50 mM CAPS (3-[cyclohexylamino]-1-propane-sulfonic acid), 1 mM dithiothreitol, and 0.3% Sarkosyl and dialyzed overnight against 20 mM Tris-HCl (pH 8.5). The fusion protein was cut with thrombin at 16°C overnight [6.5 NIH units of thrombin (Diagnostica Stago) per ml of protein (at 2,000 μg/ml)] to cleave the glutathione S-transferase (GST) moiety. The cut protein was subjected to SDS-PAGE and electroblotted on a nitrocellulose membrane for testing reactivity with a 1:1,000 dilution of anti-CTF virus mouse hyperimmune ascitic fluid.
Preparation of ELISA plates. (i) Coating with anti-His-tag antibody.
Biotinylated anti-penta-His antibodies (Qiagen) were diluted in phosphate-buffered saline (PBS) to 1 μg/ml. Wells of streptavidin-coated strips (Roche) were incubated with 100 μl of biotinylated anti-penta-His antibodies (1 h at 37°C) and washed with PBS buffer (0.05% Tween 20). A mixture of biotin and bovine serum albumin (0.05% and 50 mg/ml in PBS, respectively) was used in a subsequent blocking step (37°C for 2 h).
(ii) Coating with pVP7-6xHis and storage.
The cut protein was diluted fourfold in 20 mM Tris-HCl (pH 8.5); 100 μl was added to the wells and incubated for 1 h at room temperature, followed by washing. Microplates were dried and stored in vacuum bags at 4°C. The reactivity to a standard human serum positive for anti-CTF virus antibodies was tested over 2 months.
ELISA. (i) Protocol.
Wells were rehydrated with 20 mM Tris-HCl (pH 8.5) containing 0.05% Tween 20, and 100 μl of serum samples diluted hundredfold in 5% skim milk in PBS was added at room temperature. Plates were incubated with 100 μl of a 1:3,000 dilution in milk of anti-human Fab-peroxidase (Jackson Immunoresearch). Detection was revealed with the one-component TMB (tetramethylbenzidine) substrate chromogen (Kirkegaard & Perry), as directed by the manufacturer.
(ii) Populations tested with pVP7-6xHis ELISA.
A serum positive for CTF virus antibodies [serum 10c, obtained from the Calisher set (7)] was used as an internal standard to allow interseries comparison. The intensity of the colorimetric reaction was expressed as a normalized optical density (NOD), where NOD = [(the optical density of the sample) − (the optical density of the blank reaction)]/[(the optical density of the internal standard) − (the optical density of the blank reaction)]. Frequency distribution of NOD was analyzed with the Systat 5.03 program (Systat Inc., Evanston, Ill.).
French blood donors.
A total of 368 randomized serum samples from blood donors living in the south of France were tested. These donors had never been to the Americas, and hence the probability of contact with CTF virus was very low.
Calisher set of serum samples.
Eighteen serum samples collected 16 years ago from individuals for whom a diagnosed CTF virus infection was reported (7) were tested. All except serum 42a were convalescent-phase serum samples. The presence of antibody to CTF virus and the titer of neutralizing antibody were determined by Calisher and colleagues (7). Serum 42a was collected 17 days after disease onset and could therefore be considered an intermediate-phase serum.
Western blot analysis.
The presence of anti-CTF virus antibody was confirmed or ruled out with the previously described Western blot test (2). Criteria for a positive Western blot were reactivities to at least three viral proteins, including reactivities to the 38-kDa and 95-kDa antigens.
RESULTS
Preparation of recombinant protein.
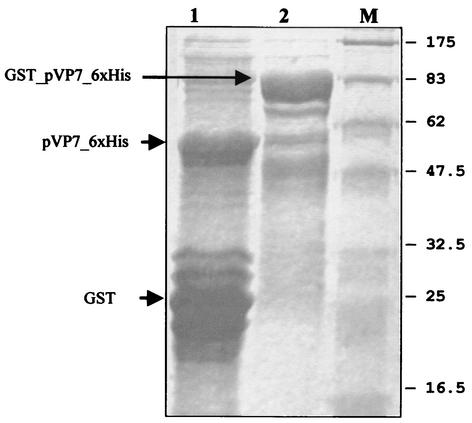
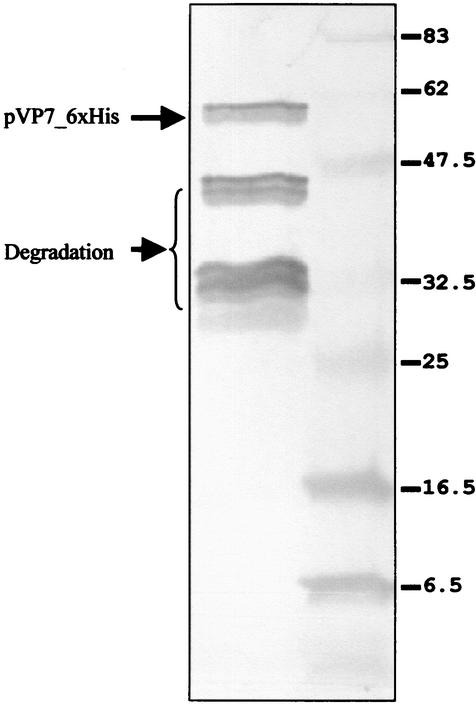
The theoretical molecular mass of the protein was calculated to be 71.5 kDa, while the apparent mass revealed by electrophoresis was 75 ± 1 kDa. This difference could be due to the lowering of mobility by the presence of 19% basic residues and 10% proline residues, known to decrease mobility in SDS-PAGE (15). The uncut fusion protein and the thrombin-cleaved proteins are shown in Fig. 1. The yield of the solubilized fusion protein was measured to be approximately 5,000 μg/ml. The protein was efficiently recognized by human and mouse antibodies to CTF virus. The Western blot reactivity of the cut protein to anti-CTF virus mouse ascites fluid is shown in Fig. 2.
FIG. 1.
Electrophoretic separation by SDS-PAGE of GST-pVP7-6xHis. Lane M shows molecular mass standards labeled (in kilodaltons); lane 1 shows the thrombin-cut protein (digestion realized at 16°C); lane 2 shows the GST-pVP7-6xHis fusion protein.
FIG. 2.
Western blot analysis of cut pVP7-6xHis. The protein was digested with thrombin at 30°C. Partial degradation products also reacted with anti-CTF virus mouse hyperimmune ascitic fluid. Sizes of molecular mass standards are shown (in kilodaltons).
Despite variation in the cleavage conditions (temperature of incubation and thrombin concentration), some degradation was observed upon treatment with thrombin (Fig. 2).
ELISA. (i) Determination of a cutoff value for ELISA.
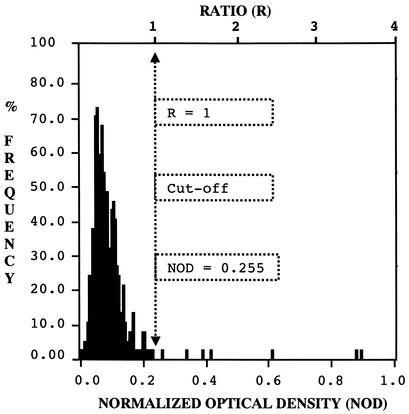
The population of 368 French blood donors was supposedly negative for anti-CTF virus antibodies. This was tested with the elaborated pVP7 ELISA. NODs followed a nearly normal distribution (Fig. 3), and the mean of all NOD values was 0.091, with a standard deviation of 0.082. According to the formula mean of all negative values + 2 standard deviations used for the calculation of the cutoff value (22), a normalized cutoff value of 0.255 was calculated. Among the tested population, seven serum samples (1.9%) had NOD values higher than the cutoff. These serum samples were tested by Western blotting against CTF virus proteins and found to be negative. Immunoblot results against the recombinant VP6 of Eyach virus were also negative. Consequently, all serum samples from the collection were considered true-negative for the presence of specific antibody to CTF virus. Based on these results, the specificity of the assay for the selected population was 98.1%.
FIG. 3.
Frequency distribution histogram of NOD values obtained with serum samples from French blood donors. The cutoff value (ratio or NOD) is indicated by a dotted vertical arrow.
ELISA results are subsequently expressed as a ratio (R, where R = NOD/cutoff).
(ii) Testing of Calisher set of serum samples.
According to the results of the Western blot analysis, all serum samples in the Calisher set had specific antibody to CTF virus. Serum 50c could not be tested by Western blotting due to insufficient volume. However, it had a low but significant titer of neutralizing antibody according to Calisher and colleagues (7). All other samples had either an IgG titer of >100 (with infected cell lysate as the antigen) or a neutralizing antibody titer of >10. Consequently, all serum samples from the collection were considered true-positive for the presence of specific antibody to CTF virus.
With the recombinant pVP7 ELISA, all serum samples had ratios ranging between 1.2 and 6.0 (Table 1). Based on these findings, the pVP7 ELISA provided 100% sensitivity for the population tested.
TABLE 1.
Results of serological assays performed on CTF virus antibody-positive sera: comparison of previously reported IgG and neutralizing antibody titers to Western blot analysis and ratios obtained with pVP7 ELISAa
| Serum no. | IgG titerb | NA titerb | WB result | Ratio | Status |
|---|---|---|---|---|---|
| 10cc | <100 | 1,280 | + | 4.0 | + |
| 21c | 400 | 128 | + | 1.5 | + |
| 22c | 400 | 128 | + | 4.8 | + |
| 23c | 800 | 320 | + | 2.9 | + |
| 25c | 1,600 | 128 | + | 5.4 | + |
| 31c | 800 | 160 | + | 6.0 | + |
| 32c | 3,200 | 128 | + | 3.5 | + |
| 33c | 400 | 320 | + | 2.1 | + |
| 34c | 400 | 160 | + | 3.6 | + |
| 37c | 800 | 160 | + | 2.2 | + |
| 38c | 3,200 | 160 | + | 5.3 | + |
| 40c | 200 | 10 | + | 2.8 | + |
| 42a | 200 | 80 | + | 1.2 | + |
| 42c | 400 | 320 | + | 2.2 | + |
| 43c | <100 | 320 | + | 3.4 | + |
| 44c | <100 | 320 | + | 3.3 | + |
| 48c | 12,800 | 1,280 | + | 6.0 | + |
| 50c | <100 | 20 | NT | 1.2 | + |
Letters following serum sample numbers indicate acute (a) and convalescent (c) phase. +, positive; −, negative; NT, not tested (not enough volume); NA, neutralizing antibody; WB, Western blot.
Data from Calisher et al. (7).
Serum used for interseries normalization.
DISCUSSION
Recombinant proteins have been used to design ELISAs for detecting antibodies to several viruses in the family Reoviridae. A recombinant VP6 rotavirus group C protein-based ELISA has been developed and used in diagnostic assays (8, 25). Among the orthoreoviruses, recombinant óB and óC proteins have been used in the diagnosis of avian reoviruses (19, 24). Serological tests based on recombinant VP7 and NS3 have also been developed for viruses of the genus Orbivirus (African horse sickness virus, epizootic hemorrhagic disease virus, and Palyam virus) (16, 21, 27).
We completely or partially expressed the VP6, VP7, VP9, VP10, VP11, and VP12 proteins of CTF virus in bacteria. The immunoreactivity of these recombinant proteins against a hyperimmune mouse ascitic fluid and a positive human serum showed that the pVP7 and pVP6 proteins were the most immunoreactive, with pVP7 showing the strongest reactivity. Because our purpose was to design a test highly specific for CTF virus, a fragment of VP7 (pVP7) that had only 49% amino acid identity with the corresponding sequence of the Eyach virus was chosen. The yield of the fusion protein GST-pVP7 (5,000 μg/ml) makes it a conveniently available antigen. Despite the expression of a truncated protein, the immunoreactivity proved to be excellent and permitted the specific detection of anti-CTF virus IgG antibodies in an ELISA format.
We analyzed a population of true-negative samples from French blood donors who never visited the Americas and a population of true-positive samples (with a positive Western blot and/or a significant titer of neutralizing antibody) with this test. A standard definition of the cutoff value resulted in a 1.9% false-positive rate for the blood donor population (confirmed by a negative Western blot) and 100% sensitivity for the population of CTF virus-infected patients. In the latter series of samples, which represent the only published reference set of human serum samples with a diagnosed CTF virus infection, three samples that had previously been declared negative for IgG but contained neutralizing antibodies (7) were found to be positive with the pVP7 ELISA (with ratios of >3). Two samples with a low titer of neutralizing antibodies were also found to be positive.
Altogether, these results suggest that this ELISA could be a useful tool for the epidemiological survey of CTF. The requirement of a Western blot confirmatory test for less than 2% of the serum samples tested is acceptable for this purpose. This test permits determination of the serological status of a population without special safety precautions and without any need to propagate the CTF virus. Besides the high yield of protein production, our method does not require extensive refolding of the protein. The immobilization of the protein through the 6xHis tag to the solid phase of the wells offers good accessibility for anti-VP7 antibodies to their target. There is no need to run a control GST ELISA (5, 26) to avoid the false-positive results caused by the presence of anti-GST antibodies, since the GST moiety is cleaved prior to coating. The dried protein plates kept under vacuum are stable over time (at least 2 months; data not shown), allowing large batches of microplates to be prepared. The use of the test for the detection of seroconversion and its adjustment to the detection of IgM antibody will require further evaluation with serum samples collected during the acute period of CTF. However, it is clear that reverse transcription-PCR detection of the CTF virus is the method of choice for diagnosis of acute CTF.
The generation of recombinant proteins for serological tests represents an interesting alternative to the conventional and hazardous method of using lysates of infected cells as the antigen. This study shows that a specific recombinant protein of the human pathogen CTF virus can be used as an antigen for detecting specific antibodies.
Acknowledgments
We acknowledge C. Calisher, N. Karabatsos, and A. J. Johnson for providing human serum samples.
This study was supported by EU Grant Reo ID number QLK2-2000-00143 and in part by the EFS-Alpes Mediterranée. The Unité des Virus Emergents is an associated research unit of the Institut de Recherche pour le Développement (IRD). This study was supported in part by the IRD.
REFERENCES
- 1.Attoui, H., P. De Micco, and X. de Lamballerie. 1997. Complete nucleotide sequence of Colorado tick fever virus segments M6, S1 and S2. J. Gen. Virol. 78:2895-2899. [DOI] [PubMed] [Google Scholar]
- 2.Attoui, H., F. Billoir, J. M. Bruey, P. de Micco, and X. de Lamballerie. 1998. Serologic and molecular diagnosis of Colorado tick fever viral infections. Am. J. Trop. Med. Hyg. 59:763-768. [DOI] [PubMed] [Google Scholar]
- 3.Attoui, H., F. Billoir, J. F. Cantaloube, P. Biagini, P. De Micco, and X. de Lamballerie. 2000. Sequence determination and analysis of the full-length genome of Colorado tick fever virus, the type species of genus Coltivirus (family Reoviridae). Biochem. Biophys. Res. Commun. 273:1121-1125. [DOI] [PubMed] [Google Scholar]
- 4.Attoui, H., F. Mohd Jaafar, P. Biagini, J. F. Cantaloube, P. de Micco, F. A. Murphy, and X. de Lamballerie. 2002. Genus Coltivirus (family Reoviridae): genomic and morphologic characterization of Old World and New World viruses. Arch. Virol. 147:533-561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brénière, S. F., M. F. Bosseno, F. Noireau, N. Yacsik, P. Liegeard, C. Aznar, and M. Hontebeyrie. 2002. Integrate study of a Bolivian population infected by Trypanosoma cruzi, the agent of Chagas disease. Mem. Inst. Oswaldo Cruz 97:289-295. [DOI] [PubMed] [Google Scholar]
- 6.Brown, S. E., and D. L. Knudson. 1995. Coltivirus infections, p. 329-342. In J. S. Porterfield (ed.), Exotic viral infections. Chapman and Hall, London, United Kingdom.
- 7.Calisher, C. H., J. D. Poland, S. B. Calisher, and L. A. Warmoth. 1985. Diagnosis of Colorado tick fever virus infection by enzyme immunoassays for immunoglobulin M and G antibodies. J. Clin. Microbiol. 22:84-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Castello, A. A., M. H. Arguelles, G. A. Villegas, A. Olthoff, and G. Glikmann. 2002. Incidence and prevalence of human group C rotavirus infections in Argentina. J. Med. Virol. 67:106-112. [DOI] [PubMed] [Google Scholar]
- 9.Chastel, C., A. J. Main, A. Couatarmanac'h, G. Le Lay, D. L. Knudson, M. C. Quillien, and J. C. Beaucournu. 1984. Isolation of Eyach virus (Reoviridae, Colorado tick fever group) from Ixodes ricinus and I. ventalloi ticks in France. Arch. Virol. 82:161-171. [DOI] [PubMed] [Google Scholar]
- 10.Draughn, D. E., O. F. Sieber, and H. J. Umlauf. 1965. Colorado tick fever encephalitis. Clin. Pediatr. 4:626-628. [DOI] [PubMed] [Google Scholar]
- 11.Emmons, R. W. 1988. Ecology of Colorado tick fever. Annu. Rev. Microbiol. 42:49-64. [DOI] [PubMed] [Google Scholar]
- 12.Emmons, R. W., and H. I. Schade. 1972. Colorado tick fever simulating acute myocardial infarction. JAMA 222:87-90. [DOI] [PubMed] [Google Scholar]
- 13.Florio, L., M. S. Miller, and E. R. Mugrage. 1950. Colorado tick fever. Isolation of the virus from Dermacentor andersoni in nature and laboratory study of the transmission of the virus in the tick. J. Immunol. 64:257-263. [PubMed] [Google Scholar]
- 14.Florio, L., M. Stewart, and E. R. Mugrage. 1946. The etiology of Colorado tick fever. J. Exp. Med. 83:1-10. [PMC free article] [PubMed] [Google Scholar]
- 15.Hames, B. D. 1998. Gel electrophoresis of proteins: a practical approach, 3rd ed., p. 13-33. Oxford University Press, Oxford, United Kingdom.
- 16.Idrissi Bourgine, S., O. Fassi Fihri, M. el Harrak, and M. M. Fassi Fehri. 1999. Use of the immunoenzyme test ELISA-NS3 to distinguish horses infected by African horsesickness virus from vaccinated horses. Res. Sci. Technol. 18:618-626. [PubMed] [Google Scholar]
- 17.Johnson, A., N. Karabtsos, and R. Lanciotti. 1997. Detection of Colorado tick fever virus by using reverse transcriptase PCR and application of the technique in laboratory diagnosis. J. Clin. Microbiol. 35:1203-1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klasco, R. 2002. Colorado tick fever. Tick-Borne Dis. 86:435-440. [DOI] [PubMed] [Google Scholar]
- 19.Liu, H. J., L. C. Kuo, Y. C. Hu, M. H. Liao, and Y. Y. Lien. 2002. Development of an ELISA for detection of antibodies to avian reovirus in chickens. J. Virol. Methods 102:129-138. [DOI] [PubMed] [Google Scholar]
- 20.Malkova, D., J. Holubova, J. M. Kolman, Z. Marhoul, F. Hanzal, H. Kulkova, K. Markvart, and L. Simkova. 1980. Antibodies against some arboviruses in persons with various neuropathies. Acta Virol. 24:298.. [PubMed] [Google Scholar]
- 21.Nagesha, H. S., A. R. Gould, J. R. White, and C. J. Duch. 1996. Expression of the major inner capsid protein of the epizootic haemorrhagic disease virus in baculovirus and potential diagnostic use. Virus Res. 43:163-169. [DOI] [PubMed] [Google Scholar]
- 22.Office International des Epizooties Standards Commission. 2000. Surra (Trypanosoma evansi), p. 686-689. In Manual of standards for diagnostic tests and vaccines, 4th ed. Office International des Epizooties, Paris, France. ([Online] www.oie.int/eng/normes/mmanual/A_summry.htm.)
- 23.Rehse-Küpper, B., J. Casals, E. Rehse, and R. Ackermann. 1976. Eyach, an arthropod-borne virus related to Colorado tick fever virus in the Federal Republic of Germany. Acta Virol. 20:339-342. [PubMed] [Google Scholar]
- 24.Shien, J. H., H. S. Yin, and L. H. Lee. 2000. An enzyme-linked immunosorbent assay for detection of antibodies to avian reovirus by using protein sigma B as the coating antigen. Res. Vet. Sci. 69:107-112. [DOI] [PubMed] [Google Scholar]
- 25.Steele, A. D., and V. L. James. 1999. Seroepidemiology of human group C rotavirus in South Africa. J. Clin. Microbiol. 37:4142-4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu, Y., A. Linde, S. Fredrikson, H. Dahl, and G. Winberg. 2002. HHV-6 A- or B-specific P41 antigens do not reveal virus variant-specific IgG or IgM responses in human serum. J. Med. Virol. 66:394-399. [DOI] [PubMed] [Google Scholar]
- 27.Yamakawa, M., and S. Furuuchi. 2001. Expression and antigenic characterization of the major core protein VP7 of Chuzan virus, a member of the Palyam serogroup orbiviruses. Vet. Microbiol. 83:333-341. [DOI] [PubMed] [Google Scholar]