Abstract
The virulence traits of the Escherichia coli strain associated with a waterborne diarrhea outbreak were examined. Forty-one of 75 students (ages 12 to 15) in Akita Prefecture, Japan, showed clinical symptoms. Seven E. coli Ouk:K-:H45 isolates were isolated from the patients as the causative agent of this outbreak. One isolate (EC-3605) showed the presence of E. coli attaching-and-effacing (eaeA) and enteroaggregative E. coli heat-stable enterotoxin-1 (astA) genes and the absence of Shiga toxin (stx1 and stx2) genes. A polymorphic enteropathogenic E. coli (EPEC) adherence factor plasmid was detected in EC-3605 with a major structural gene deletion and a regulatory gene frameshift mutation, revealing that EC-3605 represents an atypical EPEC strain harboring the astA gene. The role that atypical EPEC strains harboring the astA gene play in human disease is unclear. Our results, along with those of others, present a possibility that these strains comprise a distinct category of diarrheagenic E. coli and that astA affects the age distribution of atypical-EPEC infection.
Enteropathogenic Escherichia coli (EPEC) is a major cause of infantile diarrhea, particularly in children <2 years old (23), and is a cause of sporadic diarrhea, primarily in developed countries (31). Several outbreaks of diarrhea due to EPEC have been reported (3, 42, 45). EPEC causes characteristic attaching-and-effacing (A/E) lesions, which can be observed by intestinal biopsy in both human patients (39) and animal models (27). A/E is characterized by loss of microvilli, intimate adherence of bacteria between epithelial cell membranes (34, 41), and cytoskeletal changes, such as actin polymerization directly beneath the adherent bacteria (20). EPEC possesses a 35-kb chromosomal pathogenicity island called the locus of enterocyte effacement (LEE), which contains genes required for production of A/E lesions (26). The LEE includes the E. coli A/E (eaeA) gene that encodes intimin, a 94-kDa outer membrane protein (15, 16); a type III secretion system (13); and at least three secretion proteins: EspA (19), EspB (5), and EspD (22). In the prototype EPEC strain E2348/69, the LEE is inserted at the 82-min position in the E. coli chromosomal gene encoding the tRNA for selenocysteine (selC) (25), while in Shiga toxin-producing E. coli (STEC) O26:H−, the LEE is inserted in the pheU locus (94-min position), which encodes the tRNA for phenylalanine (37). In addition to the LEE, typical EPEC (18) strains possess a 60-MDa plasmid called the EPEC adherence factor (EAF) plasmid (29). The EAF plasmid has been shown to harbor a 14-gene bundle-forming pilus (bfp) operon that encodes a type IV pilus called the bundle-forming pilus (BFP) (38) and a subset of three genes called the plasmid-encoded regulator genes, perA, perB, and perC, which activate the bfp operon and chromosomal eaeA expression (7). The presence of the EAF plasmid in EPEC strains can be detected by the EAF probe, an ∼1-kb DNA fragment derived from pMAR 2, the 60-MDa plasmid of EPEC strain E2348/69 (28). EPEC strains that do not possess the EAF plasmid are called atypical EPEC, and there is some debate as to whether these strains are true pathogens (18).
Recently, a food-borne outbreak of diarrhea involving >100 adult patients was reported in Minnesota (9). The implicated organism was E. coli O39:NM, which hybridized with the probes for eaeA and other genes in the LEE but did not hybridize with the EAF probe (9). E. coli O39:NM also hybridized with the DNA probe for the enteroaggregative E. coli (EAggEC) heat-stable enterotoxin 1 (EAST-1) gene (astA) (9). EAST-1 is a genetically distinct toxin structurally related to heat-stable enterotoxin I (ST I) of enterotoxigenic E. coli (ETEC) (35), but little is known about its significance in the pathogenic mechanism of EAggEC. Hedberg et al. pointed out in their report (9) that this E. coli O39:NM strain should therefore be categorized as an atypical EPEC strain that harbors the astA gene. Few reports, however, are available on the role that atypical EPEC strains harboring the astA gene play in human diseases.
We isolated E. coli strains as the etiological agents from patients with diarrhea in a waterborne outbreak involving junior high school students and teachers and characterized the virulence traits of a representative isolate. We describe in this report the virulence traits of the outbreak-associated E. coli strain, which should be categorized as an atypical EPEC strain.
MATERIALS AND METHODS
Description of the diarrheal outbreak and bacterial strains.
On 30 May 2000, an outbreak of diarrhea occurred in Akita Prefecture, Japan, among students and teachers attending a fieldwork program held at a farm. Of the 75 attendees, 41 students (ages 12 to 15 years) showed clinical symptoms, including diarrhea (85% of the patients), fever (34%; 37.0 to 38.9°C), nausea (37%), vomiting (15%), and abdominal pain (90%). A marked difference in the attack rate was noted between students who drank tap water (86.1%) at the pasture and those who only washed their hands with tap water (26.3%). The tap water was provided from a brook, without chlorination, as an animal water supply. Of the 36 patients examined for diarrheagenic pathogens, 9 were positive for E.coli harboring the eaeA gene (A/E E. coli [AEEC]), which was the only pathogen isolated from the patients. The serogroup of two of the nine AEEC isolates was O8, while the remaining seven AEEC isolates could not be serogrouped using a commercially available serum kit (11). The tap water collected on 2 June was positive for the O untypeable AEEC. The eight O untypeable AEEC isolates (seven from patients and one from the tap water) displayed identical XbaI pulsed-field gel electrophoresis (45) patterns, indicating that the O untypeable AEEC strains were a single clone and were the causative agent of the diarrheal outbreak. Strain EC-3605, one of the seven AEEC patient isolates, was serotyped as Ouk:K−:H45 by Flemming Scheutz at the International Escherichia and Klebsiella Centre, Statens Serum Institute, Copenhagen, Denmark.
EC-3605 isolated from a patient with diarrhea was employed for characterization of the virulence traits. EPEC E2348/69, EAggEC 17-2, and the pJPN16 plasmid in E. coli HB101 were kindly provided by James B. Kaper, University of Maryland School of Medicine, Baltimore, Md. STEC O157:H7 EDL-931 was a kind gift from Yasuo Kudoh, Tokyo Metropolitan Research Laboratory of Public Health, Tokyo, Japan.
PCR conditions.
PCR was performed, unless otherwise stated, in a 20-μl reaction mixture containing 100 μM (each) deoxynucleoside triphosphate, 2 mM MgCl2, 0.5 μM (each) primer, 2 μl of 10-fold-concentrated polymerase reaction buffer, and a 2-μl aliquot of the boiled bacterial suspension. The mixtures were subjected to 20 cycles of amplification in a Gene Amp PCR System 2400 (Perkin-Elmer, Applied Biosystems Division, Norwalk, Conn.). The parameters for the amplification cycle were 1 cycle of preheating for 30 s at 94°C, followed by 20 cycles of denaturation for 30 s at 94°C, annealing for 30 s at 55°C, and extension for 30 s at 72°C. After the last cycle, the reaction tubes were incubated for 2 min at 72°C. The amplified products were analyzed by electrophoresis using a 2.0% agarose gel.
Examination of virulence genes by PCR.
EC-3605 was examined by PCR with the primers listed in Table 1 for the presence of the following virulence genes: Shiga toxin genes (stx1 and stx2) (12), eaeA (15), the EAF gene (29), bfpA (38), astA (35), and aggR (transcriptional activator for EAggEC aggregative adherence fimbria I expression) (30). The bfpAJY primers were designed within a putative conserved region, which was identified by comparing the bfpA sequences from EPEC E2348/69 (GenBank accession no. Z12295) and EPEC O128ab:H2 strain 20 (accession no. AF119170).
TABLE 1.
Primers used for examination of virulence traits of EC-3605
| Designation | Location | Sequence (5′ to 3′) | Target gene | Accession no. | Amplicon size (bp) | Reference |
|---|---|---|---|---|---|---|
| V1 | 213-230 | AGT TAA TGT GGT GGC GAA | ||||
| V5 | 1013-1029 | GAC TCT TCC ATC TGC CG | stx1 | M19473 | 817 | 21 |
| V3 | 289-306 | TTC GGT ATC CTA TTC CCG | ||||
| V4 | 745-762 | TCT CTG GTC ATT GTA TTA | stx2 | X07865 | 474 | 21 |
| EA-1 | 1846-1865 | AAA CAG GTG AAA CTG TTG CC | ||||
| EA-2 | 2280-2299 | CTC TGC AGA TTA ACC TCT GC | eaeA | M58154 | 454 | 45 |
| EAF1 | 546-567 | CAG GGT AAA AGA AAG ATG ATA A | ||||
| EAF25 | 922-942 | TAT GGG GAC CAT GTA TTA TCA | EAF | X76137 | 397 | 6 |
| EP-1 | 120-140 | AAT GGT GCT TGC GCT TGC TGC | ||||
| EP-2 | 423-443 | GCC GCT TTA TCC AAC CTG GTA | bfpA | Z12295 | 324 | 8 |
| bfpAJYS | 99-120 | TCT TGC TTT GAT TGA ATC TGC A | ||||
| bfpAJYAS | 273-295 | GTA AAA TCG TTG AGT CCA ATC CA | bfpA | Z12295 | 197 | This study |
| EAST-1S | 63-82 | GCC ATC AAC ACA GTA TAT CC | ||||
| EAST-1AS | 149-168 | GAG TGA CGG CTT TGT AGT CC | astA | L11241 | 106 | 45 |
| AggRks1 | 1307-1327 | GTA TAC ACA AAA GAA GGA AGC | ||||
| AggRkas2 | 1541-1560 | ACA GAA TCG TCA GCA TCA GC | aggR | Z32523 | 254 | 33 |
Southern blot analysis of plasmid DNA of EC-3605.
Plasmid DNA was extracted from EC-3605 by the method of Kado and Liu (17) and electrophoresed in a 0.7% agarose gel. Using standard techniques, the plasmid DNA was denatured in the gel, transferred to a nylon membrane, and cross-linked by UV irradiation (24). The EAF gene probe was a 1-kb SalI-BamHI fragment (1) prepared from the pJPN16 plasmid (14). The DNA probe for the bfpA gene was PCR amplified from EPEC E2348/69 with the primers bfpA ORF S (5′-AGG AAA ACA GTT TTT ATG GTT TCT-3′) and bfpA ORF AS (5′-GGC GTA TTA TGT AGA TTA CTT CAT-3′) to produce a 612-bp fragment including the bfpA open reading frame (ORF). PCR was performed as described above except that 25 amplification cycles were performed and the total reaction volume was 50 μl. The 612-bp bfpA probe was purified with a SUPREC-02 column (Takara Shuzo Co., Shiga, Japan) according to the manufacturer's instructions. Probe labeling, hybridization under high-stringency conditions, and signal detection were performed using the AlkPhos Direct Gene image kit (Amersham Pharmacia Biotech, Piscataway, N.J.) according to the manufacturer's instructions.
Cloning and sequencing of bfpA3605.
The ∼60-MDa plasmid p3605 was extracted from EC-3605 by the method of Kado and Liu (17) and digested with SalI and EcoRI according to the manufacturer's instructions. The resultant DNA fragments were cloned into pBluescript SK II by using a T4 DNA ligase ligation kit (Ligation Pack; Nippon Gene Co., Toyama, Japan). The host, E. coli strain JM109, was transformed with the recombinant plasmids by the standard CaCl2 method (24) using a transformation kit (Nippon Gene Co.). The resultant E. coli JM 109 strains harboring bfpA3605 were identified by a standard color test based on α-complementation (24) in combination with PCR with bfpAJY primers under the conditions described above. The recombinant plasmid harboring bfpA3605 was designated pJY3605. The DNA sequence of bfpA3605 in pJY3605 was determined by the Sanger dideoxynucleotide chain termination method with an ABI model 377 sequencer. A 312-bp DNA fragment containing bfpA3605 was amplified from EC-3605 by PCR under the conditions described above with the primers bfpA3605 ORF S (5′-TTC CGT GAC CTA TTA ATA CGG-3′) and bfpA3605 ORF AS (5′-CTT CCC CGA GCA TGT TGG-3′), purified with the SUPREC-02 column, and sequenced directly using the same primers in order to verify the DNA sequence data of bfpA3605 from pJY3605.
Examination of bfp and per genes in EC-3605 by PCR.
The bfpB, bfpD-F, bfpI-L, perA, perB, and perC genes were examined by PCR with the primers listed in Table 2. For detection of the bfp genes, the parameters for the amplification cycle were 1 cycle of preheating for 30 s at 94°C, followed by 20 cycles of denaturation for 30 s at 94°C, annealing for 60 s at 55°C, and extension for 60 s at 72°C. After the last cycle, the reaction tubes were incubated for 2 min at 72°C. For detection of the per genes, PCR was performed as described above.
TABLE 2.
Primers used for examination of the per and bfp genes and insertion site of the LEE
| Designation | Location | Sequence (5′ to 3′) | Target gene | Accession no. | Amplicon size (bp) | Reference |
|---|---|---|---|---|---|---|
| PerAS | 523-542 | TGT CAT CCT TAG TGC TTC AT | ||||
| PerAAS | 857-876 | GGC AAT GTT CCT TGT GTA AT | perA | Z48561 | 354 | 45 |
| PerB1 | 958-978 | GTA GTT TTT GAT GGA TGT ATG | ||||
| PerB2 | 1267-1286 | TCA CCG CCT CCT TCC ATC GT | perB | Z48561 | 329 | 2 |
| PerC1 | 1419-1439 | GAC GAG CTG CAG ATG CCT TGG | ||||
| PerC2 | 1575-1595 | GCC CCA TTT TCT TAT ATG CCT | perC | Z48561 | 177 | 2 |
| BfpB1 | 2947-2966 | CGC CAG AAG CCT TGA GAT CA | ||||
| BfpB2 | 1726-1744 | GAA CAG TGC AAC AGG CGG A | bfpB | Z68186 | 1,241 | 2 |
| BfpD | 5190-5210 | CTT ATC AGG CTG CTC GTA TAG | ||||
| BfpF | 7862-7881 | CAA GCT CAC GTG CAT CCA TC | bfpD-F | Z68186 | 2,692 | 2 |
| BfpI | 9550-9571 | TCA CTG ATT GAA GCG TCA TTA | ||||
| BfpL | 11282-11301 | GCT CGT CCG ACA GAA TAC TC | bfpI-L | Z68186 | 1,752 | 2 |
| K260 | 7328-7351 | GAG CGA ATA TTC CGA TAT CTG GTT | ||||
| K261 | 7833-7854 | CCT GCA AAT AAA CAC GGC GCA T | selC | AE000443 | 527 | 37 |
| K913 | 13-32 | CAT CGG CTG GCG GAA GAT AT | ||||
| K914 | 303-320 | CGC TTA AAT CGT GGC GTC | pheU | S67565 | 308 | 37 |
| K255 | NAc | GGT TGA GTC GAT TGA TCT CTG G | ||||
| K260 | 315-338 | GAG CGA ATA TTC CGA TAT CTG GTT | RJa | AF031371 | 418 | 37 |
| K295 | 459-478 | CGC CGA TTT TTC TTA GCC CA | ||||
| K296 | NA | CAT TCT GAA ACA AAC TGC TC | LJb | AF031372 | 418 | 37 |
RJ, right junction of LEE inserted into selC.
LJ, left junction of LEE inserted into selC.
NA, not available.
Sequencing of perA3605.
An 877-bp DNA fragment containing the perA gene of EC-3605, perA3605, was amplified from EC-3605 by PCR with the primers PerA Seq S (5′-ACA AAC GCG CAT GAA GGT GGT-3′; positions 33 to 53; Z48561) and PerA Seq AS (5′-ATA AGA TTT TAA ATA TCT CTA ACA-3′; positions 886 to 909; Z48561) using proofreading Pylobest DNA polymerase (Takara Shuzo Co.), purified using the SUPREC-02 column, and sequenced directly using the same primers.
Insertion site of LEE in EC-3605.
The insertion site of the LEE in the chromosome of EC-3605 was examined by PCR with primers K260 and K261 for the intact selC (tRNA for selenocysteine) locus, K913 and K914 for the intact pheU (tRNA for phenylalanine) locus, K255 and K260 for the right junction of the LEE inserted into the selC locus, and K295 and K296 for the left junction of the LEE inserted into the selC locus (37) (Table 2). PCR was performed as described above.
DNA sequence analysis.
DNA sequence data were analyzed with DNASIS software (Hitachi Software Engineering Co.).
Nucleotide sequence accession numbers.
The nucleotide sequences of the perA3605 and bfpA3605 genes obtained in this study were deposited in the GenBank database under accession numbers AY212287 and AY212288, respectively.
RESULTS
Virulence genes of EC-3605.
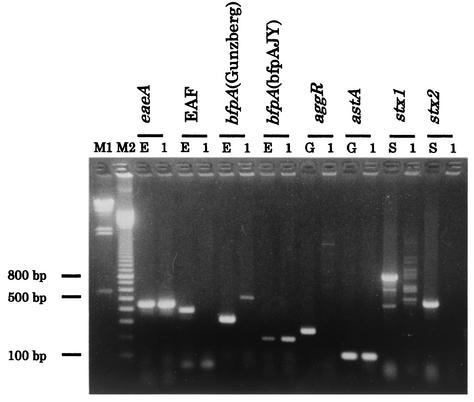
The results of the PCR examination of EC-3605 for E. coli virulence-associated genes were positive for eaeA and astA and negative for the EAF gene, aggR, stx1, and stx2. The presence of the bfpA gene in EC-3605 was determined by PCR with two sets of primers. The results using the primers described by Gunzberg et al. (8), EP-1 and EP-2, were negative, while the bfpAJY primers gave an expected 197-bp fragment (Fig. 1), suggesting that EC-3605 harbors a bfpA homologue. Southern blot hybridization analysis of the ∼60-MDa plasmid of EC-3605, p3605, for the presence of the EAF locus and a bfpA homologue using the EAF gene and bfpA probes confirmed that p3605 represents the EAF plasmid harboring the bfpA homologue, bfpA3605 (data not shown). EC-3605 was negative for the invE gene of enteroinvasive E. coli and for both the heat-stable and heat-labile enterotoxin genes of ETEC (data not shown).
FIG. 1.
Agarose gel electrophoresis of the PCR products of E. coli virulence genes showing virulence traits of EC-3605. Lanes: M1, lambda/HindIII molecular size marker; M2, 100-bp-ladder molecular size marker; 1, EC-3605; E, EPEC E2348/69; G, EAggEC 17-2; S, STEC O157: H7 EDL-931.
Characterization of the EC-3605 bfp and per genes.
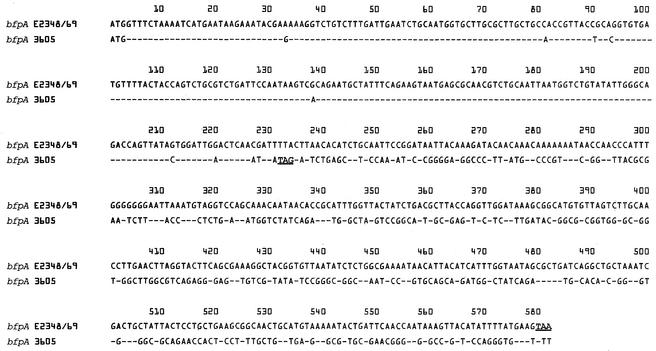
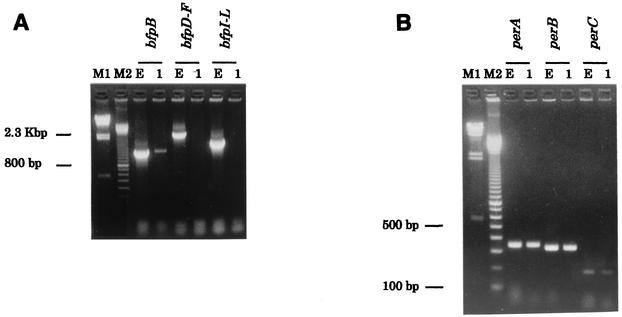
To characterize bfpA3605, we cloned it into pJY3605 and determined its DNA sequence. pJY3605 harbors an ∼2.3-kb EcoRI-SalI fragment that originated from p3605, and an ∼1-kb region flanking the EcoRI site of the 2.3-kb fragment was sequenced. A 234-bp ORF was identified in the 2.3-kb fragment. As shown in Fig. 2, the DNA sequences of the 234-bp ORF and the 5′ 234-bp region of bfpAE2348/69 are 94.9% identical, indicating that this 234-bp ORF represents bfpA3605 (AY212288). The DNA sequence downstream of the bfpA3605 ORF, however, varied significantly from the corresponding region of bfpAE2348/69, suggesting that a deletion occurred in the 3′ end of the EC-3605 bfpA. Furthermore, PCR using primers for bfpB, bfpD-F, and bfp I-L yielded no amplicons of the expected sizes from EC-3605, while PCR products of the expected sizes were amplified from E2348/69 (Fig. 3A). These results indicated that a deletion of the entire bfp operon of EC-3605 occurred, suggesting that it is unable to produce BFP, as described by Bortolini et al. (2). In fact, by performing Western blotting with polyclonal antisera raised against E2348/69 BFP, we determined that EC-3605 failed to produce BFP (data not shown). We next characterized the three genes constituting the per operon, perA, perB, and perC, in EC-3605. As shown in Fig. 3B, EC-3605 was positive for perA, perB, and perC by PCR. However, sequencing of the perA gene of EC-3605, perA3605 (AY212287), revealed a frameshift mutation caused by a single base deletion at position 325 corresponding to the perA gene of E2348/69, perAE2348/69 (Z48561), which results in a truncation of perA3605 to a 357-bp ORF compared to the 825-bp ORF of perAE2348/69 (data not shown). These results indicated that the per operon of EC-3605 is unable to activate the virulence gene promoter, as described by Okeke et al. (32).
FIG. 2.
Alignment of the bfpA nucleotide sequences of EPEC E2348/69 and EC-3605. The dashes represent consensus nucleotides. The stop codons are underlined.
FIG. 3.
Agarose gel electrophoresis of the PCR products of genes comprising the bfp operon (A) and the per operon (B). Lanes: M1, lambda/HindIII molecular mass marker; M2, 100-bp-ladder molecular size marker; E, EPEC E2348/69; 1, EC-3605.
Insertion site of the LEE in EC-3605.
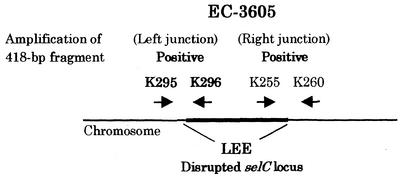
To characterize the evolutionary lineage of EC-3605, we examined the insertion site of the LEE in the chromosomal DNA of EC-3605. Figure 4 schematically presents the region surrounding the LEE in the EC-3605 chromosome. Amplicons of the expected size, 418 bp, were obtained with primers K295-K296 and K255-260 from EC-3605, indicating that the LEE is inserted within the selC locus in the EC-3605 chromosome, as well as in the E2348/69 chromosome (25). Although the data are not shown, interruption of the selC locus was confirmed in both EC-3605 and E2348/69 by PCR with primers K260 and K261. The pheU locus is interrupted by an unknown insert in EC-3605, which was evidenced by a negative result in PCR with primers K913 and K914, while the pheU locus is intact in E2348/69, because an amplicon of the expected size, 308 bp, was obtained with primers K913 and K914 (data not shown). These results indicate that both EC-3605 and E2348/69 belong to the same cluster, EPEC 1.
FIG. 4.
Schematic representation of the region surrounding the LEE in the EC-3605 chromosome. The PCR primers listed in Table 2 are indicated by arrows. The diagram is not to scale.
DISCUSSION
In this study, we characterized the virulence traits of E. coli isolated as an etiological agent in a waterborne diarrhea outbreak in Japan. The E. coli strain, EC-3605, that was isolated from patients during this outbreak was shown by PCR to be positive for the eaeA gene and by Southern hybridization to harbor the EAF plasmid. However, PCR indicated that this strain was negative for the EAF plasmid. This discrepancy suggests that the EAF locus of p3605 shows a low sequence homology, at least in the primer annealing sites, with pMAR2 from E2348/69. According to the consensus definition achieved at the Second International Symposium on EPEC (18), a typical EPEC strain produces the A/E lesion without stx genes and possesses the EAF plasmid, while an atypical EPEC strain lacks the EAF plasmid. Therefore, EC-3605 apparently represents a typical EPEC strain, although its serotype, Ouk:K-:H45, does not fit into any of the known EPEC serotypes. In addition to these virulence genes, EC-3605 harbors the astA gene, a putative heat-stable enterotoxin gene originally identified in the EAggEC strain 17-2 (35).
The importance of the EAF plasmid in typical EPEC pathogenicity is attributable to the functions of its bfp and per operons. The perA gene encodes a protein belonging to the AraC family of transcriptional activators, and the perB and perC genes encode proteins that enhance perA gene expression by an unknown mechanism (7). The per operon has been shown to increase expression of both the eaeA (7) and bfpA genes (40). Hicks et al. (10) recently showed, using in vitro organ culture with human pediatric small intestinal biopsy tissue, that BFP is involved in the formation of complex three-dimensional EPEC colonies on the intestinal epithelial tissue via bacterium-bacterium interactions, which results in the maintenance and stabilization of EPEC microcolonies. Stone et al. (38) demonstrated that 14 ORFs, those for bfpA, -G, -B, -C, -U, -D, -R, -F, -P, -H, -I, -J, -K, and -L, are sufficient to reconstitute BFP with the aid of artificial promoters, which indicates that these 14 genes are necessary for BFP biosynthesis. Recently, Bortolini et al. (2) reported that several EPEC strains harbored a truncated bfpA gene and lacked the other bfp operon genes, resulting in a lack of BFP production. The conflicting results regarding the presence of the EAF plasmid in EC-3605 observed in PCR and Southern blot hybridization suggested a sequence polymorphism between the EAF loci of p3605 and pMAR2. The conflicting results regarding the presence of the bfpA gene in EC-3605 were also observed in PCR with Gunzberg primers and bfpAJY primers, suggesting that the 3′ end of bfpA3605 is deleted, as described by Bortolini et al. (2), or that the nucleotide sequence of the EP-2 annealing site in bfpA3605 has low sequence homology with that in bfpA of the prototype EPEC E2348/69, bfpAE2348/69. In fact, we demonstrated that the entire bfp operon is deleted in EC-3605, and Western blot analysis confirmed that the EC-3605 bfp operon cannot produce BFP. Additionally, we identified a mutating frameshift that leads to truncation of the perA gene in EC-3605. This truncation could result in per operon inactivation, as described by Okeke et al. (32), in a minority of EPEC strains, including O119:H2, a subset of O128:H2, and O142:H6. Our present results indicate that even though EC-3605 harbors the EAF plasmid, the bfpA3605 and perA3605 genes are unable to function properly because of a structural gene deletion and a perA frameshift mutation, respectively, revealing that EC-3605 should functionally represent an atypical EPEC strain. It was reported previously (45) that the atypical EPEC O55:NM was an etiological agent of an infantile-diarrhea outbreak. Taken with our present results, this indicates that neither BFP nor the perA gene is essential for EPEC to cause diarrhea in humans. Diagnosis of typical EPEC strains depends on detection of eaeA and the EAF plasmid by DNA probe or PCR (31). Our present results, together with those of Bortolini et al. (2), reveal that some of the strains diagnosed to be typical EPEC strains by EAF probe harbor a nonfunctioning bfp operon. These strains should be categorized instead as atypical EPEC strains because such strains are unable to produce BFP. Such strains cannot be distinguished from “true” typical EPEC strains possessing functioning bfp and per operons by hybridization analysis using an EAF probe. A reliable and conventional method for identifying such strains needs to be developed.
EPEC infection is primarily a disease of infants <2 years old (23). An infantile-diarrhea outbreak caused by the atypical EPEC strain O55:NM (45) was also recently reported. However, a few food-borne diarrhea outbreaks involving adults caused by atypical EPEC strains have been reported. In one recent outbreak involving >100 adults, the implicated pathogen was E. coli O39:NM (9). Although the serotype O39:NM does not fit into any of the known EPEC serotypes, this strain was positive for eaeA and other genes within the LEE but negative for the EAF plasmid (9), which is representative of an atypical EPEC strain. Another diarrhea outbreak involving >600 people, including students, teachers, and auxiliary personnel, was also reported (42). The implicated pathogen in this outbreak was E. coli O111, which was eaeA positive but EAF plasmid negative (31). Interestingly, both of the outbreak-associated E. coli strains, O39:NM and O111, were astA positive. We have shown in this study that EC-3605 is also astA positive. The astA gene was first identified in EAggEC as a structural gene that encodes a distinct low-molecular-weight putative enterotoxin (35). Recently, the astA gene has been detected not only in EAggEC but also in EPEC, atypical EPEC, ETEC, STEC, and enteroinvasive E. coli strains (4, 36, 44). Although the significance of the astA gene in E. coli pathogenesis remains unclear, our findings, along with those of Hedberg et al. (9) and Viljanen et al. (42), suggest the possibility that the presence of the astA gene affects the age distribution of atypical-EPEC infection. Whether atypical EPEC strains harboring the astA gene are more frequently isolated from adults needs further elucidation.
It has recently been reported that chromosomal LEE insertion sites vary according to the evolutionary lineage of LEE-harboring E. coli strains, including EPEC and STEC. In the clusters of strains designated EPEC 1, which is composed of serotypes O127:H6 and O55:H6, and STEC 1, which is composed of serotype O157:H7, the LEE is inserted downstream of the selC locus (43). In contrast, in EPEC 2, which is composed of serotypes O128:H2, O111:NM, and O111:H2, and STEC 2, which is composed of serotypes O111:NM, O111:H8, O111:H11, O26:NM, and O26:H11, the LEE is inserted into the pheU locus (37). Our present data indicate that in EC-3605, the LEE is inserted into the selC locus. These observations indicate that EC-3605 belongs to the EPEC 1 cluster, just as EPEC E2348/69 does.
Certain serotypic and genotypic features of EC-3605 are quite similar to those of E. coli O39:NM, which is the etiological agent of food-borne diarrhea outbreaks. Neither of these strains fits into any of the known EPEC serotypes, they have virulence traits characteristic of atypical EPEC, and they harbor the astA gene. The Finnish outbreak-associated E. coli O111 strain also has the same virulence traits as E. coli O39:NM, but it belongs in the classical EPEC O serogroup (42). Interestingly, Hedberg et al. (9) also pointed out that E. coli O39:NM may be regarded as an atypical ETEC strain because it produces an adhesin, intimin, in combination with the heat-stable enterotoxin EAST-1. The significance of atypical EPEC strains that harbor the astA gene, whether they belong to the known EPEC serotypes or not, is unclear, but our results, along with those of Hedberg et al. (9) and Viljanen et al. (42), raise the possibility that these E. coli strains comprise a distinct category of diarrheagenic E. coli.
REFERENCES
- 1.Baldini, M. M., J. P. Nataro, and J. B. Kaper. 1986. Localization of a determinant for HEp-2 adherence by enteropathogenic Escherichiacoli. Infect. Immun. 52:334-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bortolini, M. R., L. R. Trabulsi, R. Keller, G. Frankel, and V. Sperandio. 1999. Lack of expression of bundle-forming pili in some clinical isolates of enteropathogenic Escherichia coli (EPEC) is due to a conserved large deletion in the bfp operon. FEMS Microbiol. Lett. 179:169-174. [DOI] [PubMed] [Google Scholar]
- 3.Bower, J. R., B. L. Congeni, T. G. Cleary, R. T. Stone, A. Wanger, B. E. Murray, J. J. Mathewson, and L. K. Pickering. 1989. Escherichia coli O114:nonmotile as a pathogen in an outbreak of severe diarrhea associated with a day care center. J. Infect. Dis. 160:243-247. [DOI] [PubMed] [Google Scholar]
- 4.De Sousa, C. P., and J. D. Dubreuil. 2001. Distribution and expression of the astA gene (EAST1 toxin) in Escherichia coli and Salmonella. Int. J. Med. Microbiol. 291:15-20. [DOI] [PubMed] [Google Scholar]
- 5.Foubister, V., I. Rosenshine, and B. B. Finlay. 1994. A diarrheal pathogen, enteropathogenic Escherichia coli (EPEC) triggers a flux of inositol phosphates in infected epithelial cells. J. Exp. Med. 179:993-998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Franke, J., S. Franke, H. Schmidt, A. Schwarzkopf, L. H. Wieler, G. Baljer, L. Beutin, and H. Karch. 1994. Nucleotide sequence analysis of enteropathogenic Escherichia coli (EPEC) adherence factor probe and development of PCR for rapid detection of EPEC harboring virulence plasmids. J. Clin. Microbiol. 32:2460-2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gomez-Duarte, O. G., and J. B. Kaper. 1995. A plasmid-encoded regulatory region activates chromosomal eaeA expression in enteropathogenic Escherichia coli. Infect. Immun. 63:1767-1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gunzberg, S. T., N. G. Tornieporth, and L. W. Riley. 1995. Identification of enteropathogenic Escherichia coli by PCR-based detection of the bundle-forming pilus gene. J. Clin. Microbiol. 33:1375-1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hedberg, C. W., S. J. Savarino, J. M. Besser, C. J. Paulus, V. M. Thelen, L. J. Myers, D. N. Cameron, T. J. Barrett, J. B. Kaper, and M. T. Osterholm. 1997. An outbreak of foodborne illness caused by Escherichia coli O39:NM, an agent not fitting into the existing scheme for classifying diarrheogenic E. coli. J. Infect. Dis. 176:1625-1628. [DOI] [PubMed] [Google Scholar]
- 10.Hicks, S., G. Frankel, J. B. Kaper, G. Dougan, and A. D. Phillips. 1998. Role of intimin and bundle-forming pili in enteropathogenic Escherichia coli adhesion to pediatric intestinal tissue in vitro. Infect. Immun. 66:1570-1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Itoh, Y., I. Nagano, M. Kunishima, and T. Ezaki. 1997. Laboratory investigation of enteroaggregative Escherichia coli O untypeable:H10 associated with a massive outbreak of gastrointestinal illness. J. Clin. Microbiol. 35:2546-2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jackson, M. P., R. J. Neill, A. D. O'Brien, R. K. Holmes, and J. W. Newland. 1987. Nucleotide sequence analysis and comparison of the structural genes for Shiga-like toxin I and Shiga-like toxin II encoded by bacteriophages from Escherichia coli 933. FEMS Microbiol. Lett. 44:109-114. [DOI] [PubMed] [Google Scholar]
- 13.Jarvis, K. G., J. A. Giron, A. E. Jerse, T. K. McDaniel, M. S. Donnberg, and J. B. Kaper. 1995. Enteropathogenic Escherichia coli contains a putative type III secretion system necessary for the export of proteins involved in attaching and effacing lesion formation. Proc. Natl. Acad. Sci. USA 92:7996-8000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jerse, A. E., W. C. Martin, J. E. Galen, and J. B. Kaper. 1990. Oligonucleotide probe for detection of the enteropathogenic Escherichia coli (EPEC) adherence factor of localized adherent EPEC. J. Clin. Microbiol. 28:2842-2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jerse, A. E., J. Yu, B. D. Tall, and J. B. Kaper. 1990. A genetic locus of enteropathogenic Escherichia coli necessary for the production of attaching and effacing lesions on tissue culture cells. Proc. Natl. Acad. Sci. USA 87:7839-7843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jerse, A. E., and J. B. Kaper. 1991. The eae gene of enteropathogenic Escherichia coli encodes a 94-kilodalton membrane protein, the expression of which is influenced by EAF plasmid. Infect. Immun. 59:4302-4309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kado, C. I., and S. T. Liu. 1981. Rapid procedure for detection and isolation of large and small plasmids. J. Bacteriol. 145:1365-1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaper, J. B. 1996. Defining EPEC. Rev. Microbiol. Sao Paulo 27:130-133. [Google Scholar]
- 19.Kenny, B., L. Lai, B. B. Finlay, and M. S. Donnenberg. 1996. EspA, a protein secreted by enteropathogenic Escherichia coli, is required to induce signals in epithelial cells. Mol. Microbiol. 20:313-324. [DOI] [PubMed] [Google Scholar]
- 20.Knutton, S., P. H. Williams, and A. S. McNeish. 1989. Actin accumulation at sites of bacterial adhesion to tissue culture cells: basis of a new diagnostic test for enteropathogenic Escherichia coli. Infect. Immun. 57:1290-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kobayashi, K. 1991. Detection of enterohemorrhagic Escherichia coli using PCR. Rinsho to Biseibutsu 18:507-513. (In Japanese.)
- 22.Lai, L. C., L. A. Wainwright, K. D. Stone, and M. S. Donnenberg. 1997. A third secreted protein that is encoded by the enteropathogenic Escherichia coli pathogenicity island is required for transduction of signals and for attaching and effacing activities in host cells. Infect. Immun. 65:2211-2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levine, M. M., and R. Edelman. 1984. Enteropathogenic Escherichia coli of classic serotypes associated with infant diarrhea: epidemiology and pathogenesis. Epidemiol. Rev. 6:31-51. [DOI] [PubMed] [Google Scholar]
- 24.Maniatis, T., E. F. Fritsch, and J. Sambrook. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 25.McDaniel, T. K., K. G. Jarvis, M. S. Donnenberg, and J. B. Kaper. 1995. A genetic locus of enterocyte effacement conserved among diverse enterobacterial pathogens. Proc. Natl. Acad. Sci. USA 92:1664-1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McDaniel, T. K., and J. B. Kaper. 1997. A cloned pathogenicity island from enteropathogenic Escherichia coli confers the attaching and effacing phenotype on E. coli K-12. Mol. Microbiol. 23:399-407. [DOI] [PubMed] [Google Scholar]
- 27.Moon, H. W., S. C. Whipp, R. A. Argenzio, M. M. Levine, and R. A. Giannella. 1983. Attaching and effacing activities of rabbit and human enteropathogenic Escherichia coli in pig and rabbit intestines. Infect. Immun. 41:1340-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nataro, J. P., M. M. Baldini, J. B. Kaper, R. E. Black, N. Bravo, and M. Levine. 1985. Detection of an adherence factor of enteropathogenic Escherichia coli with a DNA probe. J. Infect. Dis. 152:560-565. [DOI] [PubMed] [Google Scholar]
- 29.Nataro, J. P., K. O. Maher, P. Mackie, and J. B. Kaper. 1987. Characterization of plasmids encoding the adherence factor of enteropathogenic Escherichia coli. Infect. Immun. 55:2370-2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nataro, J. P., D. Yikang, D. Yingkang, and K. Walker. 1994. aggR, transcriptional activator of aggregative adherence fimbria I expression in enteroaggregative Escherichia coli. J. Bacteriol. 176:4691-4699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nataro, J. P., and J. B. Kaper. 1998. Diarrheagenic Escherichiacoli. Clin. Microbiol. Rev. 11:142-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Okeke, I. N., J. A. Borneman, S. Shin, J. L. Mellies, L. E. Quinn, and J. B. Kaper. 2001. Comparative sequence analysis of the plasmid-encoded regulator of enteropathogenic Escherichia coli strains. Infect. Immun. 69:5553-5564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ratchtrachenchai, O. A., S. Subpasu, and K. Ito. 1997. Investigation on enteroaggregative Escherichia coli infection by multiplex PCR. Bull. Dept. Med. Sci. 39:211-220. [Google Scholar]
- 34.Rothbaum, R., A. J. McAdams, R. Giannela, and J. C. Partin. 1982. A clinicopathological study of enterocyte-adherent Escherichia coli: a cause of protracted diarrhea in infants. Gastroenterology 83:441-454. [PubMed] [Google Scholar]
- 35.Savarino, S. J., A. Fasano, J. Watson, B. M. Martin, M. M. Levine, S. Guandalini, and P. Guerry. 1993. Enteroaggregative Escherichia coli heat-stable enterotoxin 1 represents another subfamily of E. coli heat-stable toxin. Proc. Natl. Acad. Sci. USA 90:3093-3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Savarino, S. J., A. McVeigh, J. Watson, A. Cravioto, J. Molina, P. Echeverria, M. K. Bhan, M. M. Levine, and A. Fasano. 1996. Enteroaggregative Escherichia coli heat-stable enterotoxin is not restricted to enteroaggregative E. coli. J. infect. Dis. 173:1019-1022. [DOI] [PubMed] [Google Scholar]
- 37.Sperandio, V., J. B. Kaper, M. R. Bortolini, B. C. Neves, R. Keller, and L. R. Trabulsi. 1998. Characterization of the locus of enterocyte effacement (LEE) in different enteropathogenic Escherichia coli (EPEC) and Shiga-toxin producing Escherichia coli (STEC) serotypes. FEMS Microbiol. Lett. 164:133-139. [DOI] [PubMed] [Google Scholar]
- 38.Stone, K. D., H.-Z. Zhang, L. K. Carlson, and M. S. Donnenberg. 1996. A cluster of fourteen genes from enteropathogenic Escherichia coli is sufficient for the biogenesis of a type IV pilus. Mol. Microbiol. 20:325-337. [DOI] [PubMed] [Google Scholar]
- 39.Taylor, C. J., A. Hart, R. M. Batt, C. McDougall, and L. McLean. 1986. Ultrastructural and biochemical changes in human jejunal mucosa associated with enteropathogenic Escherichia coli (O111) infection. J. Pediatr. Gastroenterol. Nutr. 5:70-73. [DOI] [PubMed] [Google Scholar]
- 40.Tobe, T., G. K. Schoolnik, I. Sohel, V. H. Bustamante, and J. L. Puente. 1996. Cloning and characterization of bfpTVW, genes required for the transcriptional activation of bfpA in enteropathogenic Escherichia coli. Mol. Microbiol. 21:963-975. [DOI] [PubMed] [Google Scholar]
- 41.Ulshen, M. H., and J. L. Rallo. 1980. Pathogenesis of Escherichia coli gastroenteritis in man—another mechanism. N. Engl. J. Med. 302:99-101. [DOI] [PubMed] [Google Scholar]
- 42.Viljanen, M. K., T. Peltola, S. Y. T. Junnila, L. Olkkonen, H. Jarvinen, M. Kuistila, and P. Huovinen. 1990. Outbreak of diarrhea due to Escherichia coli O111:B4 in schoolchildren and adults: association of Vi antigen-like reactivity. Lancet 336:831-834. [DOI] [PubMed] [Google Scholar]
- 43.Wieler, L. H., T. K. McDaniel, T. S. Whittam, and J. B. Kaper. 1997. Insertion site of the locus of enterocyte effacement in enteropathogenic and enterohemorrhagic Escherichia coli differs in relation to the clonal phylogeny of the strains. FEMS Microbiol. Lett. 156:49-53. [DOI] [PubMed] [Google Scholar]
- 44.Yamamoto, T., and P. Echeverria. 1996. Detection of the enteroaggregative Escherichia coli heat-stable enterotoxin 1 gene sequences in enterotoxigenic E. coli strains pathogenic to humans. Infect. Immun. 64:1441-1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yatsuyanagi, J., S. Saito, H. Sato, Y. Miyajima, K. Amano, and K. Enomoto. 2002. Characterization of enteropathogenic and enteroaggregative Escherichia coli isolated from diarrheal outbreak. J. Clin. Microbiol. 40:294-297. [DOI] [PMC free article] [PubMed] [Google Scholar]