Abstract
Although present in many copies in the mouse genome, xenotropic murine leukemia viruses cannot infect cells from laboratory mice because of the lack of a functional cell surface receptor required for virus entry. In contrast, cells from many nonmurine species, including human cells, are fully permissive. Using an expression library approach, we isolated a cDNA from HeLa cell RNA that conferred susceptibility to xenotropic envelope protein binding and virus infection when expressed in nonpermissive cells. The deduced product is a 696-aa multiple-membrane spanning molecule, is widely expressed in human tissues, and shares homology with nematode, fly, and plant proteins of unknown function as well as with the yeast SYG1 protein, which has been shown to interact with a G protein. This molecule also acts as a receptor for polytropic murine leukemia viruses, consistent with observed interference between xenotropic and polytropic viruses in some cell types. This xenotropic and polytropic retrovirus receptor (XPR1) is the fourth identified molecule having multiple membrane spanning domains among mammalian type C oncoretrovirus receptors and may play a role in G protein-coupled signal transduction, as do the chemokine receptors required for HIV entry.
Retrovirus infection is initiated by binding of the surface (SU) portion of the viral envelope (Env) glycoprotein to specific receptors on cells. This binding is thought to trigger conformational changes in the transmembrane portion of Env, leading to membrane fusion and release of capsids into the cytoplasm (1, 2). This specific interaction is the major determinant of the tissue and species tropism of retroviruses. Based on interference analysis, eight groups of retroviruses have been identified that use different receptors on human cells (3). The recent discovery of three new human-tropic retroviruses from pig and wild mouse origin (4, 5) that represent additional interference groups (6, 7) suggests that at least 11 distinct surface molecules can act as retrovirus receptors on human cells.
The retrovirus receptors identified to date include diverse cell surface proteins (2, 8–11) that fall into three groups: (i) the type I membrane-spanning receptors, which include the CD4 molecule used by HIV-1, HIV-2, and simian immunodeficiency virus, the proteins related to the low density lipoprotein receptor and the tumor necrosis factor receptor, which mediate entry of avian leukosis virus subgroup A and subgroups B, D, and E, respectively, and the receptors for bovine leukemia virus and mouse mammary tumor virus, each of which contains a single transmembrane segment and a globular external domain but no other common features that might be required for viral entry; (ii) the multiple membrane-spanning solute transporters used by murine leukemia viruses (MLV) and related retroviruses, which include the receptor for ecotropic MLV (mCAT) and amphotropic MLV (Pit2) and for gibbon ape leukemia virus (GALV) and feline subgroup B (FeLV-B) retroviruses (Pit1), all of which share common multiple-transmembrane spanning topology and transporter activity (cationic amino acid transport for mCAT and inorganic phosphate transport for Pit1 and Pit2); and (iii) the recently discovered G protein-coupled seven-transmembrane-domain chemokine receptors required for HIV and simian immunodeficiency virus infection.
The receptors for xenotropic and polytropic retroviruses present interesting puzzles. Xenotropic retroviruses were named based on the ability of these mouse viruses to infect cells from many species but not cells from laboratory mice. In contrast, polytropic viruses can infect cells from a reduced range of species but can infect cells from mice (12). Although the difference in host range of these virus groups indicates that the receptors are different, several observations suggest that these receptors are actually closely related: (i) xenotropic viruses can infect cells from some species of wild mice and can block superinfection by polytropic viruses (6, 12, 13), indicating that these viruses may use the same receptor in wild mice; (ii) the xenotropic and polytropic receptors in wild and laboratory mice have been localized to the same region of chromosome 1 and thus may be alleles of the same gene (14–16); and (iii) expression of endogenous polytropic Env glycoproteins from the Rmcf locus can block infection by xenotropic and polytropic MLVs (15). Further confusion arises from the fact that these viruses show nonreciprocal interference in cells from Mus dunni, such that xenotropic virus blocks polytropic virus infection but polytropic virus does not effectively block xenotropic virus (6, 12), suggesting that xenotropic virus may use two receptors in these cells. Molecular cloning of these receptors is required to understand these phenomena.
Identification of the receptor for the polytropic MLV also would help clarify the role of virus–receptor interaction in the disease caused by these viruses. Polytropic viruses, also called mink cell focus-forming viruses because of their ability to induce foci of transformed cells in mink cell lines, are the proximal agents responsible for leukemia induction in MLV-infected mice and are generated by recombination events that involve endogenous proviral sequences in the mouse (17). A key determinant of disease induction is the polytropic Env protein. It has been hypothesized that interaction of the Env protein with its receptor might stimulate cell division observed early in the disease process. Support for this notion comes from the ability of polytropic viruses to stimulate factor-independent growth of factor-dependent lymphoid cells by interaction with the erythropoietin receptor or the interleukin 2 receptor β-chain (18, 19), although the magnitude of the mitogenic response is controversial (20). Furthermore, the gp55 glycoprotein of the erythroleukemia-inducing strain of Friend spleen focus-forming virus, which contains the amino terminal receptor binding domain of a polytropic virus, also can strongly stimulate cell growth through the erythropoietin receptor (21). Alternatively, it has been suggested that polytropic MLV entry might be mediated by T cell antigen receptors and that Env binding to these antigen-specific mitogenic receptors might stimulate cell proliferation (22, 23). Identification of the functional receptor for polytropic MLV entry is required to address these models for leukemia induction. Here we report the expression cloning of a human cDNA that confers susceptibility to both xenotropic and polytropic MLV. The encoded protein, XPR1, contains multiple hydrophobic domains, and cells expressing the protein bind the xenotropic virus SU protein, indicating that XPR1 is a membrane-bound receptor for both of these viruses.
MATERIALS AND METHODS
Cell Culture.
Mammalian cells, including NIH 3T3 thymidine kinase-deficient mouse embryo fibroblasts (24), CHO-K1 Chinese hamster ovary (CHO) cells (ATCC CCL 61), M. dunni tail fibroblast (25), and 293 human kidney cells (ATCC CRL 1573) were grown in DMEM supplemented with 10% fetal bovine serum (HyClone). Nonessential amino acids were added to the medium for CHO cells to supply their requirement for proline.
Retroviral Vectors.
The retroviral vector LAPSN contains a human placental alkaline phosphatase cDNA linked to the retroviral long terminal repeat promoter followed by a neomycin phosphotransferase cDNA linked to the simian virus 40 early promoter (26). The retroviral vector LNCG was constructed by inserting the enhanced green fluorescent protein (GFP) cDNA (CLONTECH) into the LNCX vector (27). LNCG expresses neomycin phosphotransferase driven by the retroviral long terminal repeat promoter and GFP driven by the CMV promoter.
Helper-free retroviral vectors having amphotropic, GALV, or RD114 envelope proteins (pseudotypes) were produced by using PA317 (24), PG13 (28), and FLYRD (29) retrovirus packaging cells, respectively. Cell lines that produced helper-free vectors having a xenotropic pseudotype were generated by transduction of LGPS cells that produce Gag and Gag-Pol proteins (28) with either the LAPSN or LNCG vectors, followed by cotransfection of the cells with the plasmid pCSI.ENZB, which expresses a xenotropic Env protein, and a lower amount (1/20th) of the selectable plasmid pSV2Δ13-hyg, which encodes hygromycin phosphotransferase. The pCSI.ENZB vector was constructed by inserting the xenotropic NZB9.1 Env coding region (30) in place of the β-galactosidase coding region in the pCMVβ plasmid (CLONTECH), which contains a human CMV immediate early promoter and a simian virus 40 intron upstream of β-galactosidase and a downstream simian virus 40 polyadenylation signal. The transfected cells were selected in hygromycin, and clonal lines with highest titers (PX/LAPSN c8 and PX/LNCG c4) were selected.
The LAPSN vector with a polytropic (mink cell focus-forming) pseudotype was produced by using M. dunni cells transduced with LAPSN and infected with replication-competent 98D13 polytropic virus (13). This strain was chosen for its enhanced ability to infect human cells. Vector-containing medium was harvested 16 h after addition of fresh medium to confluent cultures of vector-producing cells, was filtered through a 0.45-μm pore-size filter, and was stored at −70°C.
Retroviral cDNA Library Screening.
A HeLa cDNA library in a murine leukemia virus-based retroviral vector plasmid with a complexity of 2.5 × 106 independent clones (31) was used to produce high-titer virus with a vesicular stomatitis virus G (VSV-G) Env pseudotype as follows. On day 1, 293 cells were seeded at 2 × 106 cells per 10-cm dish. On day 2, the cells were transfected (32) with 15 μg of the retroviral library, 15 μg of a VSV-G envelope glycoprotein expression vector (pCSI-G) [VSV-Indiana-strain G env gene (33) cloned in pCMVβ], 5 μg of the Gag-pol expression vector JK3 (32), and 1 μg of pCMV-tat (32) for transactivation of the HIV long terminal repeat of JK3. Cells were washed with PBS on day 3 and were re-fed with fresh medium. Virus-containing medium was harvested on day 4, was filtered through a 0.45-μm pore-size filter, was diluted 1:2 in culture medium, and was used to infect NIH 3T3 cells seeded the day before in five 6-cm dishes at 2.5 × 105 cells per dish. On day 5, cells in each dish were trypsinized and seeded into 2 15-cm dishes, and on day 6 the cells were exposed to 15 ml of xenotropic pseudotype LAPSN vector from PX/LAPSN producer cells in the presence of 4 μg/ml Polybrene (Sigma). On day 7, the medium was replaced with fresh culture medium containing 1 mg/ml G418 (active concentration). G418-resistant clones were isolated on day 15 and were tested for reinfectability with xenotropic pseudotype LNCG vector produced from PX/LNCG cells.
Receptor cDNA Cloning.
Genomic DNA from several clones was isolated by using the Puregene kit (Gentra Systems). The cDNA inserts from the integrated proviruses were amplified by PCR (Expand Long Template PCR System, Boehringer Mannheim; 3 min at 94°C, then 30 sec at 94°C, 30 sec at 58°C, and 5 min at 68°C for 35 cycles, then 10 min at 68°C) using the 5′LXL-C and 3′LXL-D primers (5′-CCAGCCCTCACTCCTTCTCTAG-3′ and 5′-ATGGCGTTACTTAAGCTAGCTT-GCCAAACCTAC-3′, respectively), followed by a second PCR with the nested primers 5′LXL-A and 3′LXL-B (5′-CCGGAATTCGCGGCCGCGTCGAC-3′ and 5′-GGCCGAGGCGGCCGCTTGTCGAG-3′, respectively, which both contain a NotI restriction site for convenient cloning). PCR products amplified by using primers 5′LXL-A and 3′LXL-B were cloned into pCR2.1-TOPO by using the TOPO TA cloning kit (Invitrogen). NotI-digested cDNA fragments ≥2 kilobases (kb) in size were inserted in the retroviral vector pLXSN-Not, a modified version of pLXSN (27) in which a NotI restriction site has been introduced into the unique HpaI cloning site, and were tested for their ability to confer sensitivity to xenotropic virus infection after transfer into NIH 3T3 cells.
Immunoadhesins.
The immunoadhesin consisting of the SU portion of the amphotropic Env protein linked to a human Fc fragment (ASU-hFc) has been described elsewhere (34). For the xenotropic immunoadhesin (XSU-hFc), the SU portion of the xenotropic NZB9.1 env gene (30) without the carboxy-terminal 9 residues was isolated by PCR and was fused to a human IgG-Fc constant fragment lacking the amino-terminal 3 residues. This immunoadhesin-encoding cDNA was introduced in the pCMVβ vector to promote high level expression in 293 cells. Expression and purification of the XSU-hFc immunoadhesin was performed as described for the ASU-hFc protein (34).
RESULTS
Screening for the Presence of a Xenotropic Retrovirus Receptor in a Retroviral cDNA Library.
The strategy for identification of the xenotropic retrovirus receptor involved screening for receptor activity after retrovirus-mediated transfer of a HeLa cell cDNA library to NIH 3T3 mouse cells that are not normally susceptible to xenotropic virus infection. To screen for receptor function, we used a retroviral vector (LAPSN) that expressed the selectable marker neomycin phosphotransferase and that was produced by using the helper virus-free xenotropic packaging line PX/LAPSN. We verified that human cells were highly susceptible to transduction by this xenotropic vector [vector titer was 1–3 × 106 colony-forming units (cfu)/ml on HeLa and HT-1080 human cells] but that NIH 3T3 mouse cells were totally resistant to vector transduction (vector titer <1 cfu/ml). The NIH 3T3 cells were resistant to xenotropic vector transduction even when treated with tunicamycin (vector titer <1 cfu/ml), a glycosylation inhibitor known to abrogate resistance of CHO cells to MLV infection at the receptor level (35, 36). Thus, we did not expect false positives in our receptor screen because of frequently occurring glycosylation defects such as those we observed in a previous screen for the amphotropic retrovirus receptor in CHO cells (26).
To perform the receptor screen, NIH 3T3 cells were exposed to VSV-G pseudotype retroviral vectors carrying the cDNA library. A parallel experiment using a VSV-G pseudotype vector generated from a plasmid containing a retroviral vector encoding the GFP in place of the library plasmid DNA resulted in 50% of the vector-exposed cells becoming GFP+, indicating that about half of the cells exposed to the library should have contained at least one cDNA. To identify cells that became susceptible to xenotropic vector infection, the cells were exposed to an excess of xenotropic LAPSN vector and were selected in G418. Of 36 independent G418-resistant clones isolated from multiple dishes, 34 could be transduced by a different xenotropic vector (LNCG) that expressed GFP, indicating stable expression of the xenotropic vector susceptibility phenotype.
To verify that sensitivity to xenotropic vector transduction was caused by the expression of a cDNA from the retroviral library and not by other changes in the NIH 3T3 cells, we tested whether the phenotype could be transferred to naive NIH 3T3 cells by vector rescue. Integrated proviruses from 11 reinfectable clones and from 1 clone that was not reinfectable were rescued as retrovirus particles by transfection of Gag-Pol and VSV-G expression plasmids to provide the viral structural proteins, and the rescued vectors were used to transduce naive NIH 3T3 cells. Only transfer of proviruses from the reinfectable clones rendered NIH 3T3 cells sensitive to xenotropic vector transduction (11 of 11 clones tested) (data not shown), indicating that the phenotype of these clones was conferred by vectors in the retroviral library.
Receptor cDNA Cloning.
HeLa cDNA inserts were amplified by PCR from the genomic DNA of several NIH 3T3 clones that could be transduced by xenotropic vectors after transfer of the cDNA library. Several large PCR products were cloned in the pLXSN retroviral vector and were tested for activity. A 4-kb cDNA from NIH 3T3 clone X56 exhibited receptor activity after transient transfection of NIH 3T3 cells. Two other clones with sizes of 3 and 4.3 kb showed patterns of EcoRI restriction fragments similar to that of the X56 clone but were not studied further.
Expression of the Xenotropic Receptor in Resistant Cells Confers Susceptibility to Xenotropic and Polytropic Retrovirus Infection.
To further characterize the X56 xenotropic receptor cDNA, which we renamed XPR1 (see below), a retroviral expression vector containing the cDNA [L(XPR1)SN] was introduced into NIH 3T3 cells, and the resultant cells were challenged with LAPSN vectors having various pseudotypes (Table 1). NIH 3T3 cells expressing the xenotropic receptor were highly susceptible to xenotropic vector transduction whereas cells expressing the RD114/type D retrovirus receptor RDR (31) or no exogenous receptor were not. In addition, the NIH 3T3 cells expressing XPR1 were still resistant to RD114- and GALV-pseudotype LAPSN vector transduction. Therefore, the XPR1 cDNA isolated from the HeLa retroviral library encodes a receptor specific for xenotropic-pseudotype virus infection.
Table 1.
Infection of mouse NIH 3T3 and hamster CHO cells expressing the xenotropic receptor by different LAPSN pseudotypes
| LAPSN pseudotype | LAPSN vector titer on NIH 3T3
cells expressing the following receptors,
cfu/ml
|
LAPSN vector titer on CHO cells expressing
the following receptors, cfu/ml
|
|||||
|---|---|---|---|---|---|---|---|
| None | Xenotropic | RD114/type D | None | Xenotropic | RD114/type D | Amphotropic | |
| Xenotropic | <2 | 4 × 104 | <2 | 50 | 1.5 × 106 | 30 | 20 |
| Polytropic | 1.5 × 108 | 2 × 108 | 1.5 × 108 | <2 | 2 × 104 | <2 | <2 |
| Amphotropic | 6 × 106 | 8 × 106 | 9 × 106 | <2 | <2 | <2 | 4 × 106 |
| RD114 | <2 | <2 | 2 × 104 | <2 | <2 | 3 × 103 | <2 |
| GALV | <2 | <2 | <2 | 60 | 50 | 50 | 20 |
NIH 3T3 and CHO cells expressing the XPR1 xenotropic, RD114/type D (31), amphotropic (26), or no exogenous receptor (empty vector only) were generated by transduction of the cells with L(XPR1)SN, L(RDR)SN, L(Pit2)SN, or LXSN, respectively, followed by selection in G418 to generate polyclonal populations. For assay, 105 target cells were plated in 35-mm-diameter dishes on day 1. On day 2, the medium was replaced with 1 ml of medium containing 4 μg/ml Polybrene and serial dilutions of indicated pseudotypes of the LAPSN vector. Cells were stained for alkaline phosphatase+ foci on day 4 as described (26). Results are average values from duplicate dishes in a representative experiment.
Retrovirus interference studies performed by using wild mouse (6, 12, 13) and human (data not shown) cells suggest that xenotropic and polytropic retroviruses share a common receptor for infection encoded by different alleles of the same gene in mice (15). To test the hypothesis that the human xenotropic receptor XPR1 can act as a polytropic retrovirus receptor as well, we studied the activity of the receptor in CHO cells, which are naturally resistant to xenotropic and polytropic vector transduction (Table 1). As expected, CHO cells expressing no exogenous receptor, the RD114/type D receptor, or the amphotropic receptor were fully resistant to the polytropic LAPSN vector, showing that vector transduction alone, or transfer of unrelated retrovirus receptors, does not confer susceptibility to polytropic vector transduction in CHO cells. The same set of cells were also resistant to the xenotropic LAPSN vector, although a low level of transduction was observed (20–50 cfu/ml). In contrast, the presence of the xenotropic receptor in CHO cells resulted in a >10,000-fold increase in the titer of xenotropic and polytropic vectors on these cells compared with that on CHO cells not expressing the receptor. These results indicate that the protein encoded by the X56 cDNA can be used for entry by both retroviruses. Therefore, the cloned receptor was designated XPR1 for its ability to act as a Xenotropic and Polytropic retrovirus Receptor.
The titer of the polytropic LAPSN vector on CHO cells expressing XPR1 was four orders of magnitude lower than that measured on NIH 3T3 cells (Table 1). The titer of polytropic viruses on human cells is generally lower than that measured on mouse cells (37), and we find a three- to four-order-of-magnitude lower titer on HeLa cells compared with NIH 3T3 cells when using vectors pseudotyped with the polytropic virus used here (data not shown). Thus, the low titer of the polytropic vector on CHO cells expressing XPR1 is likely caused by an intrinsic property of the receptor and not, for example, low level XPR1 expression.
XPR1 Mediates Binding of Xenotropic SU Protein to CHO Cells.
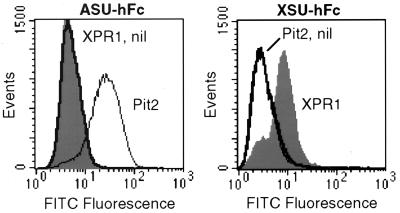
To provide evidence that XPR1 promotes entry of xenotropic retroviruses by direct binding of the xenotropic SU protein, we generated a xenotropic SU-IgG Fc fusion protein (immunoadhesin) (XSU-hFc) and a control amphotropic SU immunoadhesin (ASU-hFc) to measure SU binding to cells. Western blot analysis of conditioned medium from 293 cells transfected with ASU-hFc or XSU-hFc expression plasmids revealed single bands of the expected molecular weights that bound anti-human IgG antibody (data not shown). XSU-hFc bound specifically to polyclonal CHO cells expressing XPR1 whereas it did not bind CHO cells expressing either the amphotropic retrovirus receptor Pit2 or the empty vector (Fig. 1 Right). In the reciprocal control experiment, the same cells were used to confirm that the amphotropic envelope SU exhibits exact specificity for its cognate receptor and that there is no difference in nonspecific binding between cells containing XPR1 or the empty vector (Fig. 1 Left). These experiments indicate the XPR1 is a cell-surface protein and that it specifically binds the xenotropic SU.
Figure 1.
SU-immunoadhesin binding to CHO cells expressing XPR1 or Pit2. Cells were transduced with L(XPR1)SN (XPR1, filled histograms), L(Pit2)SN (Pit2, thin lines), or the LXSN vector without an insert (nil, thick lines) and were selected in G418 to generate polyclonal populations of vector-expressing cells. The cells were detached by treatment with 1 mM EDTA, were washed with PBS, were incubated with ASU-hFc (Left) or XSU-hFc (Right) for 30 min at 37°C, were washed twice with PBS, were incubated with rabbit F(ab′)2 anti-human IgG conjugated to fluorescein isothiocyanate (Dako), and were washed before flow cytometric analysis. Dead and clumped cells were excluded by gating on forward and high-angle light scatter and exclusion of propidium iodide (1 μg/ml), and 50,000 gated events were analyzed per sample.
The Sequence of XPR1 Reveals Multiple Membrane Spanning Domains and Relationships to Other Proteins.
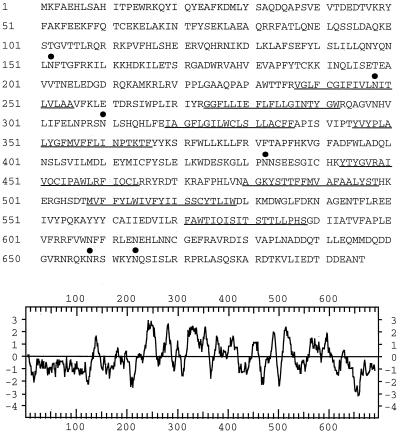
Nucleotide sequence of the XPR1 cDNA revealed the existence of a single ORF of 696 amino acids with six asparagine-linked consensus glycosylation sites (Fig. 2). Hydropathy analysis predicted that the XPR1 protein contains a long hydrophilic amino-terminal region of 236 residues followed by eight hydrophobic domains between amino acids 237 and 588 (Fig. 2), indicating that XPR1 is a membrane spanning molecule.
Figure 2.
Amino acid sequence and hydropathy analysis of the XPR1 protein. Predicted transmembrane segments are underlined, and potential consensus N-glycosylation sites are indicated by filled circles. The glycosylation site NIT at position 248 is located in the first predicted transmembrane segment. The hydropathy plot of XPR1 was generated by the program of Kyte and Doolittle (38) by using a window of 11 residues. Positive values indicate hydrophobic region, and negative values indicate hydrophilic regions.
Searches of protein databases with the XPR1 amino acid sequence failed to identify any mammalian protein related to XPR1. However, XPR1 was similar over most of its length to hypothetical proteins identified by genome sequencing of Caenorhabditis elegans, Drosophila melanogaster, Arabidopsis thaliana, and Schizosaccharomyces pombe (46, 39, 28, and 27% identity, respectively) and to a protein from Saccharomyces cerevisiae (SYG1 protein; 25% identity, 37% similarity) (39) that appears to play a role in G protein-coupled mitogenic signaling (protein relationships calculated by blastp 2.0.6, National Center for Biotechnology Information). The XPR1 hydrophilic amino-terminal region itself showed lesser identity with the Neurospora crassa NUC-2 and S. cerevisiae PHO81 and PHO85 proteins that are all involved in regulating transport of inorganic phosphate (40–42). These findings suggest a role for XPR1 in signal transduction, perhaps in the regulation of phosphate transport.
XPR1 Is Expressed in Multiple Human Tissues.
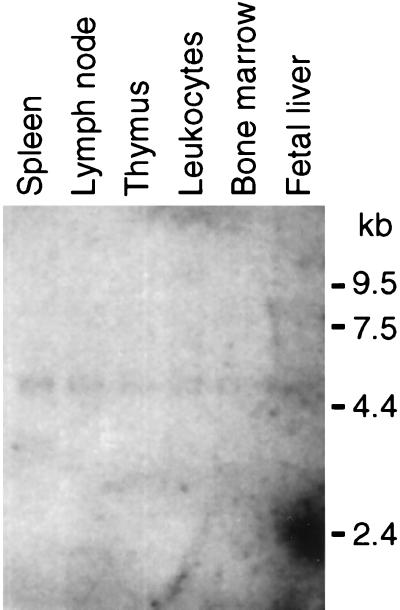
The major XPR1 RNA species in human tissues was ≈5 kb (Fig. 3). In addition to the tissue distribution shown, XPR1 also was found by Northern blot analysis of polyA+ RNA obtained from human kidney, pancreas, and skeletal muscle (data not shown), indicating that XPR1 is expressed widely in human tissues.
Figure 3.
Analysis of XPR1 transcription in human tissues. A Northern blot of polyA+ RNA from the indicated human tissues (Multiple Tissue Northern Blot 7768-1; CLONTECH) was probed with a full length XPR1 cDNA probe at high stringency. Each lane contains ≈2 μg of polyA+ RNA adjusted to give a consistent signal when hybridized to a β-actin probe. RNA size markers are shown at right. The spot at ≈2.4 kb in the far right lane is a hybridization artifact.
The Gene Encoding XPR1 Maps to Human Chromosome 1 Region q25.1.
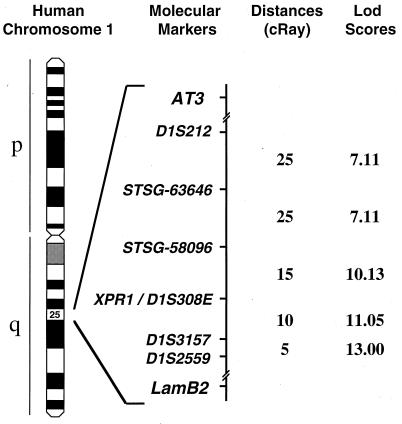
The chromosomal location of the human XPR1 gene was identified by using the Stanford Radiation Hybrid Mapping Panel G3. This panel consists of a set of 83 hamster/human hybrid cell lines containing radiation-fragmented human DNA such that each clonal cell line contains ≈18% of the human genome. The distributions of >10,000 DNA markers have been determined in DNA from these clonal lines, and a linkage map has been constructed, such that new genes can be mapped rapidly and precisely. Primers were designed to amplify a 200-bp fragment of the 3′ untranslated region of the XPR1 gene and were used to detect XPR1 in DNA from the hybrid clones by PCR. The distribution of XPR1-positive hybrids matched perfectly to that of the marker D1S308E (expressed sequence tag EST00717) (Fig. 4). However, the sequence of EST00717 was not found in the XPR1 cDNA, indicating that these cDNAs are transcribed from different genes. The spacing of markers in this chromosomal region indicates that EST00717 is within ≈200 kb of XPR1 (Fig. 4). XPR1 is located at position q25.1 of human chromosome 1 and is flanked by the genes antithrombin 3 (AT3) and lamilin B2 (LAMB2). This region of human chromosome 1 shares synteny with the distal end of mouse chromosome 1, where the xenotropic receptor gene sxv has been mapped in wild mice (15) and where its allele Rmc-1 from inbred mice has been mapped more precisely near LamB2 (16). These results support the hypothesis that XPR1 is the human homolog of the mouse polytropic and xenotropic retrovirus receptors encoded by Rmc-1 and sxv.
Figure 4.
Chromosomal localization of XPR1-encoding gene. Idiogram of human chromosome 1 shows linkage of XPR1 gene to D1S308E as determined by PCR mapping using the G3 Stanford Radiation Hybrid Panel. Distances in cRay between molecular markers and logarithm of odds scores for linkage between adjacent marker pairs are represented. 1 cRay ≈30 kb.
DISCUSSION
We report the identification of a human cDNA that confers susceptibility to xenotropic MLV infection when expressed in NIH 3T3 mouse cells and to xenotropic and polytropic MLV when expressed in CHO hamster cells. The deduced product, XPR1, is a 696-aa protein with multiple transmembrane-spanning domains. Expression of the protein in hamster cells resulted in increased binding of the xenotropic SU immunoadhesin, indicating that the xenotropic SU binds XPR1. The specificity of SU binding and the apparent localization of XPR1 to cell membranes argue for a direct role of XPR1 in xenotropic and polytropic virus binding and entry.
Xenotropic MLV are able to infect cells from a wide range of mammalian species, with the exception of cells from standard laboratory mice. A similar host range is exhibited by other retroviruses, such as GALV, the cat endogenous virus RD114, and FeLV subgroup C (1), but these viruses recognize different receptors based on previous interference studies (3). Our work shows that expression of XPR1 in NIH 3T3 cells allows infection by xenotropic MLV but not by RD114, GALV, or FeLV-C retroviruses (data not shown for FeLV-C), demonstrating that XPR1 is a specific receptor for xenotropic MLV only. This result provides another example demonstrating that cell tropism is not a good predictor of receptor utilization by retroviruses.
Xenotropic and polytropic MLV belong to two separate groups based on cell tropism but to a single group based on interference assays. The ability of XPR1 to function as a receptor for both retroviruses demonstrates that xenotropic and polytropic MLV are in the same receptor group. It is likely that most cells from different species express homologs of the XPR1 gene and that polymorphism resulting in amino acid changes may render XPR1 nonfunctional for one or the other viruses. Therefore, cell tropism provides a good measure of receptor sequence variation specific for retroviruses from a common subgroup. Our preliminary results indicate that the murine homolog of XPR1 displays amino acid divergence, which may prevent xenotropic virus binding and infection. Similar polymorphisms of receptor genes have explained why the human mCAT and the murine Pit1 receptors were not used by ecotropic MLV and GALV respectively.
Interference studies indicate that xenotropic and polytropic viruses share at least one receptor in cells from M. dunni wild mice (6, 12, 13). In addition, these retroviruses display nonreciprocal interference, suggesting that xenotropic virus is able to use two receptors to enter into wild mouse cells (6, 12). A similar feature has been described for 10A1 MLV, which is able to use both Pit1 and Pit2 for cell entry (43, 44). However, receptor mapping studies in wild mice have shown that the xenotropic receptor gene sxv and polytropic receptor gene Rmc-1 map to a single common locus on the distal end of mouse chromosome 1 (14, 15). Therefore, xenotropic MLV may use two receptors that are closely linked, or, alternatively, differences in the receptor binding affinities of xenotropic and polytropic SU proteins may explain this nonreciprocal interference phenomenon.
Structural organization of receptor-binding domains are conserved among SU from mammalian type C retroviruses (45), which include MLV, FeLV, GALV, M. dunni endogenous virus, and porcine endogenous retroviruses. They are organized as a highly conserved antiparallel β sandwich core (46), on which are connected variable loops involved in receptor binding (45, 47). This three-dimensional structure may direct the recognition of receptors displaying common structural features like the multiple transmembrane signature found in mCAT, Pit1, Pit2, and XPR1, in concert with the variable loops that may impose the receptor specificity. Therefore, unknown receptors used by M. dunni endogenous virus, porcine endogenous retroviruses subgroups A and B, and FeLV subgroups A and C also may contain multiple transmembrane domains.
A search of the sequence databases for proteins related to XPR1 revealed hypothetical proteins from C. elegans, D. melanogaster, A. thaliana, S. pombe, and S. cerevisiae that have high sequence identity to XPR1 [25–46% identity and 37–61% similarity (blastp 2.0.6)], indicating that proteins related to XPR1 are conserved in diverse organisms. The most closely related protein with known functions is the yeast protein SYG1, which interacts with a yeast G protein, a member of the class of heterotrimeric proteins consisting of α, β, and γ subunits that are involved in signal transduction (39). This particular G protein transmits the mating pheromone signal, which results in transient cell cycle arrest and differentiation. Mutants lacking the α subunit of this G protein die because of constitutive signaling by G protein β and γ heterodimers. SYG1 was identified in a screen for suppressors of lethality in a Gα-null yeast strain and appears to mediate this effect by direct interaction of the amino-terminal end of the SYG1 with the Gβ subunit (39). XPR1 and SYG1 share sequence identity of ≈30% when the proteins are aligned over their entire lengths and gaps in the alignment are allowed (gap 9.1, Genetics Computer Group, Madison, WI), with shorter stretches of 20–30 amino acids showing up to 59% identity, suggesting that XPR1 also may be involved in G protein-coupled signal transduction. However, it should be noted that neither XPR1 nor SYG1 show homology to G protein-coupled receptors such as the chemokine receptors used for entry by HIV.
The amino-terminal hydrophilic portion of XPR1 also shares sequence identity with proteins involved in the regulation of phosphate transport and alkaline phosphatase secretion, including PHO85, PHO81, and NUC-2. NUC-2 in N. crassa and PHO81 in S. cerevisiae are the most upstream genes in this regulatory pathway, and it has been suggested that these proteins are the sensors for phosphate levels, although direct proof is lacking (40–42, 48). Although the carboxy-terminal two-thirds of both NUC-2 and PHO81 contain multiple ankyrin repeats proposed to mediate interactions with other proteins, XPR1 and SYG1 show no similarity in this region, which consists of multiple membrane spanning domains. Thus, sequence similarities between XPR1 and known proteins suggest that XPR1 is a G protein-coupled signaling molecule that may be a phosphate sensor.
A model of virus-induced leukemogenesis has been proposed in which the interaction of polytropic MLV envelope with a putative antigen receptor (22) or hormone receptor (18) expressed on preleukemic cells in vivo leads to abrogation of growth factor dependency and eventual neoplastic transformation. Identification of the polytropic virus receptor XPR1 as a surface molecule that may be involved in G protein-coupled mitogenic signaling provides an alternative explanation for virus-induced leukemogenesis that should be explored.
Acknowledgments
We thank Ilya Mazo (CLONTECH) for providing the retroviral library, Mike Emerman for the JK3 and pCMV-tat plasmids, David Cosman and the Immunex Corporation for providing the human IgG Fc cDNA used in the construction of the immunoadhesins, Hans-Peter Kiem for helpful discussions on immunoadhesins, and Rebecca J. Gottschalk for excellent technical assistance. This work was supported by National Institutes of Health Grants HL54881 (to J.-L.B. and A.D.M.) and HL36444 (to A.D.M.). J.-L.B. also was supported by a fellowship from the Association Française contre les Myopathies, and J.E.J.R. was supported by Fellowship DRG081 from the Cancer Research Fund of the Damon Runyon-Walter Winchell Foundation.
ABBREVIATIONS
- MLV
murine leukemia virus
- GALV
gibbon ape leukemia virus
- SU
surface portion of the retrovirus envelope protein
- Env
retrovirus envelope protein
- CHO
Chinese hamster ovary
- VSV-G
vesicular stomatitis virus G
- kb
kilobase
- cfu
colony-forming unit
- GFP
green fluorescent protein
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Data deposition: The XPR1 sequence reported in this paper has been deposited in the GenBank database (accession no. AF099082).
References
- 1.Weiss R A. In: The Retroviridae. Levy J A, editor. Vol. 2. New York: Plenum; 1993. pp. 1–108. [Google Scholar]
- 2.Hunter E. In: Retroviruses. Coffin J M, Hughes S H, Varmus H E, editors. Plainview, NY: Cold Spring Harbor Lab. Press; 1997. pp. 71–119. [Google Scholar]
- 3.Sommerfelt M A, Weiss R A. Virology. 1990;176:58–69. doi: 10.1016/0042-6822(90)90230-o. [DOI] [PubMed] [Google Scholar]
- 4.Miller A D, Bonham L, Alfano J, Kiem H P, Reynolds T, Wolgamot G. J Virol. 1996;70:1804–1809. doi: 10.1128/jvi.70.3.1804-1809.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patience C, Takeuchi Y, Weiss R A. Nat Med. 1997;3:282–286. doi: 10.1038/nm0397-282. [DOI] [PubMed] [Google Scholar]
- 6.Miller A D, Wolgamot G. J Virol. 1997;71:4531–4535. doi: 10.1128/jvi.71.6.4531-4535.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takeuchi Y, Patience C, Magre S, Weiss R A, Banerjee P T, le Tissier P, Stoye J P. J Virol. 1998;72:9986–9991. doi: 10.1128/jvi.72.12.9986-9991.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller A D. Proc Natl Acad Sci USA. 1996;93:11407–11413. doi: 10.1073/pnas.93.21.11407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bates P. Cell. 1996;86:1–3. doi: 10.1016/s0092-8674(00)80070-7. [DOI] [PubMed] [Google Scholar]
- 10.Adkins H B, Brojatsch J, Naughton J, Rolls M M, Pesola J M, Young J A. Proc Natl Acad Sci USA. 1997;94:11617–11622. doi: 10.1073/pnas.94.21.11617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Golovkina T V, Dzuris J, van den Hoogen B, Jaffe A B, Wright P C, Cofer S M, Ross S R. J Virol. 1998;72:3066–3071. doi: 10.1128/jvi.72.4.3066-3071.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cloyd M W, Thompson M M, Hartley J W. Virology. 1985;140:239–248. doi: 10.1016/0042-6822(85)90362-9. [DOI] [PubMed] [Google Scholar]
- 13.Chesebro B, Wehrly K. Virology. 1985;141:119–129. doi: 10.1016/0042-6822(85)90188-6. [DOI] [PubMed] [Google Scholar]
- 14.Kozak C A. J Virol. 1983;48:300–303. doi: 10.1128/jvi.48.1.300-303.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kozak C A. J Virol. 1985;55:690–695. doi: 10.1128/jvi.55.3.690-695.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hunter K, Housman D, Hopkins N. Somatic Cell Mol Genet. 1991;17:169–183. doi: 10.1007/BF01232974. [DOI] [PubMed] [Google Scholar]
- 17.Hartley J W, Wolford N K, Old L J, Rowe W P. Proc Natl Acad Sci USA. 1977;74:789–792. doi: 10.1073/pnas.74.2.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li J P, Baltimore D. J Virol. 1991;65:2408–2414. doi: 10.1128/jvi.65.5.2408-2414.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsichlis P N, Bear S E. Proc Natl Acad Sci USA. 1991;88:4611–4615. doi: 10.1073/pnas.88.11.4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kozak S L, Hoatlin M E, Ferro F E, Jr, Majumdar M K, Geib R W, Fox M T, Kabat D. J Virol. 1993;67:2611–2620. doi: 10.1128/jvi.67.5.2611-2620.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li J P, D’Andrea A D, Lodish H F, Baltimore D. Nature (London) 1990;343:762–764. doi: 10.1038/343762a0. [DOI] [PubMed] [Google Scholar]
- 22.McGrath M S, Weissman I L. Cell. 1979;17:65–75. doi: 10.1016/0092-8674(79)90295-2. [DOI] [PubMed] [Google Scholar]
- 23.O’Neill H C, McGrath M S, Allison J P, Weissman I L. Cell. 1987;49:143–151. doi: 10.1016/0092-8674(87)90764-1. [DOI] [PubMed] [Google Scholar]
- 24.Miller A D, Buttimore C. Mol Cell Biol. 1986;6:2895–2902. doi: 10.1128/mcb.6.8.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lander M R, Chattopadhyay S K. J Virol. 1984;52:695–698. doi: 10.1128/jvi.52.2.695-698.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller D G, Edwards R H, Miller A D. Proc Natl Acad Sci USA. 1994;91:78–82. doi: 10.1073/pnas.91.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller A D, Rosman G J. BioTechniques. 1989;7:980–990. [PMC free article] [PubMed] [Google Scholar]
- 28.Miller A D, Garcia J V, von Suhr N, Lynch C M, Wilson C, Eiden M V. J Virol. 1991;65:2220–2224. doi: 10.1128/jvi.65.5.2220-2224.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cosset F L, Takeuchi Y, Battini J L, Weiss R A, Collins M K. J Virol. 1995;69:7430–7436. doi: 10.1128/jvi.69.12.7430-7436.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O’Neill R R, Buckler C E, Theodore T S, Martin M A, Repaske R. J Virol. 1985;53:100–106. doi: 10.1128/jvi.53.1.100-106.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rasko, J. E. J., Battini, J. L., Gottschalk, R. J., Mazo, I. & Miller, A. D. (1999) Proc. Natl. Acad. Sci. USA, in press. [DOI] [PMC free article] [PubMed]
- 32.Bartz S R, Vodicka M A. Methods. 1997;12:337–342. doi: 10.1006/meth.1997.0487. [DOI] [PubMed] [Google Scholar]
- 33.Rose J K, Bergmann J E. Cell. 1982;30:753–762. doi: 10.1016/0092-8674(82)90280-x. [DOI] [PubMed] [Google Scholar]
- 34.Kurre P, Kiem H P, Morris J, Heyward S, Battini J L, Miller A D. J Virol. 1999;73:495–500. doi: 10.1128/jvi.73.1.495-500.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miller D G, Miller A D. J Virol. 1992;66:78–84. doi: 10.1128/jvi.66.1.78-84.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miller D G, Miller A D. J Virol. 1993;67:5346–5352. doi: 10.1128/jvi.67.9.5346-5352.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Loiler S A, DiFronzo N L, Holland C A. J Virol. 1997;71:4825–4828. doi: 10.1128/jvi.71.6.4825-4828.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kyte J, Doolittle R F. J Mol Biol. 1982;157:105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- 39.Spain B H, Koo D, Ramakrishnan M, Dzudzor B, Colicelli J. J Biol Chem. 1995;270:25435–25444. doi: 10.1074/jbc.270.43.25435. [DOI] [PubMed] [Google Scholar]
- 40.Cross F. Trends Genet. 1995;11:209–211. doi: 10.1016/s0168-9525(00)89047-2. [DOI] [PubMed] [Google Scholar]
- 41.Lenburg M E, O’Shea E K. Trends Biochem Sci. 1996;21:383–387. [PubMed] [Google Scholar]
- 42.Peleg Y, Aramayo R, Kang S, Hall J G, Metzenberg R L. Mol Gen Genet. 1996;252:709–716. doi: 10.1007/BF02173977. [DOI] [PubMed] [Google Scholar]
- 43.Miller D G, Miller A D. J Virol. 1994;68:8270–8276. doi: 10.1128/jvi.68.12.8270-8276.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miller A D, Chen F. J Virol. 1996;70:5564–5571. doi: 10.1128/jvi.70.8.5564-5571.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Battini J L, Heard J M, Danos O. J Virol. 1992;66:1468–1475. doi: 10.1128/jvi.66.3.1468-1475.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fass D, Davey R A, Hamson C A, Kim P S, Cunningham J M, Berger J M. Science. 1997;277:1662–1666. doi: 10.1126/science.277.5332.1662. [DOI] [PubMed] [Google Scholar]
- 47.Battini J L, Danos O, Heard J M. J Virol. 1998;72:428–435. doi: 10.1128/jvi.72.1.428-435.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Littlewood B S, Chia W, Metzenberg R L. Genetics. 1975;79:419–434. doi: 10.1093/genetics/79.3.419. [DOI] [PMC free article] [PubMed] [Google Scholar]