Abstract
A yellow-pigmented coryneform rod was isolated from the blood of a child with acute lymphoblastic leukemia who was perfused with a central venous catheter. The culture bottles were positive twice, at a 2-month interval. The isolate was identified as a Microbacterium sp. and studied along with five other similar strains. Phenotypic, chemotaxonomic, and genetic characteristics indicated that they are closely related to Microbacterium oxydans but that they belong to a distinct species, for which the name Microbacterium paraoxydans sp. nov. is proposed. The type strain of M. paraoxydans is CF36T = DSM 15019T. The G+C content of its DNA is 69.9 mol%.
CASE REPORT
A 5-year-old boy was diagnosed with acute lymphoblastic leukemia in December 1994 and included in the Fralle 93 protocol. The child achieved hematologic remission 6 weeks after induction chemotherapy was initiated. He was seen as an outpatient and responded well to treatment in the following years. However, at a March 1997 consultation, the patient had a febrile episode when being perfused with the Port-a-cath system. Clinical examination showed no septic localization. The leukocyte count was 10,860/ml, with an absolute neutrophil count of 8,850/ml. One set of blood cultures was collected from the Port-a-cath, and the aerobic bottle culture yielded yellow-pigmented colonies of gram-positive coryneform rods. The boy received ceftriaxone (1 g) intravenously once, followed by cefadroxil 500 mg twice a day for 7 days. Because of a persistent fever, cefadroxil was given for another week and immunosuppressive drugs were discontinued during the same period. The patient's history was unremarkable for the next 2 months, and no blood culture was performed when he came for his monthly visits. In June 1997, the child was presented for consultation as subfebrile and complaining of fatigue. A physical examination revealed no focus of infection, and the leukocyte count was not elevated. One set of blood cultures was taken from the Port-a-cath, and the aerobic vial revealed the same gram-positive coryneform rods, subsequently identified as Microbacterium sp. Catheter-related bacteremia was diagnosed, and the central venous catheter was removed. Ampicillin (100 mg/kg/day) was given intravenously perioperatively, with the first dose given 24 h prior to surgery. The patient's clinical condition improved rapidly afterwards. The Port-a-cath was cultured on Columbia blood agar. Within 24 h at 37°C, a pure culture of yellow-pigmented colonies of a nonfermentative, gram-positive, somewhat discolored rod had grown. Unfortunately, subcultures were lost and no further examination could be performed.
The strain isolated from the patient's blood cultures, labeled CF36, was a yellow-pigmented, motile coryneform with an oxidative metabolism, proteolytic activity, and cellular fatty acids of the branched type. These general features are suggestive of the genus Microbacterium, which includes both fermentative and oxidative species, the latter formerly called Aureobacterium (13).
The 16S rRNA gene (rDNA) sequence was studied by using one set of primers for amplification. PCR products were purified from agarose gel with a QIAquick gel extraction kit (Qiagen, Westburg, The Netherlands). Sequence analysis was performed with a 3100 automatic DNA sequencer (Perkin-Elmer Applied Biosystems, Foster City, Calif.) by using the ABI Prism Big Dye Terminator Cycle Sequencing Ready Reaction kit. Each sequence was compared to the sequence data available in databases by using BLAST (10). A phylogenetic tree was constructed by the neighbor-joining method (8, 11).
16S rDNA sequencing based on 1,490 nucleotides of strain CF36 revealed the highest similarity levels of 99.5% with the sequence of M. luteolum DSM 20143T and 99.3% with the sequence of M. oxydans DSM 20578T. It was also closely related to the sequences of M. saperdae (99.2%) and M. liquefaciens (99.1%) (Table 1). Four other similar strains were collected from various samples from other patients that were labeled CF7 isolated from one blood culture, CF34 from a bronchial aspirate, CF130 from one blood culture, and CF40 from an unknown human source. One additional strain, CF128, was obtained from a vegetable sample. The five strains showed 16S rDNA sequences similar to that of the type strain of M. oxydans, ranging from 99.3 to 99.4% similarity, whereas their rates of similarity to CF36 were 99.9 to 100%. Nine other clinical isolates exhibited 99.9 to 100% similarity to the type strain of M. oxydans.
TABLE 1.
16S rDNA sequence similarity between M. paraoxydans CF36T and other Microbacterium speciesa
| Microbacterium sp. | Accession no. | 16S rDNA similarity to CF36T (%) |
|---|---|---|
| Microbacterium arabinogalactanolyticum | Y17228 | 97.7 |
| Microbacterium arborescens | X77443 | 95.1 |
| Microbacterium barkeri | X77446 | 94.2 |
| Microbacterium gubbeenense | AF263563 | 93.2 |
| Microbacterium imperiale | X77442 | 95.0 |
| Microbacterium esteraromaticum | Y17231 | 98.0 |
| Microbacterium foliorum | AJ249780 | 98.5 |
| Microbacterium phyllosphaerae | AJ277840 | 98.3 |
| Microbacterium keratanolyticum | Y17233 | 98.0 |
| Microbacterium liquefaciens | X77444 | 99.1 |
| Microbacterium oxydans | Y17227 | 99.3 |
| Microbacterium saperdae | Y17236 | 99.2 |
| Microbacterium luteolum | Y17235 | 99.5 |
| Aureobacterium resistens | Y14699 | 98.3 |
| Microbacterium testaceum | X77445 | 98.6 |
| Microbacterium dextranolyticum | Y17230 | 97.2 |
| Microbacterium laevaniformans | Y17234 | 97.6 |
| Microbacterium maritypicum | AB004713 | 97.8 |
| Microbacterium flavescens | Y17232 | 97.2 |
| Microbacterium lacticum | X77441 | 97.5 |
| Microbacterium schleiferi | Y17237 | 97.7 |
| Microbacterium aurum | Y17229 | 96.9 |
| Microbacterium terregens | Y17239 | 95.7 |
| Microbacterium halophilum | AB004715 | 97.7 |
| Microbacterium thalassium | AB004713 | 97.9 |
| Microbacterium ketosireducens | AB004724 | 97.4 |
| Microbacterium terrae | Y17238 | 96.9 |
| Microbacterium kitamiense | AB013907 | 97.2 |
| Microbacterium aurantiacum | AB004726 | 97.0 |
| Microbacterium chocolatum | AB004725 | 96.7 |
| Microbacterium trichothecenolyticum | Y17240 | 97.1 |
| Microbacterium hominis | AB004727 | 97.0 |
1,490 nucleotides were compared.
These results prompted us to perform an extensive study of the following six strains: CF36, CF7, CF34, CF40, CF128, and CF130.
Cellular fatty acids, determined as described previously (14), showed the same profile in all strains. The average percentages of the main cellular fatty acids were as follows: 40.3% anteiso-C15:0 (range, 37.1 to 46.0%), 27% anteiso-C17:0 (range, 22.9 to 31.3%), 15.7% iso-C16:0 (range, 13.8 to 19.3%), 9.3% iso-C15:0 (range, 4.6 to 13.9%), and 4.2% iso-C17:0 (range, 3.0 to 7.2%).
Analysis of CF36 and CF7 cell wall peptidoglycan was performed at the Deutsche Sammlung von Mikroorganismen und Zellkulturen (DSMZ; Braunschweig, Germany) by N. Weiss with a thin-layer chromatography method as outlined by Schleifer and Kandler (12). The peptidoglycan was of the B2β type with d-ornithine as the diamino acid.
DNA-DNA hybridization was carried out by P. Schumann at the DSMZ as described by De Ley et al. (3) with the modifications of Escara and Hutton (4) and Huss et al. (7). The DNA homology rate of CF36 with M. luteolum LMG 16207T was only 33.7%, and that with M. oxydans DSM 20578T was 46.6%, but high levels of DNA relatedness of 78.1, 81.8, and 78,2% were obtained with strains CF7, CF34, and CF40, respectively. This was consistent with their assignment to a single species. The G+C content of strain CF36 DNA was 69.9 mol% as determined at the DSMZ.
The six strains were gram-positive, motile, coryneform rods. They grew well on Columbia blood agar at 37°C, and the color of the colonies was yellow. The strains were strictly aerobic, catalase positive, and oxidase negative. They were able to hydrolyze esculin and gelatin. Although 16S rDNA sequencing showed close relationships to M. luteolum, M. saperdae, and M. liquefaciens, these species were phenotypically quite different. None of them grew at 37°C, M. luteolum and M. liquefaciens were nonmotile, and M. luteolum and M. saperdae were nonproteolytic. These findings are in agreement with data in the literature (2). The phenotypic properties of the strains were most similar to those of M. oxydans. Glucose was oxidatively acidified on low-peptone phenol red agar (15). All of the strains grew at 40°C, whereas none of the M. oxydans isolates were capable of growing at that temperature. Moreover, salicin was not acidified, in contrast to M. oxydans. The new strains could also be differentiated from M. oxydans by API 50CH assimilation strips (bioMérieux). Lactose was utilized, and 2-ketogluconate was not, while M. oxydans had the opposite reactions. When API ZYM strips (bioMérieux) were used, negative reactions for esterase lipase (C8), α-chymotrypsin, and β-glucosidase separated the six strains from M. oxydans. Phenotypic characteristics differentiating the new strains from related oxidative, motile, proteolytic Microbacterium species growing at 37°C are reported in Table 2.
TABLE 2.
Differentiation of M. paraoxydans from other oxidative, proteolytic, motile Microbacterium species growing at 37°Ca
| Test | Result
|
|||||
|---|---|---|---|---|---|---|
| Microbacterium paraoxydans (n = 6) | Microbacterium oxydans (n = 10)b | Microbacterium barkeri DSM 20145T | Microbacterium foliorum LMG 19580T | Microbacterium phyllosphaerae LMG 19581T | Microbacterium maritypicum LMG 8374T | |
| Growth at 40°C | + | − | + | − | − | − |
| Acid from salicin | − | + | + | + | + | + |
| Assimilation API 50CH | ||||||
| 2-Ketogluconate | − | + | + | − | − | + |
| d-Arabinose | + | + | − | − | − | − |
| Lactose | + | − | + | − | − | − |
| Rhamnose | + | + | − | − | (+) | − |
| Raffinose | − | − | + | (+) | + | + |
| N-Acetylglucosamine | + | + | + | − | − | − |
| α-Methyl-d-glucoside | +/(+) | + | − | − | − | − |
| API ZYM | ||||||
| Esterase lipase (C8) | − | + | +w | + | + | + |
| α-Chymotrypsin | − | + | − | − | − | − |
| β-Glucosidase | − | + | + | + | + | + |
+, positive; −, negative; (+), delayed positive; +w, weak reaction. These data are from this study.
Strain DSM 20578T and nine clinical isolates.
Antibiotic susceptibility was tested on Mueller-Hinton agar with E-test strips (PDM, Solna, Sweden). The MIC ranges (micrograms per milliliter) were as follows: penicillin, 1.5 to 2; ampicillin, 1 to 1.5; cefotaxime, 3 to 6; cephalothin, 16 to 24; ciprofloxacin, 1 to 1.5; gentamicin, 2 to 3; vancomycin, 3 to 4; clarithromycin, <0.016.
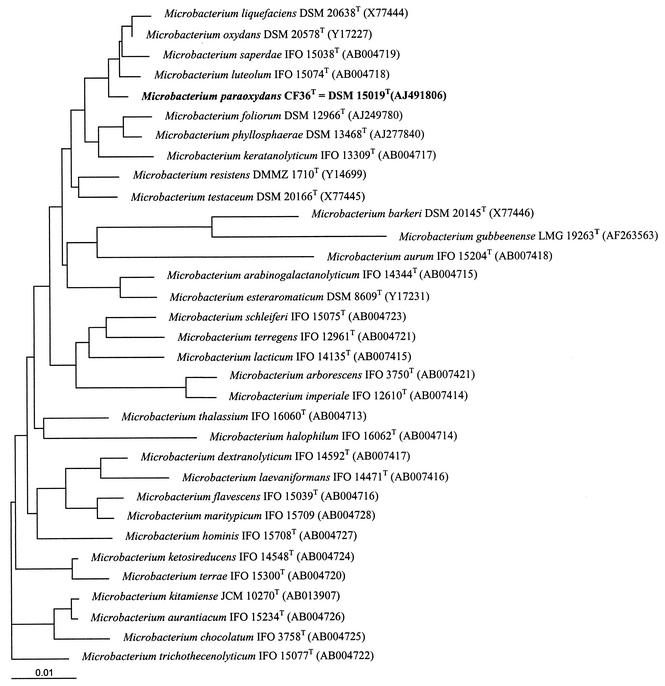
Phenotypic, chemotaxonomic, and genetic studies suggest that the six study strains belong to the genus Microbacterium but constitute a new species, for which the name Microbacterium paraoxydans is proposed and which is closely related to M. oxydans, M. saperdae, M. luteolum, and M. liquefaciens (Fig. 1).
FIG. 1.
Unrooted tree showing the phylogenetic position of M. paraoxydans CF63T within the genus Microbacterium. The bar represents one nucleotide substitution per 100 nucleotides.
Only few reports deal with the isolation of Microbacterium species from clinical material. Most were initially assigned to CDC group A-4 or A-5, and even more recent isolates were rarely identified at the species level (1, 5, 6, 9). In only a small number of cases did the isolate prove to be the causative agent of the infection. A case of endophthalmitis caused by a Microbacterium was reported by Funke et al., and on the basis of 16S rDNA sequencing, the strain was thought to belong to an undescribed species (6). In a study of Microbacterium spp. encountered in clinical specimens, several strains were identified as M. arborescens and M. imperiale but no genetic confirmation was performed (5). A nosocomial outbreak of Microbacterium-related bacteremia was reported in cancer patients, but the causative organism was not identified to the species level. A central venous catheter was the major risk factor in that study (1). More recently, two cases of catheter-related Microbacterium bacteremia, identified by 16S rDNA sequencing, were reported. One isolate was 99.4% related to M. oxydans, and the other was 98.7% related to M. trichothecenolyticum, but formal identification at the species level was not achieved (9).
Strain CF36 was isolated from blood cultures of the patient at an interval of several weeks, and although the organism isolated from the removed catheter could only be partially identified, it is likely that the repeated bacteremia was catheter related, confirming that intravascular catheters represent a risk factor of infection by these organisms. Their origin may be the patient's skin, but since the natural habitat of microbacteria is the inanimate environment, the possibility cannot be ruled out that contaminated perfusion fluid seeded the patient's catheter.
During the past decades, we have collected 30 Microbacterium isolates of human origin at our laboratory. All of these strains were identified by a set of phenotypic and chemotaxonomic characteristics and by 16S rDNA sequencing. M. oxydans was, by far, the most frequently isolated species, accounting for about a third of the strains (9 out of 30), followed by M. paraoxydans (5 strains); M. aurum and M. lacticum were each represented by 4 strains. The remaining eight strains were scattered over several species, with one M. foliorum isolate, one M. schleiferi isolate, one M. testaceum isolate, and five isolates that did not fit any described species.
Although microbacteria are rarely involved in human diseases, the number of relevant isolates found in nosocomial settings is increasing. While more than 30 species have been recognized in the genus, it is likely that only a limited number of them are isolated from clinical specimens. A more accurate identification of human isolates may help us better understand which species are prone to become opportunistic pathogens.
Description of Microbacterium paraoxydans sp. nov.
Cells of Microbacterium paraoxydans (pa-ra-o′-xy-dans, because the organism resembles M. oxydans) are small, gram-positive, coryneform rods that grow aerobically at 20, 37, and 40°C. Colonies are bright yellow, smooth, and sometimes sticky and reach a diameter of 2 mm after 48 h of incubation at 37°C on blood agar. Strains are motile by peritrichous flagella. They are catalase positive, and oxidase negative. Glucose, sucrose, maltose, galactose, fructose, mannose, and mannitol are oxidatively acidified, but salicin is not. Esculin is hydrolyzed with some delay. DNase, gelatin, and casein hydrolyses are positive. There is no decomposition of tyrosine.
In the API 50CH system, the following compounds are assimilated: glycerol, d-arabinose, ribose, glucose, galactose, fructose, mannose, rhamnose, mannitol, α-methyl-d-glucoside, N-acetylglucosamine, esculin, salicin, cellobiose, maltose, lactose, sucrose, trehalose, melezitose, gentiobiose, d-turanose, l-fucose and gluconate. Sorbose, sorbitol, amygdalin, xylitol, and d-fucose are assimilated by some strains. When API ZYM strips are used, leucine arylamidase, phosphoamidase, and α-glucosidase are positive. Acid and alkaline phosphatases, esterase (C4), β-galactosidase, N-acetylglucosaminidase, α-mannosidase, and α-fucosidase are variable. Esterase lipase (C8), valine arylamidase, cystine arylamidase, trypsin, α-chymotrypsin, β-glucuronidase, and β-glucosidase are negative. d-ornithine is the diamino acid of the peptidoglycan, and the main cellular fatty acids are anteiso-C17:0, anteiso-C15:0, and iso-C16:0. The strains are isolated from various body sites. The type strain is CF36T (= DSM 15019T = CCUG 46601T). Two other strains have been deposited in the DSMZ and the CCUG (Culture Collection of the University of Göteborg, Göteborg, Sweden): CF7 (= DSM 15020 = CCUG 46602) and CF40 (= DSM 15021 = CCUG 46603). The G+C content of the DNA of the type strain is 69.9 mol%. It was isolated from human blood.
Nucleotide sequence accession number.
The 16S rDNA sequence of strain CF36 was deposited in the EMBL (European Molecular Biology Laboratory) Data Library under accession number AJ491806.
REFERENCES
- 1.Alonso-Echanove, J., S. S. Shah, A. J. Valenti, S. N. Dirrigl, L. A. Carson, M. J. Arduino, and W. R. Jarvis. 2001. Nosocomial outbreak of Microbacterium species bacteremia among cancer patients. J. Infect. Dis. 184:754-760. [DOI] [PubMed] [Google Scholar]
- 2.Behrendt, U., A. Ulrich, and P. Schumann. 2001. Description of Microbacterium foliorum sp. nov. and Microbacterium phyllosphaerae sp. nov., isolated from the phyllosphere of grasses and the surface litter after mulching the sward, and reclassification of Aureobacterium resistens (Funke et al. 1998) as Microbacterium resistens comb. nov. Int. J. Syst. Evol. Microbiol. 51:1267-1276. [DOI] [PubMed] [Google Scholar]
- 3.De Ley, J., H. Cattoir, and A. Reynaerts. 1970. The quantitative measurement of DNA hybridization from renaturation rates. Eur. J. Biochem. 12:133-142. [DOI] [PubMed] [Google Scholar]
- 4.Escara, J. F., and J. R. Hutton. 1980. Thermal stability and renaturation of DNA in dimethylsulphoxide solutions: acceleration of renaturation rate. Biopolymers 19:1315-1327. [DOI] [PubMed] [Google Scholar]
- 5.Funke, G., E. Falsen, and C. Barreau. 1995. Primary identification of Microbacterium spp. encountered in clinical specimens as CDC coryneform group A-4 and A-5 bacteria. J. Clin. Microbiol. 33:188-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Funke, G., G. Haase, N. Schnitzler, N. Schrage, and R. R. Reinert. 1997. Endophthalmitis due to Microbacterium species: case report and review of Microbacterium infections. Clin. Infect. Dis. 24:713-716. [DOI] [PubMed] [Google Scholar]
- 7.Huss, V. A. R., H. Festl, and K. H. Schleifer. 1983. Studies on the spectrometric determination of DNA hybridization from renaturation rates. J. Syst. Appl. Microbiol. 4:184-192. [DOI] [PubMed] [Google Scholar]
- 8.Kimura, M. 1980. A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 16:111-120. [DOI] [PubMed] [Google Scholar]
- 9.Lau, S. K., P. C. Woo, G. K. Woo, and K. Y. Yuen. 2002. Catheter-related Microbacterium bacteremia identified by 16S rRNA gene sequencing. J. Clin. Microbiol. 40:2681-2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pearson, W. R., and D. J. Lipman. 1998. Improved tools for biological sequence comparison. Proc. Natl. Acad. Sci. USA 85:2444-2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saitou, N., and M. Nei. 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4:406-425. [DOI] [PubMed] [Google Scholar]
- 12.Schleifer, K. H., and O. Kandler. 1972. Peptidoglycan types of bacterial cell walls and their taxonomic implications. Bacteriol. Rev. 36:407-477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takeuchi, M., and K. Hatano. 1998. Union of the genera Microbacterium Orla-Jensen and Aureobacterium Collins et al. in a redefined genus Microbacterium. Int. J. Syst. Bacteriol. 48:739-747. [DOI] [PubMed] [Google Scholar]
- 14.Wauters, G., A. Driessen, E. Ageron, M. Janssens, and P. A. D. Grimont. 1996. Propionic acid-producing strains previously designated as Corynebacterium xerosis, C. minutissimum, C. striatum and CDC group I2 and group F2 coryneforms belong to the species Corynebacterium amycolatum. Int. J. Syst. Bacteriol. 46:653-657. [Google Scholar]
- 15.Wauters, G., B. Van Bosterhaut, M. Janssens, and J. Verhaegen. 1998. Identification of Corynebacterium amycolatum and other nonlipophilic fermentative corynebacteria of human origin. J. Clin. Microbiol. 36:1430-1432. [DOI] [PMC free article] [PubMed] [Google Scholar]