Abstract
A case of Scedosporium apiospermum keratitis was successfully treated with oral voriconazole and penetrating keratoplasty. Voriconazole levels in the aqueous humor were 53% of the levels in plasma and exceeded the MIC for the isolate by sevenfold.
CASE REPORT
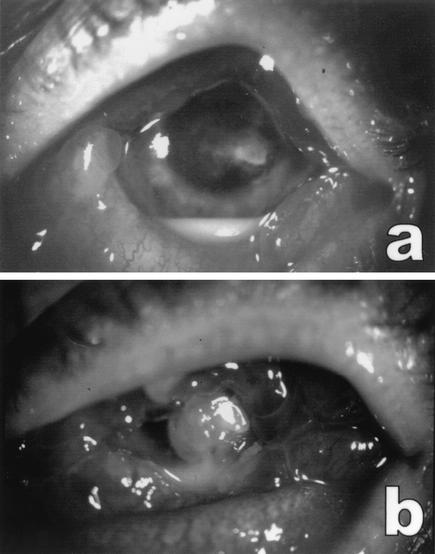
A 47-year-old male presented with a painful corneal ulcer of his left eye, present for 2 weeks. Previous locally applied treatment with chloramphenicol, gentamicin, and tobramycin combined with dexamethasone had been ineffective. At presentation, an infiltrate about 2 mm in diameter was present in the central part of the corneal stroma, surrounded by stromal edema, partially covered with epithelium. The anterior chamber showed severe inflammation and a hypopyon (3 mm). The conjunctiva was heavily injected (Fig. 1a). The patient revealed that he was owner of a worm-breeding station and that he did not use contact lenses.
FIG. 1.
Left eye of patient. (a) On presentation. An infiltrate was present in the central part of the cornea and a hypopyon was visible in the anterior chamber. The conjunctiva was heavily injected. (b) Three days after presentation. Shown are increased corneal infiltrate and hypopyon in the anterior chamber, after treatment with amphotericin B and itraconazole eyedrops. Note the severe edema and hyperemia of the conjunctiva.
Corneal scrapings, obtained the same day, showed no bacteria, but possible fungal structures in the Gram stain were seen, which was confirmed by Blankophor P staining. Blankophor P is an optical brightener, similar to calcofluor white, with high affinity for β-glycosidically linked polysaccharides, and is used to trace glucan and chitin in the fungal cell wall (11). The scrapings were inoculated onto blood agar, brain heart infusion agar, and Sabouraud agar and broth and were incubated at 29°C. After 2 days of incubation, fungal colonies were cultured. At the surface a white cottony aerial mycelium was seen; the reverse of the culture was also white. A lactophenol cotton blue preparation showed septate hyphae with short, slender conidiophores, bearing single conidia. The conidia were oval and unicellular, with the larger end toward the apex, and a cut-off appearance near the base. The mold was identified as Scedosporium apiospermum. Scedosporium prolificans was excluded, because typical morphological features such as swollen elongated annellides bearing multiple conidia were not present.
Therapy was changed to topical amphotericin B (0.3%), applied hourly. Tobramycin four times daily and 1% atropine twice daily (b.i.d.) were added. Corticosteroid treatment was discontinued immediately. By the next day, the aspect of the cornea had not changed. On the 3rd day, however, the patient complained of an excessive increase in pain and we saw increased inflammation of the anterior chamber and increased hypopyon. The infiltrate had enlarged, and two satellite infiltrates had developed. In view of the possible resistance of S. apiospermum, itraconazole (200 mg b.i.d.) was administered systemically and itraconazole 1% (intravenous fluid) was applied topically, pending approval for compassionate voriconazole use. The next day, however, infiltration of the cornea and the hypopyon had increased twofold (Fig. 1b). Therefore, a therapeutic penetrating keratoplasty was performed that same day.
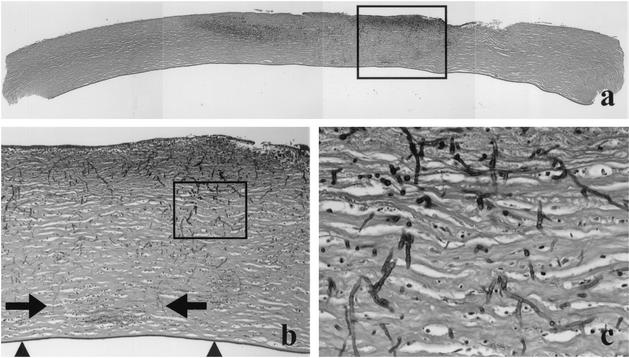
The resected cornea button was cut in three pieces that were fixed in formalin and embedded in paraffin. Subsequently, hematoxylin-and-eosin staining and periodic acid-Schiff (PAS) staining were performed on 4-μm-thick paraffin sections. Microscopic examination revealed desquamation of large parts of the corneal epithelium and an acute inflammatory infiltrate with necrosis in the central anterior part of the cornea stroma, accompanied by multiple fungal elements that were highlighted in the PAS stain (Fig. 2a to c). The hyphae showed septation and branching. Some hyphae were found close to Descemet's membrane. However, penetration of this membrane was not found, nor did the inflammatory infiltrate or fungal elements reach the peripheral rim of the cornea button.
FIG. 2.
(a) Composition of a cross-section of the cornea button; the darker areas in the cornea stroma represent inflammatory infiltrate with necrosis and fungi. Note that the periphery of the cornea does not show this change. (b) Shown is a higher magnification of the area indicated by the rectangle in panel a. It illustrates that some fungi (arrows) and inflammatory cells reach close to Descemet's membrane (arrowheads); however, penetration of this membrane was not found. (c) Shown is the area indicated by the rectangle in panel b. The fungi appear as septated, branching hyphae with variable diameter. PAS staining is shown in all panels. Original magnifications, ×50 (a), ×100 (b), and ×400 (c).
The aqueous humor stained with Blankophor P showed no fungal structures, and cultures remained sterile. The corneal biopsy, however, showed abundant septate mycelium structures, and the culture was positive with S. apiospermum. Cultures for other pathogens, including Acanthamoeba, remained negative.
Itraconazole topical therapy was discontinued after 3 days following the corneal transplantation, since white crystals appeared at the anterior stroma at the site of the epithelial defect. These crystals were assumed to be itraconazole precipitates, as this drug is not water soluble. The crystal precipitate disappeared very slowly during the succeeding months. Oral voriconazole therapy (two loading doses of 6 mg/kg of body weight, followed by 4 mg/kg b.i.d.) was started 3 days after surgery and was continued for 3 months. Slow improvement of the eye occurred in the following months. The fungal infection did not reactivate in the donor cornea or in the remnants of the recipient cornea.
The in vitro activities of amphotericin B, voriconazole, miconazole, fluconazole, itraconazole, posaconazole, ravuconazole, and terbinafine against the strain were tested with a microdilution technique, according to NCCLS M38-P guidelines (8). After incubation for 70 to 74 h at 35°C, final reading of the microtitration plate was performed visually. The MIC end point for amphotericin B was defined as the lowest concentration at which there was 100% inhibition of growth. For voriconazole, miconazole, fluconazole, itraconazole, posaconazole, ravuconazole, and terbinafine, the MIC end point was 50% inhibition of growth, compared to that of the drug-free control. The minimum fungicidal concentration (MFC) was determined by plating 50 μl of the contents from each clear well onto Sabouraud dextrose agar plates. The MFC was defined as the lowest concentration at which ≥99% of the inoculum was killed (3). In addition, a two-dimensional checkerboard titration with serial dilutions with combinations of voriconazole and terbinafine was performed to determine if synergy between the two drugs existed. The fractional inhibitory concentration index was calculated with the method described by Walsh et al. (15). The MICs of the drugs, compared with those of other clinical S. apiospermum isolates from our laboratory, are shown in Table 1 (6). All drugs exhibited fungistatic activity against the isolate. There was no synergistic interaction between voriconazole and terbinafine (fractional inhibitory concentration index, 1.03).
TABLE 1.
In vitro susceptibility of the S. apiospermum strain to various antifungal agents, compared with the MIC90s for 13 previously tested S. apiospermum strains from our laboratoryb
| Drug | Result (μg/ml) for:
|
|||
|---|---|---|---|---|
| Patient's isolate
|
Clinical S. apiospermum isolates (n = 13)
|
|||
| MIC | MFC | MIC90 | Range | |
| Amphotericin B | 4 | >16 | 4 | 0.015-16 |
| Miconazole | 0.25 | >16 | 1 | 0.06-64 |
| Fluconazole | 16 | >64 | NDa | ND |
| Itraconazole | 1 | >16 | 2 | 0.03-32 |
| Voriconazole | 0.25 | >16 | 0.25 | 0.03-32 |
| Posaconazole | 0.5 | >8 | 1 | 0.007-8 |
| Ravuconazole | 0.25 | >16 | ND | ND |
| Terbinafine | >8 | ND | >32 | 2-32 |
| 5-Fluorocytosine | >64 | ND | ND | ND |
| Voriconazole-terbinafine | 0.25 | ND | ND | ND |
ND, not done.
See reference 6 concerning the 13 strains. MIC90, MIC at which 90% of the isolates tested are inhibited.
After 12 days of treatment with oral voriconazole, a puncture of the anterior chamber was performed and, at the same time, a blood sample was taken to determine the concentration of voriconazole. A bioassay agar diffusion method was used as described by Perea et al. (10). The assay was performed in duplicate for the aqueous humor and in triplicate with plasma. Inhibition zones were read after incubation for 16 h at 28°C. The calibration curve was plotted on a logarithmic scale to determine the concentrations in the samples. The mean voriconazole concentration in plasma was 3.4 μg/ml and in the aqueous humor was 1.8 μg/ml (53% of the level in plasma).
Environmental sampling was performed in order to trace the source of the infection. Cultures were obtained from materials related to the profession of the patient, including dry and wet feed, peat, cellulose, wheat, soil, and water. Furthermore, eyedrop fluid and container were cultured, since the patient indicated that he wiped the snout of the eyedrop container manually after administering the eyedrops. S. apiospermum was not cultured from any of the materials.
S. apiospermum is a ubiquitous filamentous fungus in nature and can be recovered from soil, decaying matter, and polluted water (9, 16). It may cause localized and disseminated opportunistic infections in immunocompromised patients and in immunocompetent patients after massive exposure and trauma (16).
Keratitis caused by S. apiospermum is rare, although several cases have been reported (1, 13, 16). S. apiospermum keratitis is often associated with trauma or exposure to vegetation or other organic matter (9, 16). Keratitis caused by simultaneous Acanthamoeba and S. apiospermum infection, usually in patients with contact lenses, or by contact with polluted water has been reported (4, 12). In the present case, the patient had no history of trauma, but he had contact with many potential organic sources. Unfortunately, we were unable to identify the source of infection.
Scedosporium is known to be resistant to many antifungals, and S. prolificans is generally more resistant than S. apiospermum (2, 6). Our strain was inhibited by a concentration of 1 μg of itraconazole, posaconazole, ravuconazole, miconazole, or voriconazole per ml. The MICs are within the range of those found with other S. apiospermum strains previously tested in our laboratory (Table 1) (6) and those found in other in vitro studies (1, 5, 9). The antifungals exhibited fungistatic activity, which is in accordance with previous in vitro studies (2, 5). No synergistic activity between voriconazole and terbinafine was detected, although synergistic interaction of this combination has been shown in vitro previously in S. prolificans (7).
We found that, after oral administration of 200 mg of voriconazole b.i.d. for 12 days, the concentration in the aqueous humor was more than 50% of the concentration in plasma and almost seven times higher than the MIC for the strain. It has been shown that voriconazole concentrations in cerebrospinal fluid are also 50 to 70% of the levels in plasma (14). Furthermore, treatment with voriconazole was successful in patients with central nervous system aspergillosis and S. apiospermum brain abscess (9). In a recent study, voriconazole could be detected in the aqueous humor of rabbit eyes, after administration of 5-μg (50 μg/ml) or 10-μg (100 μg/ml) voriconazole eyedrops b.i.d. for 11 days. There was significant variability among individual animals, but the voriconazole concentration might still be high enough to treat fungal infection (17).
It is not clear yet which therapy is optimal to treat S. apiospermum infections. Usually, surgical intervention with adjuvant antifungal therapy is recommended (9). It is possible that transplantation of the cornea alone would have been sufficient to prevent further spread of the infection in our patient, because Descemet's membrane was not perforated and because the periphery of the cornea was free of inflammation with fungi. However, since miconazole eyedrops were not available and since we were aware of the possible toxic effect of systemically administered miconazole and of the previously reported treatment failure of S. apiospermum corneal infections, we decided to administer voriconazole orally.
Voriconazole, together with penetrating keratoplasty, was effective for the treatment of S. apiospermum keratitis. High levels of the drug were found in the aqueous humor following oral administration, indicating a possible role for voriconazole in the treatment of fungal eye infections.
REFERENCES
- 1.Diaz-Valle, D., J. M. Benitez del Castillo, E. Amor, N. Toledano, M. Moriche Carretero, and T. Diaz-Valle. 2002. Severe keratomycocsis secondary to Scedosporium apiospermum. Cornea 21:516-518. [DOI] [PubMed] [Google Scholar]
- 2.Espinel-Ingroff, A. 2001. In vitro fungicidal activities of voriconazole, itraconazole, and amphotericin B against opportunistic moniliaceous and dematiaceous fungi. J. Clin. Microbiol. 39:954-958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Espinel-Ingroff, A., A. Fothergill, J. Peter, M. G. Rinaldi, and T. J. Walsh. 2002. Testing conditions for determination of minimum fungicidal concentrations of new and established antifungal agents for Aspergillus spp.: NCCLS collaborative study. J. Clin. Microbiol. 40:3204-3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Froumis, N. A., B. J. Mondino, and B. J. Glasgow. 2001. Acanthamoeba keratitis associated with fungal keratitis. Am. J. Ophthalmol. 131:508-509. [DOI] [PubMed] [Google Scholar]
- 5.Johnson, E. M., A. Szekely, and D. W. Warnock. 1998. In-vitro activity of voriconazole, itraconazole and amphotericin B against filamentous fungi. J. Antimicrob. Chemother. 42:741-745. [DOI] [PubMed] [Google Scholar]
- 6.Meletiadis, J., J. F. G. M. Meis, W. Mouton, J. L. Rodriquez-Tudela, J. P. Donnelly, and P. E. Verweij. 2002. In vitro activities of new and conventional antifungal agents against clinical Scedosporium isolates. Antimicrob. Agents Chemother. 46:62-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meletiadis, J., J. W. Mouton, J. F. G. M. Meis, and P. E. Verweij. 2003. In vitro drug interaction modeling of combinations of azoles with terbinafine against clinical Scedosporium prolificans isolates. Antimicrob. Agents Chemother. 47:106-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Committee for Clinical Laboratory Standards. 1998. Reference method for broth dilution antifungal susceptibility testing of conidium-forming filamentous fungi; proposed standard. Document M38-P. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 9.Nesky, M. A., E. C. McDougal, and J. E. Peacock, Jr. 2000. Pseudallescheria boydii brain abscess successfully treated with voriconazole and surgical drainage: case report and literature review of central nervous system pseudallescheriasis. Clin. Infect. Dis. 31:673-677. [DOI] [PubMed] [Google Scholar]
- 10.Perea, S., G. J. Pennick, A. Modak, A. W. Fothergill, D. A. Sutton, D. J. Sheehan, and M. G. Rinaldi. 2000. Comparison of high-performance liquid chromatographic and microbiological methods for determination of voriconazole levels in plasma. Antimicrob. Agents Chemother. 44:1209-1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rüchel, R., and M. Schaffrinski. 1999. Versatile fluorescent staining of fungi in clinical specimens by using the optical brightener Blankophor. J. Clin. Microbiol. 37:2694-2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rumelt, S., I. Cohen, E. Lefler, and U. Rehany. 2001. Corneal co-infection with Scedosporium apiospermum and Acanthamoeba after sewage-contaminated ocular injury. Cornea 20:112-116. [DOI] [PubMed] [Google Scholar]
- 13.Sridhar, M. S., P. Garg, A. K. Bansal, and S. Sharma. 2000. Fungal keratitis after laser in situ keratomileusis. J. Cataract Refract. Surg. 26:613-615. [DOI] [PubMed] [Google Scholar]
- 14.Verweij, P. E., K. Brinkman, H. P. H. Kremer, B.-J. Kullberg, and J. F. G. M. Meis. 1999. Aspergillus meningitis: diagnosis by non-culture-based microbiological methods and management. J. Clin. Microbiol. 37:1186-1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walsh, T. J., J. Peter, D. A. McGough, A. W. Fothergill, M. G. Rinaldi, and P. A. Pizzo. 1995. Activities of amphotericin B and antifungal azoles alone and in combination against Pseudallescheria boydii. Antimocrob. Agents Chemother. 39:1361-1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu, Z., H. Ying, S. Yiu, J. Irvine, and R. Smith. 2002. Fungal keratitis caused by Scedosporium apiospermum. Cornea 21:519-523. [DOI] [PubMed] [Google Scholar]
- 17.Zhou, L., R. D. Glickman, N. Chen, W. E. Sponsel, J. R. Graybill, and K.-K. Lam. 2002. Determination of voriconazole in aqueous humor by liquid chromatography-electrospray ionization-mass spectrometry. J. Chromatogr. B 776:213-220. [DOI] [PubMed] [Google Scholar]