Abstract
We conducted an exploratory investigation in a community in Haiti to determine the prevalence of Cyclospora cayetanensis infection and to identify potential risk factors for C. cayetanensis infection. In 2001, two cross-sectional stool surveys and a nested case-control study were conducted. In 2002, a follow-up cross-sectional stool survey was conducted among children ≤10 years of age. Stool specimens from study participants and water samples from their wells were examined for Cyclospora and other intestinal parasites. In stools, the prevalence of infection with Cyclospora in persons of all ages decreased from 12% (20 of 167 persons) in February 2001 to 1.1% (4 of 352 persons) in April 2001, a 90.8% decrease. For children ≤10 years of age, the prevalence rates were 22.5% (16 of 71 children) in February 2001, 3.0% (4 of 135 children) in April 2001, and 2.5% (2 of 81 children) in January 2002. Use of the water from the artesian well in the northern region of the community versus the one in the south was the only risk factor associated with Cyclospora infection in multivariate analyses (odds ratio, 18.5; 95% confidence interval, 2.4 to 143.1). The water sample from one of the nine wells or water sources tested (one sample per source) in January 2001, shortly before the investigation began, was positive for Cyclospora by UV fluorescence microscopy and PCR. None of the water samples from the 46 wells or water sources tested during the investigation (one sample per source per testing period, including the artesian wells) were positive for Cyclospora. Further studies are needed to assess the role of water as a possible risk factor for Cyclospora infection in Haiti and other developing countries.
The modes of transmission and the risk factors for the cases of Cyclospora cayetanensis infection that have been diagnosed and investigated in the United States are well defined (14). Most of these cases have been associated either with foreign travel or with food-borne outbreaks caused by produce imported from countries where Cyclospora is endemic (14). Several reported U.S. cases might have been associated with exposure to contaminated drinking or recreational water or to sewage (13, 18, 25).
In contrast, little is known about the modes of transmission or risk factors for Cyclospora infection in settings where Cyclospora is endemic. Specifically, few studies to address these and other epidemiologic issues have been conducted in Haiti. From January 1997 to January 1998, stool specimens were collected during six different periods from a convenience sample of mothers and children living in and around Leogane, Haiti (9). Cyclospora infection was most prevalent in January (16 of 140 persons; 11.4%) and March (18 of 116; 15.5%) and was most common in children ≤10 years of age (41 of 49 children; 83.7%) (9). From 1990 to 1993, in a clinic-based study in Port-au-Prince, Haiti, 51 (11.3%) of 450 human immunodeficiency virus-positive persons who had had diarrhea for at least 3 weeks were infected with Cyclospora (20). However, the seasonality of infection was not addressed.
In 2001 and 2002, we conducted an exploratory investigation to determine the prevalence of Cyclospora infection and to identify the potential risk factors for Cyclospora infection, which could be evaluated further in later studies.
MATERIALS AND METHODS
Epidemiologic investigation.
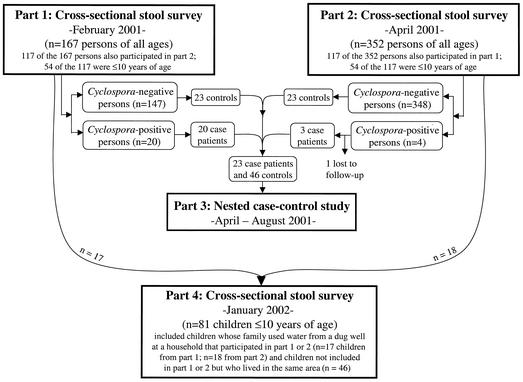
The investigation described here, which had four parts, was approved by the institutional review boards of the Hospital St. Croix (Leogane, Haiti) and the Centers for Disease Control and Prevention (CDC). In a community outside of Leogane, Haiti, which is near the hospital, two cross-sectional surveys (parts 1 and 2) and one nested case-control study (part 3) were conducted in 2001 and a follow-up cross-sectional survey (part 4) was conducted in 2002 (Fig. 1).
FIG. 1.
Flow diagram of parts 1, 2, 3, and 4 of an investigation conducted in a community in Haiti from January 2001 to January 2002. For the cross-sectional stool survey of January 2002, stool containers were also given to children who used water from a dug well at a household that participated in part 1 and/or 2 in 2001, regardless of whether the child had participated in that part(s).
Parts 1, 2, and 3 (2001).
Part 1, a cross-sectional stool survey, was conducted in February 2001 (Fig. 1). The locations of the houses in the community were mapped, the houses were numbered, and some were randomly selected for the investigation (see below). All persons in the selected houses were asked to participate. Informed consent was obtained from at least one adult member of the household for the entire household and all parts of the investigation. Part 2, another stool survey, was conducted in April 2001. In both surveys, stool collection containers were given to everyone in the selected houses, and basic demographic information was collected. The study population for these two surveys consisted of persons who provided stool specimens during part 1 and/or 2.
From April to August 2001, all persons with stool specimens positive for Cyclospora in part 1 and/or 2 and a sample of controls with stool specimens negative for Cyclospora were enrolled in part 3, a nested case-control study (Fig. 1). We used a structured questionnaire about potential risk factors for infection with Cyclospora, such as sources for drinking and other water; consumption of specific uncooked vegetables, fruits, and herbs; household sanitation; socioeconomic indicators (e.g., the quality of and the materials used for household construction); and the presence of animals. On the basis of discussions with community members, we assumed that exposure variables, such as water sources, were relatively constant throughout the year and therefore did not limit questions to a particular period.
Case patients and controls were selected without regard to whether the person was symptomatic or infected with other parasites. A patient with a case of cyclosporiasis was defined as a person with a stool specimen positive for Cyclospora during at least one of the two stool surveys. A control was defined as a person with stool specimens negative for Cyclospora. Two controls were randomly selected and frequency matched by age to each case patient. One of the two controls for every case patient had been tested only once because the control had been enrolled during part 2.
Part 4 (2002).
In January 2002, another cross-sectional stool survey, part 4, was conducted among children ≤10 years of age (Fig. 1). Part 4 included only these persons because most (20 of 24; 83.3%) of the case patients identified in 2001 were in this age group.
After each stool survey, mebendazole was offered to persons infected with intestinal helminths. Treatment for Cyclospora infection was not provided because in a previous study some children had reactions to sulfa drugs that were more troublesome than any symptoms that they might have had from Cyclospora infection (9).
Laboratory and environmental investigations.
The consistency of each stool specimen was recorded, and a portion of each specimen was concentrated by the formalin-ethyl acetate procedure (10). The sediment from the concentrate was subsequently examined by UV fluorescence microscopy to detect Cyclospora. Wet-mount preparations of the stool specimens were examined for helminths and protozoa. Additionally, Meridian Meriflour direct fluorescent-antibody assay kits were used to identify the protozoa Giardia lamblia and Cryptosporidium parvum (11). Specimens were not tested for bacterial or viral pathogens.
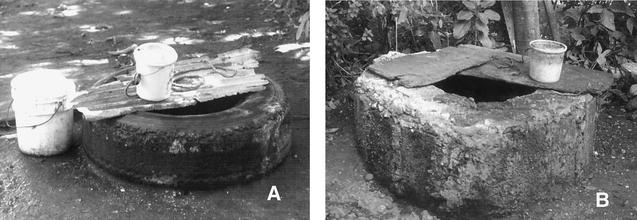
To identify potential sources of Cyclospora, water samples (10 liters per sample) were collected from manually dug, surface-water wells throughout the community (see below), from the two artesian wells in the community, from a river, and from a spring that fed into the river (Fig. 2). The manually dug wells were either unlined with a tire ring at ground level to indicate the location of the well; unlined with no tire ring; or lined with concrete, with a concrete ring at ground level (Fig. 3). For the dug wells, we collected water in the same way in which it was collected by persons in the community: water was collected from the surface by dropping buckets tied to a rope into the well and retrieving the bucket. In January 2001, shortly before the investigation began, water samples (one per source) were collected from nine arbitrarily, but not randomly, selected dug wells or water sources. During part 3, in 2001, water samples (one per well) were collected from the water flowing out of the two artesian wells and from the dug wells used by case patients and one of the controls per case patient. Water samples were then processed by calcium carbonate flocculation (24) and tested for Cyclospora, Cryptosporidium, Giardia, Ascaris, Trichuris, and hookworm. The processed water samples were examined by the same methodology used for the stool specimens.
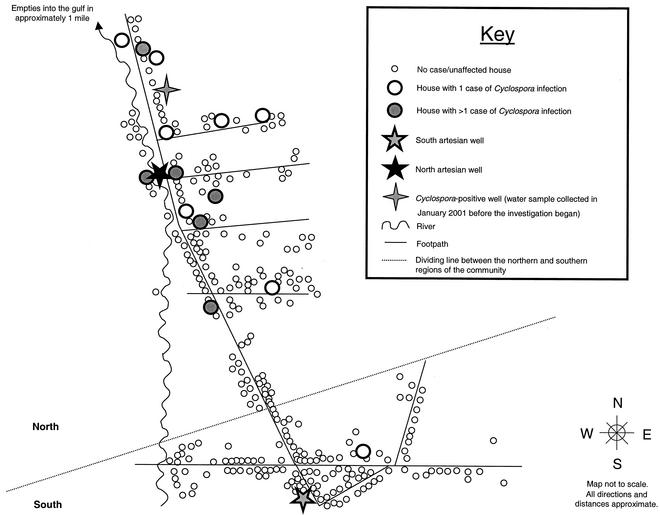
FIG. 2.
Map of the study community in Haiti that illustrates the distribution of cases of Cyclospora infection identified during the investigation. The map includes all households that were mapped during the investigation, regardless of whether the household participated in any of the studies. Although it appears that the northern region of the community is much larger than the southern region, the numbers of houses in each region were comparable. Most households in the community had dug wells, and some (an unknown percentage) of the dug wells were covered when not in use.
FIG. 3.
An unlined dug well with a tire ring (A) and a concrete-lined dug well with a concrete ring (B). The construction and quality of the different types of wells varied throughout the community. Examples of variation included the height of the concrete ring; the types, quality, and numbers of tires used for the tire rings; and the materials, if any, used to cover the wells when not in use. Both community members and research staff typically used buckets tied to ropes, such as the ones shown in panels A and B, to retrieve water from the wells.
In part 4, in 2002, we used U.S. Environmental Protection Agency Method 1623 (23) to collect and process water samples. The samples (18.9 liters per sample) were collected from selected dug wells, the two artesian wells, and five additional randomly selected water sources within the community (e.g., the river) (one sample was collected from each source). The samples were then tested for the same organisms, by the same methodologies, listed above. Because of field conditions, a manual pump, which did not account for flow rate, was used to push water through the filters (Envirocheck High Volume sampling capsules; Pall Corporation, Ann Arbor, Mich.). Samples were held at 4°C for over 72 h before processing; immunomagnetic separation was not done with the processed samples. When the samples were collected in 2001 and 2002, additional information about the parameters of the wells, such as type (e.g., unlined dug well), depth, and diameter, was obtained.
If a processed water sample was positive for Cyclospora by UV fluorescence microscopy, it also was examined by PCR. A volume of 300 μl of water concentrated from 500 μl was processed for PCR by using selected reagents from the FastDNA kit (Bio 101-Qbiogene, Vista, Calif.), as described elsewhere (6). Nested PCR amplification was performed by using primers CYCF1 and CYCR2 for the first step and primers CYCF3 and CYCR4 for the second step, as described elsewhere (7). The nested PCR amplifies a specific fragment of 294 bp of the small-subunit rRNA-coding region of at least four Cyclospora spp. (i.e., C. cayetanensis, C. colobi, C. cercopitheci, and C. papionis).
Statistical analyses.
A sample size of 400 persons in both parts 1 and 2 would be sufficient, with 80% power, to identify enough case patients for part 3 (i.e., to detect an odds ratio [OR] of 3.5 between the case patients and the controls for the key variables in the questionnaire, assuming a 7% prevalence rate of Cyclospora infection in the community and a 33% increase in the standard error because of clustering of case patients).
Univariate and multivariate ORs, 95% confidence intervals (CIs), and standard errors were calculated by using the SUDAAN program (release 7.5; Research Triangle Institute, Research Triangle Park, N.C.) to account for clustering for part 3 of the investigation. The Stat Exact program (version 5.0) was used to calculate 95% CIs for very small sample sizes or when the calculated proportion was close to 0 or 100%. The chi-square, Fisher exact, and Wilcoxon rank sum tests were used to determine differences between the types of participants (e.g., case patients versus non-case patients). Logistic regression was performed by using SAS Proc Genmod program (version 8.0 for Windows; SAS Institute Inc., Cary, N.C.); the generalized estimating equations procedure was used to adjust for correlations among persons in the same household. An exchangeable correlation structure was assumed.
RESULTS
Epidemiologic investigation.
The environments of the southern and northern regions of the community were distinct. In the southern region, houses were close together and little vegetation was present. Houses usually were made from cement, and most of the dug wells were lined with cement and had concrete rings. In contrast, the community was more rural in the northern region, where houses were more dispersed and more vegetation was present. Houses usually were made from sticks and adobe and had dirt floors. Most (an unknown percentage) of the dug wells were unlined and had tire rings.
Parts 1, 2, and 3 (2001).
The population of the community was about 1,500. In 2001, in parts 1 and 2, stool specimens were collected from a total of 402 (75.6%) of the 532 persons who agreed to participate in the investigation. The median age of the participants was less than that of the nonparticipants (16 years [range, 1 to 86 years] versus 22 years [range, 1 to 80 years], respectively; P = 0.002); 57.0% (229 of 402) of the participants and 51.5% (67 of 130) of the nonparticipants were female (P = 0.28).
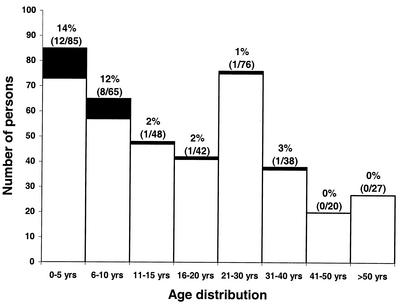
Figure 1 shows the study design and the study population for parts 1 and 2. Twenty-four persons (6.0%; 95% CI, 2.4 to 9.5%) had stool specimens positive for Cyclospora; 18 (75.0%; 95% CI, 54.4 to 95.6%) of the 24 were identified only during part 1, 4 (16.7%; 95% CI, 4.7 to 37.4%) were identified only during part 2, and 2 (8.3%; 95% CI, 1 to 27%) were identified in both surveys. The median age of the case patients (n = 24) was less than that of the non-case patients (n = 377) (5.5 years [range, 1 to 32 years] versus 16 years [range, 1 to 86 years], respectively; P < 0.001) (Fig. 4). Twenty of the 24 infected persons were ≤10 years of age; comparable percentages of children in the age groups of 0 to 5 and 6 to 10 years were infected (i.e., 14 and 12%, respectively; Fig. 4). Fifty percent (12 of 24) of the case patients and 57.4% (217 of 378) of the non-case patients were female (P = 0.48).
FIG. 4.
Age distribution among case patients (n = 24; black boxes) and non-case patients (n = 378; white boxes) who participated in part 1 and/or 2. The age of one non-case patient was unknown. Fifty (65.8%) of the 76 persons in the 21- to 30-year age range were women; they are the predominant figures in Haitian households and thus the adults most likely to be home. The percentages (numerator/denominator) of persons in each age group infected with Cyclospora are provided. The prevalence for children ≤10 years of age (i.e., those in age groups of 0 to 5 and 6 to 10 years) was 13.3% (20 of 150).
The 24 case patients were 3.1 times more likely than the non-case patients to have watery versus soft or firm stools (P = 0.13). Infection with Giardia was the most prevalent, while infection with Cryptosporidium was the least prevalent (Table 1).
TABLE 1.
Prevalences of infection with intestinal parasites in the study population in a community in Haiti in 2001 and 2002
| Parasite | Infection prevalence (no. [%]; 95% CI) ina:
|
|||
|---|---|---|---|---|
| February and April 2001b (persons of all ages [n = 402]) | February and April 2001b (persons ≤10 years of age [n = 150]) | January 2002c (persons ≤10 years of age [n = 81]) | ORd (95% CI) | |
| Giardia lamblia | 110 (27.4; 20.7-33.9) | 68 (45.3; 35.4-55.3) | 20 (24.7; 15.4-34) | 2.5 (1.3-4.8) |
| Trichuris trichuria | 91 (22.6; 16.3-28.9) | 44 (29.3; 20.3-38.4) | 14 (17.3; 9.1-25.5) | 2.0 (1.0-4.1) |
| Ascaris lumbricoides | 24 (6.0; 3.4-8.5) | 13 (8.7; 4.7-13.4) | 5 (6.2; 2-13.8) | 1.4 (0.5-4.8) |
| Cyclospora cayetanensis | 24 (6.0; 2.4-9.5) | 20 (13.3; 5.8-20.8) | 2 (2.5; 0.3-8.6) | 0.5 (0.1-5.4) |
| Hookworm | 16 (4.0; 1.8-6.1) | 2 (1.3; 0.2-4.7) | 1 (1.2; 0-6.7) | 1.1 (0.1-30.6) |
| Cryptosporidium parvum | 3 (0.7; 0.2-2.2) | 0 (0) | 1 (1.2; 0-6.7) | 0 (0.0-9.4) |
During a mass treatment program for lymphatic filariasis, which was conducted in and around the study community, single doses of albendazole and diethylcarbamazine were provided to persons >2 years of age, excluding women of child-bearing age, in October 2000, before part 1 of the investigation, and in October 2001, before part 4. Although we do not have pretreatment prevalence data for the intestinal parasites listed in the table, stool specimens were collected from a convenience sample of children ≤10 years of age in the study community in January 1997, 1998, and 1999. The median prevalence of Giardia infection was 33.3% (range, 29.1 to 58.3%), that of Trichuris infection was 50% (range, 32.7 to 56.4%), that of Ascaris infection was 20% (range, 4.2 to 23.1%), that of Cyclospora infection was 11.4% (range, 6.5 to 13.2%), and that of hookworm infection was 12.5% (range, 5.5 to 17.9%). Data were not collected for Cryptosporidium. Although Entamoeba coli was found in some stool specimens, it was not listed in the table because it is not pathogenic.
Of the total of 402 persons who participated in part 1 and/or 2, 186 (46.3%) were infected with at least one intestinal parasite at some point during part 1 and/or 2. Of the 448 total participants in the entire investigation (2001 and 2002), 110 (24.6%) were found at least once during the investigation to be infected with at least one parasite. Data for the prevalence of infection in 2001 include information collected during parts 1 (February) and 2 (April).
In part 4, 35 (43.2%) of the 81 children who participated were infected with at least one intestinal parasite.
Comparisons of the prevalences of infection with the intestinal parasites in 2001 and 2002 in persons ≤10 years of age.
A total of 69 persons (17.2% of the study population as a whole [n = 402]) were enrolled in part 3, a nested case-control study (Fig. 1), which included 23 case patients (1 of the original 24 case patients was lost to follow-up) and 46 controls. The 23 case patients clustered by household (i.e., they lived in 12 households). The median number of case patients per household was one (range, one to five); 6 (50.0%) of the 12 households had more than one case patient. The case patients' households clustered in the northern region of the community (Fig. 2).
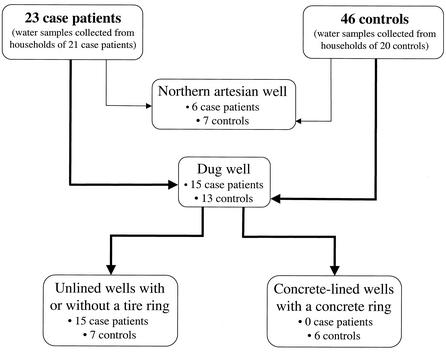
In univariate analyses, adjusted for clustering, we identified two factors significantly associated with Cyclospora infection. The use of water from the artesian well in the northern region (versus the use of water from the artesian well in the southern region) for any purpose was most strongly associated with infection (OR, 18.5; 95% CI, 2.4 to 143.1). Additionally, case patients were more likely to have unlined dug wells, with or without tire rings, than concrete-lined dug wells with cement rings (univariate OR, undefined; P = 0.05). Of the 23 case patients, 15 reported the use of water from an unlined dug well, which either had a tire ring or did not have such a ring (only one unlined dug well did not have a tire ring). The water from these wells was used for cleaning, bathing, washing of clothes, and cooking but reportedly not for drinking. Of the 13 (of 46) controls who used a dug well, 7 used unlined dug wells with a tire ring and 6 used concrete-lined dug wells with a concrete ring (Fig. 3 and 5). None of the other parameters recorded about the wells were associated with infection. Living in a stick and adobe house versus a cement house was a factor that approached statistical significance (OR, 3.8; 95% CI, 0.8 to 17.9). Eating uncooked food items and having animals did not approach statistical significance. The only factor associated with infection in multivariate analyses was using water from the artesian well in the northern region (OR, 18.5; 95% CI, 2.4 to 143.1). This factor also was significant in multivariate analyses for infection with Giardia (OR, 3.5; 95% CI, 1.4 to 8.9).
FIG. 5.
Flow diagram of the types of wells used by case patients and controls. Case patients were more likely to have unlined dug wells, with or without tire rings, than concrete-lined dug wells with cement rings (univariate OR, undefined; P = 0.05). Only one case patient had an unlined dug well without a tire ring.
Part 4 (2002).
A total of 150 children ≤10 years of age participated in part 1 and/or 2 in 2001. In 2002, stool specimens were collected from 81 (67.5%) of the 120 children who received stool containers for this survey (see the legend to Fig. 1). The median age of the participants was greater than that of the nonparticipants (5 years [range, 1 to 10 years] versus 4 years [range, 1 to 9 years], respectively; P = 0.50); 53.2% (42 of 79) of the participants and 50.0% (19 of 38) of the nonparticipants were female (P = 0.75). Infection with Giardia was the most prevalent, while infection with Cryptosporidium and hookworm were the least prevalent (Table 1).
Two children (2.5%; 95% CI, 0.3 to 8.6%) 1 and 10 years of age had stool specimens positive for Cyclospora; their family members had not had positive stool specimens in previous surveys. These two children lived in different households but used water from the same unlined dug well, which had a tire ring.
Laboratory and environmental investigations.
We collected water samples from 55 wells or other water sources (one sample per source per collection period), including the 2 artesian wells, throughout the community; 9 of these samples were collected in January 2001, 1 month before the investigation began, and 46 samples were collected during the investigation. One (11.1%) of the nine samples collected in January 2001 was positive for Cyclospora by UV fluorescence microscopy and PCR analyses. The positive sample was collected in the northern region from an unlined dug well with a tire ring (Fig. 2). However, none of the case patients had used this well. During part 3 in April 2001, water samples were collected from the two artesian wells and the wells of nine case households and seven control households. None of these samples were positive for any of the parasites for which we tested.
In January 2002, in part 4, water samples were collected from 32 wells or other water sources (e.g., a river). The 32 samples included water from some of the dug wells from which water also had been collected in part 3, specifically, wells used by eight of the nine case households and six of the seven control households. Additionally, we collected water samples from the two artesian wells and from four of the nine dug wells or other water sources that were sampled before the investigation began. None of these samples were positive for any of the parasites for which we tested. Additionally, water from the well used by both case patients identified during part 4 was negative for Cyclospora.
DISCUSSION
In 2001 and 2002, we conducted an exploratory investigation with four parts in a Haitian community to determine the prevalence of and to identify the potential risk factors for Cyclospora infection (Fig. 1). The main findings from our investigation are as follows. The prevalence of Cyclospora infection in persons of all ages in this community was not uniform from month to month and was higher in children ≤10 years of age than in older persons. However, infection with Giardia was the most prevalent parasitic infection overall and in children ≤10 years of age. Additionally, the key risk factors for infection with Cyclospora included using water from the artesian well in the northern region of the community, which was the only factor associated with infection in multivariate analyses; having a shallow, unlined dug well, with or without a tire ring, which was statistically significant in univariate analyses only; and living in a stick and adobe house rather than a cement house, which was a factor that approached statistical significance in univariate analyses. Eating uncooked food items and having animals did not approach statistical significance.
The prevalence of infection with Cyclospora in all age groups was higher in February 2001 (20 of 167 persons; 12.0%) than in April 2001 (4 of 352; 1.1%). Additionally, the prevalence rates for children ≤10 years of age were 22.5% (16 of 71) in February 2001, 3.0% (4 of 135) in April 2001, and 2.5% (2 of 81) in January 2002. The difference in prevalence rates for children between February 2001 and January 2002 show that infection rates can be highly variable from year to year. The prevalence rate in February 2001 for persons of all ages was comparable with the rates for January and March 1997 (in a previous study), two of the cooler, drier months of the year (9). A marked seasonality of the prevalence of Cyclospora infection has been identified in multiple countries (e.g., Guatemala, Peru, Indonesia, and Nepal). However, the seasonality is not uniform among these countries and defies easy explanation (14).
Most (22 of 26; 84.6%) of the cases that we identified during our investigation were in children ≤10 years of age. Previous studies in Guatemala and Peru, where Cyclospora is endemic, have also shown this (1, 2, 17). Together, the various studies suggest that some immunity to Cyclospora develops over time or that risk factors for infection decrease as persons age.
Although our investigation focused on Cyclospora infection, we also determined the prevalence of infection with other intestinal parasites. Of the 448 total participants, 110 (24.6%) were found at least once in the investigation to be infected with at least one parasite (Table 1); all of the types of infections tended to cluster in the northern region of the community. Infection with Giardia was the most prevalent, while infection with Cryptosporidium was the least prevalent (Table 1). The prevalence of Giardia infection in developing countries has ranged from about 13 to 43% (16, 19). For example, a community-based study of persons of all ages in Bangladesh found the prevalence to be 13% (46 of 358). A higher prevalence (i.e., 19 of 89; 21%) was found in children from 5 through 10 years of age (12). Most studies looking at the prevalence of Cryptosporidium infection in developing countries have involved persons with diarrhea. In those studies, the prevalence of Cryptosporidium infection has ranged from about 3 to 32% (5, 22). In Venezuela, a community-based study of persons of all ages found the overall prevalence of infection with Cryptosporidium to be 9.9% (21 of 212). However, the prevalence in children up through 15 years of age was 13.2% (16 of 121) (4). Therefore, whereas our findings for Giardia infection were consistent with those from past studies, we found lower rates of Cryptosporidium infection; one possible explanation is that we included persons regardless of their symptom status rather than primarily symptomatic persons.
The prevalences of infection with some of the intestinal parasites could have been affected by a mass treatment program for lymphatic filariasis that was conducted in our study community and nearby communities (Table 1). This program, which included treatment with albendazole and diethylcarbamazine, provided medications in October 2000, before part 1 of our investigation, and in October 2001, before part 4 of our investigation.
Because water and sewage contaminated with Cyclospora have previously been identified as risk factors for infection in multiple countries in both outbreak and nonoutbreak situations (1, 13, 15, 18, 21, 25; J. G. Rabold, C. W. Hoge, D. R. Shlim, C. Kefford, R. Rajah, and P. Echeverria, Letter, Lancet 344:1360-1361, 1994), we explored the possibility that water was a potential risk factor for infection. In our investigation, two water-related variables (i.e., using water for any purpose from the artesian well in the northern region of the community and having an unlined dug well, with or without a tire ring) were risk factors for infection. Exposure to water from the artesian well was the only variable significantly associated with infection in both univariate and multivariate analyses; it also was significant in multivariate analyses for Giardia infection. Whether the water per se was a true risk factor for Cyclospora infection is unclear, in part because we did not collect water quality data for the artesian well and were unable to evaluate the well's vulnerability to contamination. Water from the well might have been contaminated after collection by persons infected with Cyclospora (e.g., persons with contaminated hands could have contaminated the water collection vessels, which typically consisted of 5-gallon buckets with wide mouths and no lids). However, we might have modeled type I error, such that the identification of the artesian well as a risk factor was a false-positive result.
Another hypothesis is that the use of water from the northern artesian well was a proxy for the environment around the well or the socioeconomic status of the persons who used it rather than a true risk factor for infection. The area around the well was a social gathering place where persons bathed, did laundry, and played. In addition, persons, especially children, might have defecated near the well or in the fields nearby because latrines were uncommon in this area. The fact that all of the parasitic infections for which we tested were more prevalent in the northern region than in the southern region (data not shown) supports the conclusion that this region had poor sanitation. The helminths Trichuris, Ascaris, and hookworm require periods of about 1 to 3 weeks in the environment, after excretion, to become infectious. For Cyclospora oocysts, the required period outside the host is thought to be days to weeks. Therefore, feces containing these parasites probably would have to be deposited and then remain at least days to weeks in the environment before a person could become infected. Although the case patients infected with Cyclospora were clustered in households, this clustering is unlikely, because of the biology of Cyclospora, to have resulted from direct person-to-person spread but might reflect shared risk factors, such as living in the same contaminated environment.
Having an unlined dug well, with or without a tire ring, was also a risk factor for infection with Cyclospora, but only in univariate analyses. We identified one unsporulated Cyclospora oocyst in one water sample, which was collected in January 2001, before the investigation began, in the northern region, from an unlined dug well with a tire ring (Fig. 2). Only one stool specimen was collected (April 2001) from a member of the household that had used water from the “contaminated” well (January 2001), and that specimen was negative for Cyclospora. Even if the water sample and stool specimen had been collected simultaneously, we would not have known if water from the well caused the infection. Reportedly, no one drank water from the dug wells. However, we could not verify this because our investigation was not an observational study. None of the water samples collected during the investigation in 2001 and 2002 were positive for Cyclospora or any of the other parasites for which we tested.
Several factors might have limited our ability to detect parasites in the water. First, water samples might not have been collected at the optimal time (i.e., they were not collected in February 2001, when we identified the largest number of cases of cyclosporiasis). Second, we collected only one sample per well during each collection period. Third, Cyclospora oocysts might have settled to the bottoms of the wells and might not have been retrieved when we collected the samples by simply dropping buckets tied to ropes into wells, which was how the local residents collected water. Dug wells lined with dirt probably were more likely than those lined with cement to have oocysts stuck to their sides, and this technique for collecting water might not have been optimal for collection of oocysts stuck to the sides of wells. Even heavy rains, had they occurred in the few days before water collection, probably would not have stirred up Cyclospora oocysts that had settled to the bottoms of dug wells. However, rains could have caused contamination of the wells by surface water. Although it rained one night before some of the well-water samples were collected, we did not identify any parasites in the samples collected after the rainfall. Fourth, the volume of water collected for each sample (i.e., 10 liters by one technique and 18.9 liters by the other technique) might have been too small to collect sufficient organisms to be detectable by our testing techniques (i.e., examination of processed samples by UV fluorescence microscopy and PCR if the slide was positive), especially if the water source was not heavily contaminated. Fifth, the methods used to identify Cyclospora and other protozoan parasites, such as Cryptosporidium, in environmental samples (e.g., water) are improving but still might be suboptimal for the detection of low levels of parasites, which may be sufficient to infect humans (23).
Whereas studies conducted in Guatemala (1) and Peru (3) suggested that having certain animals (e.g., dogs, chickens, and ducks) increased the risk for human infection with Cyclospora (1, 3), this was not true in our investigation. In a survey conducted in and around Leogane, Haiti, from 1997 to 1998, Cyclospora was not identified in the feces of any of the 327 stool specimens collected from 11 types of domestic animals (e.g., dogs, pigs, goats, and chickens) (8).
Our investigation had several limitations, besides those discussed above. We identified fewer cases than we had anticipated. Five possible explanations are that we conducted the investigation somewhat later than we had hoped (i.e., because of findings from past studies in Haiti, our goal had been to finish stool collection before April); we had fewer participants than we had expected (the sampling of the wells was so time-consuming that we could not request participation in the investigation from the planned number of households); we might have missed some infected persons by testing only one specimen per participant in each part of the investigation and by examining stool specimens by techniques that might not have detected low levels of Cyclospora; immunity from prior infections might have limited the number of cases that we detected even in young children; and environmental conditions in 2001 might have been less conducive than those in previous years to the survival of oocysts and therefore to the infection of persons. Not only did we not detect as many cases as we had planned but also our cases were suboptimal, because we could not distinguish prevalent from incident ones and could not consider clustered cases independent in the analyses. Because of such limitations, our investigation was not powered to detect subtle risk factors for infection with Cyclospora.
Our investigation also had several strengths. A major strength was our study design, especially the following features: the investigation was community based rather than clinic based; the households of the participants were randomly selected, in contrast to previous research in Haiti by CDC and others (9), which was based on convenience samples; the participation rate of those selected for the investigation was high (i.e., 75.6%, which included 35% [532 of 1,500] of the entire community); the stool specimens obtained to determine prevalence data for infections with various parasites were collected from persons regardless of their symptom status; and, for the first time in Haiti, we attempted to identify risk factors for Cyclospora infection (9, 20). To our knowledge, our investigation is one of the very few community-based studies that have addressed the epidemiology of Cyclospora infection in developing countries (3, 17). Therefore, although our investigation was exploratory in nature and our risk-factor data were not definitive, our investigation contributes to a small but important body of literature. Further studies are needed both to assess the role of water and other variables as risk factors for infection in Haiti and other developing countries and to evaluate whether variables associated with socioeconomic status could predispose persons to infection. Finally, studies to address the intriguing question of why Cyclospora infection is seasonal, with different patterns in different parts of the world, are needed.
Acknowledgments
We thank David Addiss, Michael Beach, Jean Marc Brisseau, Chris Fiack, Allen Hightower, Stephanie Johnston, Patrick Lammie, Marybeth Lovegrove, Els Mathieu, Eva Nace, Greg Noland, George Punkodsky, Jeanne Radday, Steven St. Hilaire, Kettlie Toussaint, Charles Washington, and Kimberly Won for help with the investigation.
This research was supported in part by the Emerging Infectious Diseases Fellowship Program, which is administered by the Association of Public Health Laboratories and funded by CDC, and the Atlanta Research and Education Foundation of the Veterans Affairs Medical Center of Atlanta.
Adriana S. Lopez and Jean M. Bendik contributed equally to the investigation.
REFERENCES
- 1.Bern, C., B. Hernandez, M. B. Lopez, M. J. Arrowood, M. A. de Mejia, A. M. de Merida, A. W. Hightower, L. Venczel, B. L. Herwaldt, and R. E. Klein. 1999. Epidemiologic studies of Cyclospora cayetanensis in Guatemala. Emerg. Infect. Dis. 5:766-774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bern, C., B. Hernandez, M. B. Lopez, M. J. Arrowood, A. M. de Merida, and R. E. Klein. 2000. The contrasting epidemiology of Cyclospora and Cryptosporidium among outpatients in Guatemala. Am. J. Trop. Med. Hyg. 63:231-235. [PubMed] [Google Scholar]
- 3.Bern, C., Y. Ortega, W. Checkley, J. M. Roberts, A. G. Lescano, L. Cabrera, M. Verastegui, R. E. Black, C. Sterling, and R. H. Gilman. 2002. Epidemiologic differences between cyclosporiasis and cryptosporidiosis in Peruvian children. Emerg. Infect. Dis. 8:581-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chacin-Bonilla, L., M. Mejia De Young, G. Cano, N. Guanipa, J. Estevez, and E. Bonilla. 1993. Cryptosporidium infections in a suburban community in Maracaibo, Venezuela. Am. J. Trop. Med. Hyg. 49:63-67. [DOI] [PubMed] [Google Scholar]
- 5.Chen, X. M., J. S. Keithly, C. V. Paya, and N. F. LaRusso. 2002. Cryptosporidiosis. N. Engl. J. Med. 346:1723-1731. [DOI] [PubMed] [Google Scholar]
- 6.da Silva, A. J., F. J. Bornay-Llinares, I. N. Moura, S. B. Slemenda, J. L. Tuttle, and N. J. Pieniazek. 1999. Fast and reliable extraction of protozoan parasite DNA from fecal specimens. Mol. Diagn. 4:57-64. [DOI] [PubMed] [Google Scholar]
- 7.Eberhard, M. L., A. J. da Silva, B. G. Lilley, and N. J. Pieniazek. 1999. Morphologic and molecular characterization of new Cyclospora species from Ethiopian monkeys: C. cercopitheci sp. n., C. colobi sp. n., and C. papionis sp. n. Emerg. Infect. Dis. 5:651-658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eberhard, M. L., E. K. Nace, and A. R. Freeman. 1999. Survey for Cyclospora cayetanensis in domestic animals in an endemic area in Haiti. J. Parasitol. 85:562-563. [PubMed] [Google Scholar]
- 9.Eberhard, M. L., E. K. Nace, A. R. Freeman, T. G. Streit, A. J. da Silva, and P. J. Lammie. 1999. Cyclospora cayetanensis infections in Haiti: a common occurrence in the absence of watery diarrhea. Am. J. Trop. Med. Hyg. 60:584-586. [DOI] [PubMed] [Google Scholar]
- 10.Eberhard, M. L., N. J. Pieniazek, and M. J. Arrowood. 1997. Laboratory diagnosis of Cyclospora infections. Arch. Pathol. Lab. Med. 121:792-797. [PubMed] [Google Scholar]
- 11.Garcia, L. S., and R. Y. Shimizu. 1997. Evaluation of nine immunoassay kits (enzyme immunoassay and direct fluorescence) for detection of Giardia lamblia and Cryptosporidium parvum in human fecal specimens. J. Clin. Microbiol. 35:1526-1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gilman, R. H., K. H. Brown, G. S. Visvesvara, G. Mondal, B. Greenberg, R. B. Sack, F. Brandt, and M. U. Khan. 1985. Epidemiology and serology of Giardia lamblia in a developing country: Bangladesh. Trans. R. Soc. Trop. Med. Hyg. 79:469-473. [DOI] [PubMed] [Google Scholar]
- 13.Hale, D., W. Aldeen, and K. Carroll. 1994. Diarrhea associated with cyanobacterialike bodies in an immunocompetent host: an unusual epidemiological source. JAMA 271:144-145. [PubMed] [Google Scholar]
- 14.Herwaldt, B. L. 2000. Cyclospora cayetanensis: a review, focusing on the outbreaks of cyclosporiasis in the 1990s. Clin. Infect. Dis. 31:1040-1057. [DOI] [PubMed] [Google Scholar]
- 15.Hoge, C. W., D. R. Shlim, R. Rajah, J. Triplett, M. Shear, J. G. Rabold, and P. Echeverria. 1993. Epidemiology of diarrhoeal illness associated with coccidian-like organism among travellers and foreign residents in Nepal. Lancet 341:1175-1179. [DOI] [PubMed] [Google Scholar]
- 16.Islam, A. 1990. Giardiasis in developing countries, p. 235-266. In E. A. Meyer (ed.), Giardiasis. Elsevier Science Publishers B.V. (Biomedical Division), Amsterdam, The Netherlands.
- 17.Madico, G., J. McDonald, R. H. Gilman, L. Cabrera, and C. R. Sterling. 1997. Epidemiology and treatment of Cyclospora cayetanensis infection in Peruvian children. Clin. Infect. Dis. 24:977-981. [DOI] [PubMed] [Google Scholar]
- 18.Ooi, W. W., S. K. Zimmerman, and C. A. Needham. 1995. Cyclospora species as a gastrointestinal pathogen in immunocompetent hosts. J. Clin. Microbiol. 33:1267-1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ortega, Y. R., and R. D. Adam. 1997. Giardia: overview and update. Clin. Infect. Dis. 25:545-550. [DOI] [PubMed] [Google Scholar]
- 20.Pape, J. W., R. I. Verdier, M. Boncy, J. Boncy, and W. D. Johnson. 1994. Cyclospora infection in adults infected with HIV: clinical manifestations, treatment, and prophylaxis. Ann. Intern. Med. 121:654-657. [DOI] [PubMed] [Google Scholar]
- 21.Sherchand, J. B., J. H. Cross, M. Jimba, S. Sherchand, and M. P. Shrestha. 1999. Study of Cyclospora cayetanensis in health care facilities, sewage water and green leafy vegetables in Nepal. Southeast Asian J. Trop. Med. Public Health 30:58-63. [PubMed] [Google Scholar]
- 22.Ungar, B. L. P. 1990. Cryptosporidiosis in humans (Homo sapiens), p. 59-82. In J. P. Dubey, C. A. Speer, and R. Fayer (ed.), Cryptosporidiosis of man and animals. CRC Press, Inc., Boca Raton, Fla.
- 23.U.S. Environmental Protection Agency. 1999. Method 1623: Cryptosporidium and Giardia in water by filtration/IMS/FA. Office of Water, US Environmental Protection Agency, Washington, DC. [Online.] http://www.epa.gov/nerlcwww/1623ap01.pdf.
- 24.Vesey, G., J. S. Slade, M. Byrne, K. Shepherd, and C. R. Fricker. 1993. A new method for the concentration of Cryptosporidium oocysts from water. J. Appl. Bacteriol. 75:82-86. [DOI] [PubMed] [Google Scholar]
- 25.Wurtz, R. M., F. E. Kocka, C. S. Peters, C. M. Weldon-Linne, A. Kuritza, and P. Yungbluth. 1993. Clinical characteristics of seven cases of diarrhea associated with a novel acid-fast organism in the stool. Clin. Infect. Dis. 16:136-138. [DOI] [PubMed] [Google Scholar]