Abstract
Human and bovine tuberculosis have long been detected by skin testing with purified protein derivative (PPD), a complex mix of partly denatured mycobacterial antigens with suboptimal specificity. In the present study, skin tests based on ESAT-6, a recombinantly produced antigen highly specific for tuberculosis infection, were investigated. Although ESAT-6 was strongly recognized in vitro and induced high levels of gamma interferon, initial investigations demonstrated that higher doses of ESAT-6 than of PPD were needed to induce substantial delayed-type hypersensitivity reactions. Also, the kinetics of the skin test response differed for the two reagents; PPD showed maximal response at 72 h, but the response to ESAT-6 often peaked later at 96 h. Tests based on an optimized strategy (400 μg of ESAT-6 measured between 72 and 96 h), in cattle infected with Mycobacterium bovis (n = 22) and animals sensitized by exposure to environmental mycobacteria showed ESAT-6 to have a promising diagnostic potential (sensitivity, 82%; specificity, 100%; optimal cutoff, 3 mm), compared with PPD (sensitivity, 86%; specificity, 90%; optimal cutoff, 4 mm). Larger investigations are required to refine cutoff points for any new diagnostic test, but the present results indicate great potential for skin tests based on specific antigens for accurate in vivo diagnosis of tuberculosis.
Tuberculosis is on the increase. It has been estimated that there are eight million new cases of infection with Mycobacterium tuberculosis in the human population annually (33). In cattle, infection with the very closely related M. bovis has been increasing in several national herds (15) and represents a zoonotic risk to human health and a significant barrier to trade. Consequently, better methods of tuberculosis diagnosis and control are required urgently. Such advances require a better understanding of the immune responses and antigenic targets recognized during tuberculosis infection.
Current methods for the control of bovine tuberculosis require identification and removal of infected individuals from the herd. Diagnosis is usually based on tuberculin skin tests involving the measurement of increases in skin fold thickness 72 h after intradermal injection of mycobacterial extracts termed purified protein derivatives (PPDs). Increases in skin thickness from a few millimeters to 20 mm or more are recorded regularly in cattle positive to the test. However, PPD does not always allow discrimination between cattle infected with virulent M. bovis and noninfected cattle sensitized by environmental mycobacteria. This is because of the complexity of these reagents and the sharing of antigenic components between pathogenic and nonpathogenic mycobacteria (2). Furthermore, immunization with M. bovis BCG is known to induce skin reactivity to PPD, and this factor has limited the potential of vaccination strategies for the control of bovine tuberculosis. Because of environmental sensitization, it has been estimated that an intradermal test based solely on PPD prepared from M. bovis (PPDB) would give false-positive results in up to 12% of the cattle in the United Kingdom and Ireland (20). Thus, the single intradermal comparative tuberculin test (SICTT), which compares skin responses to injections of PPDB and PPDA, the latter prepared from M. avium, is used where this is a problem. In the SICTT, for an animal to be classified as positive, the responses to PPDB must be greater than parallel, contemporaneous responses to PPDA by defined scales. Nevertheless, although the results of the SICTT constitute a good indication of mycobacterial exposure, even this test does not always discriminate between cattle with tuberculosis and those exposed to nonpathogenic organisms. Similar comparative tests have been proposed to allow discrimination between human patients infected with tuberculosis and those sensitized with M. avium (28). It can be envisaged, however, that a skin test based on a tuberculosis-specific reagent would provide clear benefits in the diagnosis of disease.
A replacement antigen for PPD, on which a test of improved specificity could be based, has been a long-standing research goal. Attempts to identify candidate antigens have led to the purification and characterization of many proteins from M. tuberculosis and M. bovis (1, 5, 8, 10, 19, 29, 34). Several such proteins have been investigated as candidate skin test reagents in guinea pig models of tuberculosis. These include MPT64 (6, 14, 16, 22), MPT59 (14), 38-kDa antigen (12-14), KatG, MPT32, MTC28, MPT51, MPT70, 19-kDa antigen, MPT63 (16), and ESAT-6 (7). In the guinea pig model some of these antigens have given very promising results. In this regard, Elhay et al. (7) found that skin testing with recombinant ESAT-6 and MPT64 could differentiate guinea pigs infected with M. tuberculosis from animals given M. bovis BCG or M. avium.
Efforts to replicate results obtained in guinea pigs for the diagnosis of human tuberculosis have so far been disappointing (30), and so far no purified antigens have been successfully tested as skin test reagents in cattle. Recently, a major research focus for tuberculosis diagnosis has been the gamma interferon (IFN-γ) test (32), where low-molecular-weight antigens such as ESAT-6 and CFP10 have been identified as having great potential (23, 25, 27). ESAT-6 has been considered to be particularly interesting because, although the esat-6 gene is present in M. tuberculosis and virulent M. bovis, it is absent from M. bovis BCG and major environmental mycobacteria (11). Indeed, this antigen has been shown to be a practical possibility for in vitro diagnosis of bovine tuberculosis, having an estimated sensitivity of up to 88% coupled with very high specificity (4, 24).
This report details the responses which can be elicited by the intradermal injection of ESAT-6 in cattle infected with M. bovis. The potential of skin testing with this antigen to discriminate cattle naturally infected with M. bovis from tuberculosis-free cattle, naturally sensitized to PPD by exposure to environmental mycobacteria, is also reported.
MATERIALS AND METHODS
Cattle.
All animal experiments were conducted following guidelines established by the ethical committees of the appropriate institution.
Experimental infection of cattle with M. bovis.
Groups of up to six Friesian-cross animals approximately 6 months of age were obtained from tuberculosis-free herds which had maintained that status for at least the previous 5 years and were used for individual skin test experiments. The calves were negative for in vitro immune responses to M. bovis antigens (IFN-γ release and lymphocyte proliferation) prior to experimental challenge. They were housed in isolation and were infected via the respiratory route with virulent M. bovis (T/91/1378; Veterinary Sciences Division, Belfast, United Kingdom) as described previously (21). Following infection, the animals developed measurable in vitro cellular immune responses to PPDB as indicated by the results of the IFN-γ test by approximately 4 weeks postchallenge. Skin tests were performed within 30 weeks of infection. Uninfected calves from the same source were kept in conventional housing for use as controls.
PPD-responsive natural cases of bovine tuberculosis.
Nine mixed-breed cattle approximately 2 years of age, which had been selected based on SICTT positivity in herds known to be infected with M. bovis, were transported to the cattle holding facility at Abbotstown, Dublin, Ireland. These animals had been used for two assays to control the quality of PPDB over a period of 7 months. No skin tests had been performed for 5 months prior to the present experiment.
Cattle sensitized to PPD by exposure to environmental mycobacteria.
Six cattle of mixed breeds were obtained from a tuberculosis-free source where cattle had previously and repeatedly exhibited a high incidence of nonspecific reactions to PPD due to environmental sensitization. No skin tests had been performed on these animals for 3 months prior to this experiment, but significant immune responsiveness to PPDA had been confirmed by in vitro tests for release of IFN-γ.
IFN-γ test.
In vitro T-cell responsiveness to mycobacterial antigens was assessed by measuring specific release of IFN-γ. Whole-blood cultures were established in microtiter plates and individual wells were stimulated with antigen (PPD and ESAT-6; 4 μg/ml) for 24 h as previously described (24). Levels of IFN-γ in culture supernatants were measured by enzyme-linked immunosorbent assay (CSL, Parkville, Australia) and expressed as optical density indices (ODI), which are defined as the ratio of OD from stimulated cultures to OD from nonstimulated (phosphate-buffered saline) cultures.
Postmortem examinations.
All the animals used in the experiments reported here were euthanized and subjected to postmortem examination. Animals were confirmed as being infected with tuberculosis on the basis of the presence of macroscopically and microscopically apparent caseous lymphadenitis followed by formal cultural isolation of M. bovis from the affected tissues. The animals which were naturally sensitized to PPD by exposure to environmental mycobacteria were confirmed to be free of M. bovis infection on the basis of the absence of detectable tuberculous lesions at slaughter and by cultural examination.
Recombinant ESAT-6.
The ESAT-6 reagent used for skin testing in this study was a dimeric recombinant construct, which was cloned, expressed, and purified directly from the culture supernatant of Lactococcus lactis strain PSM631 grown in a defined medium containing no components of animal origin. PSM631 (Biotechnological Institute, Horsholm, Denmark), a mutant strain of MG1363 (9), was transformed with pAMJ752ESAT-6. A growth-phase-dependent promoter (P170) up-regulated the synthesis of ESAT-6 at a constant pH of 6.0 during the transition to stationary phase (17), and a signal sequence from the usp45 gene promoted secretion (26). Compared to the monomeric molecule, dimeric ESAT-6 contains six additional amino acids: aspartic acid, threonine, arginine and serine in the N-terminal end and arginine and serine between the two monomers.
Skin testing.
Intradermal tests were performed in the skin of the neck (midcervical) using several sites per animal. Skin sites were clipped free of hair, and pretest skin fold thicknesses were measured using calipers. The skin test reagents to be investigated as appropriate for each experiment (ESAT-6, PPDB, or PPDA) were then injected intradermally at separate sites using 27-gauge hypodermic needles. PPDs were obtained from VLA, Weybridge, United Kingdom. At various times postinjection (up until 120 h), skin fold thicknesses were again measured and responses were recorded as increases in skin thickness in millimeters.
Statistical interpretation.
All analyses were performed using one-way analysis of variance with Tukey-Kramer multiple comparisons test or a two-tailed paired t test (GraphPad Instat; GraphPad Software, San Diego, Calif.).
RESULTS
Comparison of skin test and IFN-γ responses to ESAT-6 and PPDB in cattle experimentally infected with M. bovis.
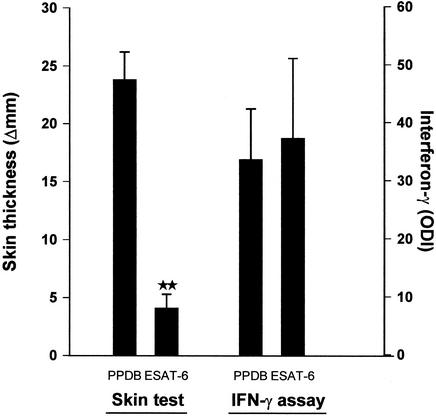
Dimeric recombinant ESAT-6 was expressed in L. lactis and tested in parallel with PPDB for cell-mediated immunity recognition in vitro and in vivo. Blood samples were obtained from five animals which had been experimentally infected with M. bovis and whole blood IFN-γ responses to ESAT-6 and PPDB evaluated after 24 h of incubation. All of the animals responded strongly to both ESAT-6 and PPDB in vitro with IFN-γ levels to the two antigens of similar magnitude in the range from 30 to 40 ODI (Fig. 1). Having ensured that the all animals were ESAT-6 responsive, skin test responses to the two reagents were then investigated. The animals were tested on separate skin sites with equal quantities (100 μg) of each reagent, and responses were measured at 72 h. The animals responded strongly to PPDB, but skin test responses to ESAT-6 were significantly lower (Fig. 1). The mean skin response to ESAT-6 was 4.1 mm, compared to almost 25 mm seen in reactions to PPDB. However, reactions of a few millimeters can easily be recognized and measured in cattle, and the response to ESAT-6 was specific, as no detectable delayed-type hypersensitivity responses were found in naïve animals (results not shown).
FIG. 1.
Comparison of skin test responses and in vitro IFN-γ responses induced by PPDB and ESAT-6 in five animals experimentally infected with M. bovis. Blood samples were stimulated in vitro with antigen (4 μg/ml) for IFN-γ responses, and antigens (100 μg) were injected intradermally at separate sites in the cervical skin for skin test responses. Results are presented as means (+ standard errors of the mean [error bars]) of ODI for IFN-γ responses and increases in skin thickness at 72 h postinjection for skin test responses. **, mean skin responses to PPDB and ESAT-6 significantly different (P < 0.01).
Titration of ESAT-6 skin responses in cattle experimentally infected with M. bovis.
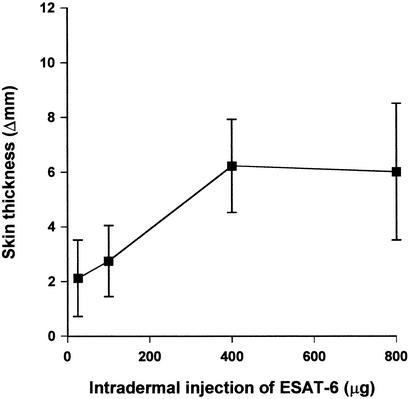
PPDB is normally used at a dose of 100 μg for skin tests in cattle, but the optimal dose for a small purified molecule like ESAT-6 may be completely different. Dimeric ESAT-6 was therefore titrated in the dose range 25 to 800 μg, and the effect of the quantity of ESAT-6 injected on the magnitude of the skin response was monitored in four M. bovis-infected cattle (Fig. 2). Increasing the dose of ESAT-6 from 25 to 400 μg resulted in a threefold increase in skin response, an effect which, although not statistically significant, turned reactions from barely measurable to clearly positive as measured at 72 h (from a mean increase in skin thickness of 2 to 6 mm). Increasing the dose beyond 400 μg did not produce a greater increase in skin thickness. Therefore, 400 μg of ESAT-6 was chosen for further investigation.
FIG. 2.
Titration of skin response to increasing quantities of ESAT-6 in four animals experimentally infected with M. bovis. Different cervical skin sites were used for individual doses, and results are presented as means (+ standard errors of the mean [error bars]) of increases in skin thickness at 72 h postinjection.
Kinetics of response to ESAT-6 and PPDB in experimentally infected cattle.
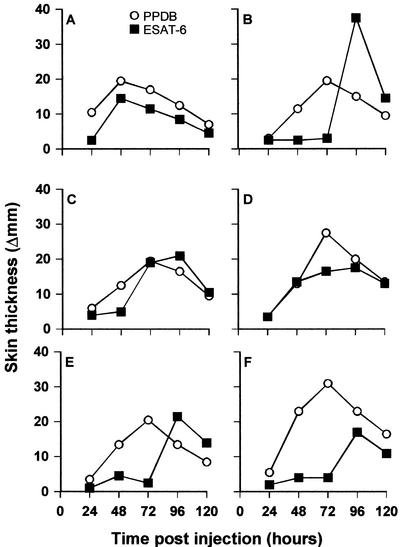
Skin test reactions induced by PPD normally peak after 72 h and are therefore routinely read at this time point. To evaluate if skin test responses to a highly purified reagent follow the same kinetics, six cattle which had been experimentally infected with M. bovis received intradermal injections at separate sites with 400 μg of ESAT-6 and 100 μg of PPDB. Skin thicknesses were measured and compared for the two preparations up until 120 h postinjection (Fig. 3). In general, responses to PPDB peaked at 72 h postinjection, in accordance with conventional application of the SICTT. Skin test responses to ESAT-6 were somewhat delayed compared to PPD and peaked at 96 h after injection in four out of six animals. In some animals, responses to ESAT-6 varied strikingly depending on the time of measurement with responses which were negligible at 72 h becoming clearly positive at 96 h postinjection.
FIG. 3.
Kinetics of skin responses to PPDB and ESAT-6 in six animals experimentally infected with M. bovis. Each animal was injected intradermally at separate skin sites with 100 μg of PPDB and 400 μg of ESAT-6. Results are presented as increases in skin thickness between 24 and 120 h postinjection.
Skin test responses to ESAT-6 and PPD in cattle infected with M. bovis and in cattle exposed to environmental mycobacteria.
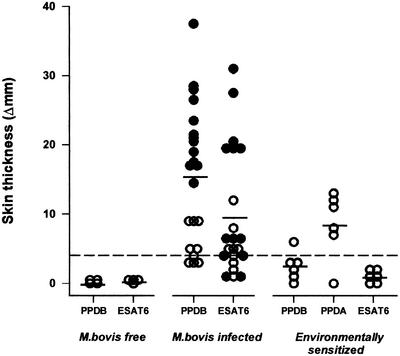
The diagnostic potential of skin testing with ESAT-6 using the optimized preparation was evaluated by tests performed in several categories of animal (Fig. 4). Because of the findings of the kinetic studies, skin test responses were read up to three times for each animal (between 48 and 96 h), and maximal responses are presented in the figure. As expected, noninfected cattle, which were from a tuberculosis-free source, did not respond to either ESAT-6 or PPDB. Of the 22 animals which were infected with M. bovis (experimentally or naturally) 19 gave positive (≥4 mm) skin responses to PPDB, with increases in skin thicknesses ranging up to 37.5 mm. Considering a similar increase in skin thickness as a positive response, 17 of these animals also gave positive skin test reactions with ESAT-6, with responses up to 31 mm. Interestingly, the naturally infected cattle had lower overall skin responsiveness, but similar numbers (six out of nine) were positive (≥4 mm) for both skin test reagents. When cattle from a source known to produce nonspecific responses to PPD were investigated, none had positive responses to ESAT-6. This was despite showing large responses to PPDA (up to 13 mm). Indeed, one of these animals responded positively to PPDB (6 mm) but remained negative for ESAT-6.
FIG. 4.
Skin responses to PPD and ESAT-6 for animals which were (i) M. bovis-free (n = 4), (ii) M. bovis-infected (experimentally [n = 13] [closed symbols] or naturally [n = 9] [open symbols]), or (iii) environmentally sensitized (n = 6). Responses are presented as the maximal increases in skin thickness detected between 48 and 96 h postinjection.
The effects of selecting different cutoff points on test parameters were investigated (Table 1). Considering the M. bovis-infected cattle of the present study, PPDB had a sensitivity between 81.8 and 100%, while ESAT-6 had a sensitivity between 63.6 and 81.8% using the same cutoffs. However, in terms of accuracy within noninfected cattle, ESAT-6 performs much more effectively, having a specificity of 100% at all cutoffs, compared with a best value of 90% for PPDB. These observations indicate promising diagnostic potential for ESAT-6 as a skin test reagent using a cutoff of 3 mm (sensitivity, 82%; specificity, 100%), compared with PPDB with the 4-mm cutoff (sensitivity, 86%; specificity 90%). Importantly, lowering the cutoff for ESAT-6 to 3 mm did not jeopardize test specificity, while a similar stringent test for PPDB resulted in an unsatisfactory specificity of 70%.
TABLE 1.
Effect of selected cutoff points on ability of skin tests with PPDB and ESAT-6 to correctly identify tuberculosis- free and tuberculosis-infected cattle
| Cutoff value (mm) | % Test specificity for tuberculosis-free cattlea
|
% Test sensitivity for tuberculosis-infected cattleb
|
||
|---|---|---|---|---|
| PPDB | ESAT-6 | PPDB | ESAT-6 | |
| ≥3 | 70 | 100 | 100 | 81.8 |
| ≥4 | 90 | 100 | 86.4 | 77.3 |
| ≥5 | 90 | 100 | 81.8 | 63.6 |
Animals from M. bovis-free or environmentally sensitized sources (n = 10).
Animals naturally or experimentally infected with M. bovis (n = 22).
DISCUSSION
The tuberculin skin test has been a useful diagnostic and epidemiological tool for tuberculosis monitoring in humans and cattle for many years (20). Despite this, there are major practical and theoretical problems with the skin test as it exists at present. Lack of absolute diagnostic accuracy is associated with false-positive reactions and indefinite responses. PPD itself is a poorly defined cocktail of antigens, meaning that the present test does not discriminate clearly between individuals infected with tuberculosis and those sensitized by vaccination or exposure to environmental mycobacteria (3). The present study focused on ESAT-6 since the esat-6 gene is known to be selectively expressed in M. tuberculosis and virulent M. bovis and this protein has been shown recently to have a major potential for in vitro diagnosis of tuberculosis in both humans and cattle (23, 25, 27).
One obvious difference between PPD and ESAT-6 is physical composition: PPD is a complex, partly denatured mix of protein and nonprotein components (2), while ESAT-6 is a highly purified single polypeptide. In the present investigation, ESAT-6 was initially found to give much lower levels of skin responses than equal quantities of PPD, although the preparations gave the same level of in vitro responses. This may be due to the small size of the ESAT-6 molecule, which, compared to PPD, may lead to rapid diffusion away from the injection site and necessitate that relatively large quantities of that protein are required. However, in addition to size, another factor which may be involved in the mechanism as to why more ESAT-6 than PPDB is required to elicit a significant skin test response is the crude nature of PPD versus a purified molecule like ESAT-6. In this regard, one particularly interesting finding of the present study is the delayed kinetics of skin test responses to ESAT-6 compared to PPD. This may be due to proinflammatory factors present in PPD which are missing from ESAT-6. PPD is derived from partly degraded mycobacteria, and it is well known that the mycobacterial cell wall contains a variety of highly stimulatory lipids and sugars which may influence cytokine and chemokine networks (18, 31). Although the full explanation for the present observation is unclear, the indication is that reading skin test responses at a single time point, and in particular at the standard 72 h, is likely to compromise the sensitivity of a skin test based on purified antigens like ESAT-6.
Overall, the results of the present study indicate significant potential for ESAT-6 as a skin test reagent for tuberculosis. 77% of cattle infected with M. bovis (experimentally or naturally) reacted positively to ESAT-6 while 86% reacted to PPDB (change in skin fold thickness ≥ 4 mm). Importantly, ESAT-6 did not give positive skin responses in cattle sensitized with environmental mycobacteria. Indeed, one such animal had positive responsiveness to PPDB, but did not respond to ESAT-6. This indicates that the ESAT-6 reagent has potential in the development of a skin test with greatly enhanced specificity. In some circumstances, for example as a screening test in regions with no known tuberculosis but a high prevalence of environmental mycobacteria, a slight decrease in test sensitivity may be acceptable when the benefit is enhanced specificity or circumvention of the need for a comparative test. There has been considerable interest in tuberculosis diagnosis using blood tests and specific antigens. However, a skin test based on reagents such as ESAT-6 may be of great benefit as it requires no additional laboratory facility or transportation of fresh blood sample requiring urgent processing.
In conclusion, this study has shown that it is possible to generate skin responses with highly purified antigens like ESAT-6 in cattle. In view of the many problems of cross-reactivity and nonspecificity of complex antigens such as PPD, it is encouraging that a single antigen can be used to induce highly specific skin responses. Investigations are merited to determine if it is feasible to develop tests with sufficient sensitivity based on ESAT-6 alone or if combinations of specific antigens are needed. Also, large and carefully controlled trials are indicated to further investigate the practical applicability of these new approaches and accurately determine their diagnostic performance.
Acknowledgments
We thank D. Mackie, D. Bryson, and B. Gangadharan for their assistance with these studies. Frances Quigley and staff of the mycobacteriology section at Abbotstown are thanked for culturing tissues. Rodat Cunningham is also thanked for assistance in preparation of the manuscript.
Financial support was from the Department of Agriculture and Rural Development in Northern Ireland, the Department of Agriculture and Food in Dublin, Statens Seruminstitut in Denmark, and EU project ICA4-CT-2000-30023.
REFERENCES
- 1.Andersen, A. B. 1994. Mycobacterium tuberculosis proteins. Danish. Med. Bull. 41:205-215. [PubMed] [Google Scholar]
- 2.Andersen, A. B., and P. Brennan. 1994. Proteins and antigens of Mycobacterium tuberculosis, p. 307-332. In B. R. Bloom (ed.), Tuberculosis: pathogenesis, protection and control. American Society for Microbiology, Washington, D.C.
- 3.Andersen, P., M. E. Munk, J. M. Pollock, and T. M. Doherty. 2000. Specific immune-based diagnosis of tuberculosis. Lancet 356:1099-1104. [DOI] [PubMed] [Google Scholar]
- 4.Buddle, B. M., T. J. Ryan, J. M. Pollock, P. Andersen, and G. W. de Lisle. 2001. Use of ESAT-6 in the interferon-gamma test for diagnosis of bovine tuberculosis following skin testing. Vet. Microbiol. 80:37-46. [DOI] [PubMed] [Google Scholar]
- 5.Closs, O., M. Harboe, N. H. Axelsen, K. Bunch-Christensen, and M. Magnusson. 1980. The antigens of Mycobacterium bovis, strain BCG, studied by crossed immunoelectrophoresis: a reference system. Scand. J. Immunol. 12:249-263. [DOI] [PubMed] [Google Scholar]
- 6.De Bruyn, J., R. Bosmans, M. Turneer, M. Weckx, J. Nyabenda, J. P. Van Vooren, P. Falmagne, H. G. Wiker, and M. Harboe. 1987. Purification, partial characterization, and identification of a skin-reactive protein antigen of Mycobacterium bovis BCG. Infect. Immun. 55:245-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elhay, M. J., T. Oettinger, and P. Andersen. 1998. Delayed-type hypersensitivity responses to ESAT-6 and MPT64 from Mycobacterium tuberculosis in the guinea pig. Infect. Immun. 66:3454-3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fifis, T., J. S. Rothel, and P. R. Wood. 1994. Soluble Mycobacterium bovis protein antigens: studies on their purification and immunological evaluation. Vet. Microbiol. 40:65-81. [DOI] [PubMed] [Google Scholar]
- 9.Gasson, M. J. 1983. Plasmid complements of Streptococcus lactis NCDO 712 and other lactic streptococci after protoplast-induced curing. J. Bacteriol. 154:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harboe, M., S. Nagai, M. E. Patarroyo, M. L. Torres, C. Ramirez, and N. Cruz. 1986. Properties of proteins MPB64, MPB70 and MPB80 of Mycobacterium bovis BCG. Infect. Immun. 52:293-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harboe, M., T. Oettinger, H. G. Wiker, I. Rosenkrands, and P. Andersen. 1996. Evidence for occurrence of the ESAT-6 protein in Mycobacterium tuberculosis and virulent Mycobacterium bovis and for its absence in Mycobacterium bovis BCG. Infect. Immun. 64:16-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harboe, M., and H. G. Wiker. 1992. The 38kDa protein of Mycobacterium tuberculosis: a review. J. Infect. Dis. 166:874-884. [DOI] [PubMed] [Google Scholar]
- 13.Haslov, K., A. B. Andersen, L. Ljungqvist, and M. W. Bentzon. 1990. Comparison of the immunological activity of five defined antigens from Mycobacterium tuberculosis in seven inbred guinea pigs. The 38kDa antigen is immunodominant. Scand. J. Immunol. 31:503-514. [DOI] [PubMed] [Google Scholar]
- 14.Haslov, K., R. Andersen, S. Nagai, A. Gottschau, T. Sorensen, and P. Andersen. 1995. Guinea-pig cellular immune-responses to proteins secreted by Mycobacterium tuberculosis. Infect. Immun. 63:804-810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krebs, J. R., R. Andersen, T. Clutton-Brock, I. Morrison, D. Young, and C. Donnelly. 1997. Bovine tuberculosis in cattle and badgers. MAFF publications, London, United Kingdom.
- 16.Lyashchenko, K., C. Manca, R. Colangeli, A. Heijbel, A. Williams, and M. L. Gennaro. 1998. Use of Mycobacterium tuberculosis complex-specific antigen cocktails for a skin test specific for tuberculosis. Infect. Immun. 66:3606-3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Madsen, S. M., J. Arnau, A. Vrang, M. Givskov, and H. Israelsen. 1999. Molecular characterization of the pH-inducible and growth phase-dependent promoter P170 of Lactococcus lactis. Mol. Microbiol. 32:75-87. [DOI] [PubMed] [Google Scholar]
- 18.Masihi, K. N., W. Brehmer, I. Azuma, W. Lange, and S. Muller. 1984. Stimulation of chemiluminescence and resistance against aerogenic influenza infection by synthetic muramyl dipeptide combined with trehalose dimycolate. Infect. Immun. 43:233-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Minden, P., P. J. Kelleher, J. H. Freed, L. D. Nielsen, P. J. Brennan, L. McPheron, and J. K. McClatchy. 1984. Immunological evaluation of a component isolated from Mycobacterium bovis BCG with a monoclonal antibody to M. bovis BCG. Infect. Immun. 46:519-525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Monaghan, M. L., M. L. Doherty, J. D. Collins, J. F. Kazda, and P. J. Quinn. 1994. The tuberculin test. Vet. Microbiol. 40:111-124. [DOI] [PubMed] [Google Scholar]
- 21.Neill, S. D., J. Hanna, O' Brien, and R. M. McCracken. 1988. Excretion of Mycobacterium bovis by experimentally infected cattle. Vet. Rec. 123:340-343. [DOI] [PubMed] [Google Scholar]
- 22.Oettinger, T., A. Holm, I. M. Mtoni, A. B. Andersen, and K. Haslov. 1995. Mapping of the delayed-type hypersensitivity-inducing epitope of secreted protein MPT64 from Mycobacterium tuberculosis. Infect. Immun. 63:4613-4618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pollock, J. M., and P. Andersen. 1997. The potential of the ESAT-6 antigen secreted by virulent mycobacteria for specific diagnosis of tuberculosis. J. Infect. Dis. 175:1251-1254. [DOI] [PubMed] [Google Scholar]
- 24.Pollock, J. M., R. M. Girvin, K. A. Lightbody, R. A. Clements, S. D. Neill, B. M. Buddle, and P. Andersen. 2000. Assessment of defined antigens for the diagnosis of bovine tuberculosis in skin test-reactor cattle. Vet. Rec. 146:659-665. [DOI] [PubMed] [Google Scholar]
- 25.Ravn, P., A. Demissie, T. Eguale, H. Wondwosson, D. Lein, H. A. Amoudy, A. S. Mustafa, A. K. Jensen, A. Holm, I. Rosenkrands, F. Oftung, J. Olobo, F. von Reyn, and P. Andersen. 1999. Human T cell responses to the ESAT-6 antigen from Mycobacterium tuberculosis. J. Infect. Dis. 179:637-645. [DOI] [PubMed] [Google Scholar]
- 26.Van Asseldonk, M., G. Rutten, M. Oteman, R. J. Siezen, W. M. de Vos, and G. Simons. 1990. Cloning of usp45, a gene encoding a secreted protein from Lactococcus lactis subsp. lactis MG1363. Gene 95:155-160. [DOI] [PubMed] [Google Scholar]
- 27.van Pinxteren, L. A. H., P. Ravn, E. M. Agger, J. Pollock, and P. Andersen. 2000. Diagnosis of tuberculosis based on the two specific antigens ESAT-6 and CFP10. Clin. Diagn. Lab. Immunol. 7:155-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.von Reyn, C. F., D. E. Williams, C. R. Horsburgh, A. S. Jaeger, B. J. Marsh, K. Haslov, and M. Magnusson. 1998. Dual skin testing with Mycobacterium avium sensitin and purified protein derivative to discriminate pulmonary disease due to M. avium complex from pulmonary disease due to Mycobacterium tuberculosis. J. Infect. Dis. 177:730-736. [DOI] [PubMed] [Google Scholar]
- 29.Wiker, H. G., K. P. Lyashchenko, A. M. Aksoy, K. A. Lightbody, J. M. Pollock, S. V. Komissarenko, S. O. Bobrovnik, I. N. Kolesnikova, L. O. Mykhalsky, M. L. Gennaro, and M. Harboe. 1998. Immunochemical characterization of the MPB70/80 and MPB83 proteins of Mycobacterium bovis. Infect. Immun. 66:1445-1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilcke, J. T. R., B. N. Jensen, P. Ravn, A. B. Andersen, and K. Haslov. 1996. Clinical evaluation of MPT-64 and MPT-59, two proteins secreted from Mycobacterium tuberculosis, for skin test reagents. Tuber. Lung Dis. 77:250-256. [DOI] [PubMed] [Google Scholar]
- 31.Wilson, M., R. Seymour, and B. Henderson. 1998. Bacterial perturbation of cytokine networks. Infect. Immun. 66:2401-2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wood, P. R., and J. S. Rothel. 1994. In vitro immunodiagnosis assays for bovine tuberculosis. Vet. Microbiol. 40:125-135. [DOI] [PubMed] [Google Scholar]
- 33.World Health Organisation. 1992. Memorandum from a W. H. O. meeting: tuberculosis control and research strategies for the 1990s. Bull. W. H. O. 70:17-21. [PMC free article] [PubMed] [Google Scholar]
- 34.Young, D. B., S. H. E. Kaufmann, P. W. M. Hermans, and J. E. R. Thole. 1992. Mycobacterial protein antigens: a compilation. Mol. Microbiol. 6:133-145. [DOI] [PubMed] [Google Scholar]