Abstract
The second case of phaeohyphomycosis caused by Veronaea botryosa in China, in a 12-year-old boy from Jiangsu Province, is presented. Based on direct examination of the scrapings from crusted lesions; histologic examination of the biopsy tissue showing septate, phaeoid hyphal elements; and the culture exhibiting sympodial, conidiogenous cells producing predominantly two-celled, cylindric conidia, the etiologic agent was identified as V. botryosa.
CASE REPORT
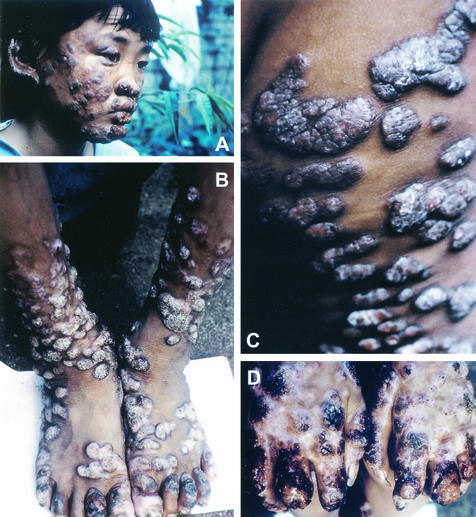
A 12-year-old boy from Jiangsu Province, China, received a seemingly innocuous scratch on his right elbow at the age of 6 years. Later, several 10- to 12- by 6- to 8-mm papules with exudates appeared sporadically on his extremities and became crusted. Within a period of 2 years, the infection had spread to other parts of the body—namely, face, hands, legs, scrotum, and buttocks—except for the chest and back (Fig. 1). The lesions became nodular, forming thick, crusted areas, especially on the patient's face, the backs of his hands, and the crural area. Adjacent lesions often coalesced to form plaques. Superficial lymph node swellings were not observed. The peripheral blood was found to be normal with positive HbsAg. No abnormal findings were noted in a urine test, chest X ray, and abdominal echo examination. Histopathologic and mycological examinations were out and the patient received treatment, including antifungal therapy, over a period of many months, without significant improvement. Effective treatment is still being sought.
FIG. 1.
Verrucous crusty lesions caused by V. botryosa covering face (A), legs and feet (B), buttocks (C), and hands (D) of a 12-year-old Chinese boy.
Histopathologic examination.
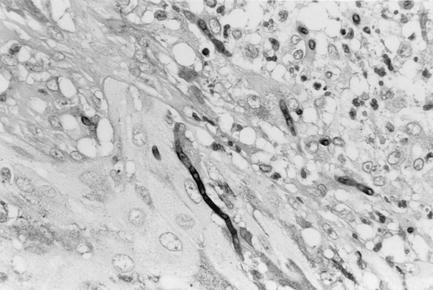
Biopsy tissue slides were stained by the hematoxylin and eosin stain and periodic acid Schiff's reagent procedures. Microscopic examination of hematoxylin- and eosin-stained slides revealed hyperkeratosis and incrustation accompanying parakeratosis and containing neutrophils and yellow-brown-pigmented, septate hyphal elements of various lengths. The epidermis was thickened irregularly and showed pseudoepitheliomatous hyperplasia. High-density inflammatory cells were seen in the upper and middle layers of the dermis. Microabscesses, composed of neutrophil aggregates surrounding hyphal elements, were present, and a granulomatous reaction consisting of giant cells containing fungal hyphae was observed. The hyphal elements of various lengths were septate, branched, or unbranched and were pale brown (Fig. 2).
FIG. 2.
Tissue section showing septate hyphal elements of V. botryosa with periodic acid Schiff's reagent. Magnification, ×465.
Mycological examination.
Microscopic examination of the scrapings from crust of the lesions in potassium hydroxide mounts showed numerous septate, branched, yellowish-brown-pigmented hyphae 2.5 to 4.0 μm in diameter. Scrapings from the crusty lesions and biopsy tissue were cultured on in-house-prepared nutritional media (3), namely, Sabouraud dextrose agar containing chloramphenicol (Sab+C) and Sab+C containing cycloheximide. The culture plates were incubated at 25 and 37°C in the dark and were observed every 2 days. Numerous velvety, olivaceous gray colonies were observed after 7 to 10 days of incubation on all plates. Bacterial and mycobacterial cultures were negative.
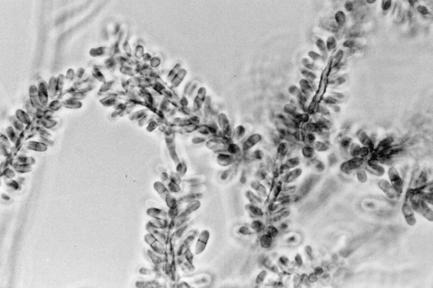
A subculture of the isolate was studied in greater details at the Centers for Disease Control and Prevention (CDC). Colonies on Sab+C and potato dextrose agar (Difco Laboratories, Detroit, Mich.) were velvety to lanate, grayish brown to black, and reached 22 to 25 mm in diameter in 10 days at 25°C. Growth at 37°C was slow, reaching 9 to 11 mm in diameter after 2 weeks. No growth was evident at 40°C. Slide cultures on potato dextrose agar were incubated at 25°C for 10 days and were examined using lactophenol cotton blue. Vegetative hyphae were septate, branched, melanized, and 2.5 to 4.5 μm wide, bearing lateral and terminal conidiophores. The conidiophores were erect, straight, or flexous, unbranched or occasionally branched, rarely geniculate, smooth walled, septate, and pale brown, reaching up to 250 to 200 μm in length and 2.5 to 4.0 μm in width. Conidiogenous cells were terminal or lateral and cylindrical in the apical area, bearing numerous scars. Conidia were produced sympodially and were smooth walled and cylindrical, with rounded apices, and truncate at the bases. They were hyaline to pale brown, zero to three septate (the majority being one septate), and measured 4.5 to 10 μm in length by 2 to 4 μm wide (Fig. 3). The isolate (CDC B-5937) hydrolyzed urea and starch and liquefied gelatin. It was identified as Veronaea botryosa. It has been deposited in the University of Alberta Microfungus Collection and Herbarium, Edmonton, Alberta, Canada (UAMH 9743) and at the Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands (CBS 102593).
FIG. 3.
Slide culture of V. botryosa (CDC B-5937) on potato dextrose agar showing sympodial conidiogenous cells with one-septate, cylindrical conidia. Magnification, ×650.
Treatment.
The patient received treatment in China with a Chinese herbal medication (“Lanmei su” tablets) for >1 year without any marked improvement. Because of the unsatisfactory response, the family contacted the senior author (A.M.) for treatment. As extensive areas of the body were involved with lesions, the recommended treatment consisted of bathing; disinfection of the affected parts with a low concentration of povidone-iodine; local heat treatment, especially of the lesions on the backs of both hands and the lower extremities; local injections of amphotericin B in the facial area; and oral administration of terbinafine. The patient was treated with terbinafine (125 mg/day) for 6 months followed by itraconazole (100 mg/day) for 6 months. At the end of treatment, slight clinical improvement was noted. The last follow-up was done on 20 March 2002. A local Chinese doctor reported enlargement and increase in the number and size of the facial nodules, although the nodular lesions on the hands and legs had become more flattened. The patient eats food with some difficulty due to the painful lesions on his lips. His therapy was stopped for 3 months because of the unsatisfactory results, and he is currently without any treatment due to financial difficulties. His family is now looking for help to receive treatment abroad.
Discussion.
More than 130 fungal species belonging to 70 diverse genera have been reported as causative agents of human and animal phaeohyphomycosis (2, 9). In spite of the global distribution of V. botryosa in soil samples from Brazil (11), China (3, 12), Egypt, and India; on plant materials from Italy; as an air contaminant in New Zealand; from water in Poland (http://www.cabri.org/CABRI/home/Hypercat/fun/caf544.htm); and from alligator farms in Queensland, Australia (http://www.rirde.gov.au), the human infections caused by V. botryosa have been few. There are only four known human infections caused by V. botryosa described in the literature. Because of its rarity as a human pathogen, we deem it worthwhile to report this case. The first case of subcutaneous infection in a 24-year-old male farmer from Henan Province, China, was described in 1990 (15, 16). The patient had black verrucous nodules and cysts on the back of his left hand, forearm, and both cheeks. The second infection caused by V. botryosa presented as nodular, ulcerating lesions on the thumb and fifth finger of the right hand of a 28-year-old Libyan woman with a flexure deformity living in Tripoli (1). There were ulcerating lesions of the nasal mucosa and in the mouth, especially on the palate. In 1998, Medina et al. from the Philippines reported a 34-year-old medical worker with erythematous, pruritic papules on the right deltoid and left shin caused by V. botryosa (A. L. Medina, J. A. D. Redondo, and L. M. Nebrida, Proc. 4th China Japan Int. Congr. Mycol., p. 88, 1998). The fourth case, from France, involved a 57-year-old liver transplant recipient undergoing immunosuppressive therapy who developed multiple painless dermal nodules that coalesced and spontaneously yielded pus. A skin biopsy when cultured yielded V. botryosa. Prolonged treatment with itraconazole healed all cutaneous lesions (7). The present case represents the second case of subcutaneous phaeohyphomycotic infection due to V. botryosa from China involving extensive areas of the body. The distribution of the lesions over different areas of the body in this case was probably due to self-implantation and frequent scratching rather than involvement of lymph nodes. In the present case, the lymph nodes were not swollen, and no abnormalities were found in the internal organs. The patient was checked for metastasis to the internal organs, but no particular abnormality was found. His immune status was not checked because it was not possible to carry out special tests of his immune system in China. However, his past history did not reveal any bacterial or viral infections, and his immune system seemed to be normal. His growth rate (bodyweight and height) was the same as that of any healthy child.
The prepubertal age of the patient in the present case seems to have had some effect on the extensive spread of the lesions over various parts of his body. In the previously described four cases caused by V. botryosa (1, 7, 15, 16; Medina et al., Proc. 4th China Japan Int. Congr. Mycol.), all of the patients were adults, and their lesions were localized. The influence of the age of the patient on the extensive nature of the lesions was also pointed out by de Hoog et al. (4) in infections caused by the phaeohyphomycotic agent Exophiala spinifera. The subcutaneous infections in children (8, 10, 13, 14) involved extensive areas of the body, causing granulomatous crusty lesions. The infections in those cases became chronic and involved lymph nodes, severe osteomyelitis, and eventual fatal outcomes. The lack of early diagnosis and tardy initiation of appropriate therapy may also have influenced the extensive nature of the lesions in our case.
The epidemiology and ecology of V. botryosa are not well known. V. botryosa has been isolated from soil and rotting plant material in China (3, 12), and based on its occurrence as a saprophyte in soil and plant material and as a rare human pathogen, de Hoog et al. (3) classified V. botryosa as a biosafety level 1 pathogen. Since more cases of human infections due to V. botryosa have now been described (1, 7, 15, 16; Medina et al., Proc. 4th China Japan Int. Congr. Mycol.), we recommend that V. botryosa be classified as a biosafety level 2 pathogen. The genus Veronaea has nine species, with V. botryosa as the type species (5, 6). Only V. botryosa is known to cause human infections. The morphological descriptions of the remaining eight species are based on examination of either plant material, such as dead leaves, plant stems, or wood, or animal dung on which they were growing. Of the eight species, Veronaea coprophila (now known to be synonymous with V. botryosa) and Veronaea carlinae produce cylindrical-to-ellipsoidal conidia with one to three septations, which thus resemble those of V. botryosa. However, nothing is known regarding their temperature tolerance, growth rates at different temperatures, and other culture features, since they have not been isolated and grown in culture. V. botryosa resembles species of Rhinocladiella in some respects. The conidiogenous cells of V. botryosa and Rhinocladiella spp. are sympodial. However, the conidia produced by species of Rhinocladiella are one celled, while those produced by V. botryosa are predominantly two celled.
REFERENCES
- 1.Ayadi, A., M. R. Huerre, and C. de Bievre. 1995. Phaeohyphomycosis caused by Veronaea bothryosa. Lancet 346:1703-1704. [DOI] [PubMed] [Google Scholar]
- 2.Chabasse, D. 2002. Les phaeohyphomycetes agents de phaeohyphomycosis: des champignons emergents. J. Mycol. Med. 12:65-85. [Google Scholar]
- 3.de Hoog, G. S., J. Guarro, J. Gene, and M. J. Figueras. 2000. Atlas of clinical fungi. Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands.
- 4.de Hoog, G. S., N. Poonwan, and A. H. G. Gerrits van den Ande. 1999. Taxonomy of Exophiala spinifera and its relationship to E. jeanselmei. Stud. Mycol. 43:133-142. [Google Scholar]
- 5.Ellis, M. B. 1971. Dematiaceous hyphomycetes. Commonwealth Agricultural Bureaux, Farnham Royal, Slough, United Kingdom.
- 6.Ellis, M. B. 1976. More dematiaceous hyphomycetes. Commonwealth Agricultural Bureaux, Farnham Royal, Slough, United Kingdom.
- 7.Foulet, F., C. Duvoux, C. de Bievre, C. Hezode, and S. Bretagne. 1999. Cutaneous phaeohyphomycosis caused by Veronaea botryosa in a liver transplant recipient successfully treated with itraconazole. Clin. Infect. Dis. 29:689-690. [DOI] [PubMed] [Google Scholar]
- 8.Lacaz, C. S., E. Porto, J. G. Andrade, and F. D. Q. Telles Filho. 1984. Feohifomicose disseminada por Exophiala spinifera. Ann. Bras. Dermatol. 59:238-243. [Google Scholar]
- 9.Matsumoto, T., and L. Ajello. 1998. Agents of phaeohyphomycosis, p. 503-524. In L. Collier, A. Balows, and M. Sussman (ed.), Topley and Wilson's microbiology and microbial infections, vol. 4. Oxford University Press, New York, N.Y.
- 10.Mirza, S. H., A. Hannan, A. Ahmad, and M. Ahmad. 1993. Subcutaneous phaeohyphomycosis. J. Infect. 27:75-78. [DOI] [PubMed] [Google Scholar]
- 11.Montenegro, M. R., M. Miyaji, M. Franco, K. Nishimura, K. I. Coelho, Y. Horie, R. P. Mendes, A. Sano, K. Fukushima, and D. Fecchio. 1996. Isolation of fungi from nature in the region of Botucatu, state of Sao Paulo, Brazil, an endemic area of paracoccidioidomycosis. Mem. Inst. Oswaldo Cruz 91:665-670. [DOI] [PubMed] [Google Scholar]
- 12.Nishimura, K., M. Miyaji, H. Taguchi, D. L. Wang, R. V. Li, and Z. H. Meng.1989. An ecological study on pathogenic dematiaceous fungi in China, p. 17-20. In Current problems of opportunistic fungal infections. Proceedings of the 4th International Symposium of the Research Center for Pathogenic Fungi and Microbial Toxicoses, Chiba University, Chiba, Japan.
- 13.Padhye, A. A., L. Ajello, F. W. Chandler, J. E. Banos, E. Hernandez-Perez, J. Llerena, and L. M. Linares. 1983. Phaeohyphomycosis in El Salvador caused by Exophiala spinifera. Am. J. Trop. Med. Hyg. 32:799-803. [DOI] [PubMed] [Google Scholar]
- 14.Rajam, R. V., K. C. Kandhari, and M. J. Thirumalachar. 1958. Chromoblastomycosis caused by a rare yeast-like dematiaceous fungus. Mycopathol. Mycol. Appl. 9:5-19. [DOI] [PubMed] [Google Scholar]
- 15.Wang, D. L., R. Y. Li, X. H. Wang, and H. E. Zhang. 1991. Studies on Veronaea botryosa agent of the first human case. Acta Mycol. Sinica 10:159-165. [Google Scholar]
- 16.Zhang, H. E., D. L. Wang, and R. Y. Li. 1990. Report of the case of phaeohyphomycosis caused by Veronaea botryosa. Chinese J. Dermatol. 23:96-98. [Google Scholar]