Abstract
The performances of the Etest and the disk diffusion methods for testing of the susceptibilities of 235 Candida glabrata isolates to fluconazole and voriconazole were compared with that of the National Committee for Clinical Laboratory Standards (NCCLS) approved standard broth microdilution (BMD) method. The NCCLS method used RPMI 1640 broth medium, and MICs were read after incubation for 48 h at 35°C. Etest MICs were determined with RPMI 1640 agar containing 2% glucose (RPG agar) and with Mueller-Hinton agar containing 2% glucose and 0.5 μg of methylene blue per ml (MBE agar) and were read after incubation for 48 h at 35°C. Disk diffusion testing was performed with MBE agar, 25-μg fluconazole disks, and 1-μg voriconazole disks and by incubation at 35°C for 24 h. Overall agreements between the Etest and the BMD MICs obtained with RPG and MBE agars were 91 and 96%, respectively, for fluconazole and 93 and 95%, respectively, for voriconazole. Categorical agreements between the agar-based methods and BMD were 52.3 to 64.7% with fluconazole and 94.8 to 97.4% with voriconazole. The vast majority of the discrepancies by the disk diffusion and Etest methods with fluconazole were minor errors. The agar-based methods performed well in identifying isolates with resistance to fluconazole and decreased susceptibility to voriconazole.
The agar-based methods for performing fluconazole and voriconazole susceptibility testing with Candida spp. include both the disk diffusion and the Etest stable-agar-gradient MIC methods (1, 3, 4, 6, 8, 9, 12, 16, 17). Barry et al. (3) demonstrated that both the disk diffusion and the Etest methods were accurate and precise when they were used to determine the fluconazole susceptibilities of 495 isolates of Candida spp. Although published data for the disk diffusion and Etest methods with voriconazole are limited (4, 9, 12), the results of both methods show good agreement with those of the reference broth microdilution (BMD) method.
The studies to date that have documented the efficacies of agar-based methods for the testing of susceptibilities to fluconazole and voriconazole have generally included adequate numbers of Candida albicans isolates but relatively few C. glabrata isolates (1, 3, 8, 17). Among the four most common species of Candida causing bloodstream infections (BSIs; C. albicans, C. glabrata, C. parapsilosis, and C. tropicalis) (10), C. glabrata alone tends to be less susceptible to fluconazole, with a significant percentage of isolates classified as susceptible-dose dependent (S-DD; MIC, 16 to 32 μg/ml) or resistant (R; MIC, ≥64 μg/ml) (11). This relative lack of susceptibility to fluconazole means that BSIs due to C. glabrata must be treated with high doses of fluconazole (800 mg/day) or an alternative agent pending the results of antifungal susceptibility testing (14). Voriconazole may be useful as an alternative agent, given its excellent activity against C. glabrata isolates that are susceptible (S) or S-DD to fluconazole, but it is not reliably active in vitro against fluconazole-R strains (11). For these reasons, antifungal susceptibility testing may play a very important role in optimizing the treatment of BSIs due to C. glabrata, and new testing methods (i.e., agar-based methods) should be rigorously examined with a large number of clinically important isolates of this species (15).
The purpose of this study was to expand the evaluation of agar-based methods for determining the in vitro susceptibilities of C. glabrata to fluconazole and voriconazole by testing an international collection of 235 BSI isolates obtained from more than 60 medical centers worldwide. The fluconazole and voriconazole Etest MICs obtained with two different media and the disk diffusion zone diameters obtained with each agent were compared to the MICs determined by the National Committee for Clinical Laboratory Standards (NCCLS) reference BMD method, the M27-A method (7).
MATERIALS AND METHODS
Organisms.
A total of 235 clinical isolates of C. glabrata were obtained from 61 medical centers worldwide in 2001. All were incident clinical isolates obtained from cultures of blood from 235 different patients with candidemia. Isolates were identified with the Vitek and API yeast identification systems (bioMerieux, Inc., Hazelwood, Mo.), and identification tests with these systems were supplemented by conventional methods as needed (18). Isolates were stored as water suspensions until use. Prior to testing, each isolate was passaged on potato dextrose agar (Remel, Lenexa, Kans.) and CHROMagar (Hardy Laboratories, Santa Monica, Calif.) to ensure purity and viability.
Susceptibility testing.
Reference antifungal susceptibility testing of C. glabrata was performed by the BMD method described by NCCLS (7). Reference powders of fluconazole and voriconazole were obtained from Pfizer Pharmaceuticals (Groton, Conn.).
Fluconazole and voriconazole Etest strips were provided by AB BIODISK (Solna, Sweden). MICs were determined by the Etest as described previously (8, 9) with RPMI 1640 agar with 2% glucose (RPG agar; Remel), an inoculum suspension adjusted to the turbidity of a 0.5 McFarland standard (∼106 cells/ml), and incubation at 35°C for 48 h. In addition, a second medium prepared as described by Barry et al. (3) with Mueller-Hinton agar (Difco Laboratories) supplemented with 2% glucose and methylene blue (0.5 μg/ml) (MBE agar) was used for both Etest and disk diffusion testing (see below). The MICs of both fluconazole and voriconazole obtained with both RPG and MBE agars were read as the lowest concentration at which the border of the elliptical inhibition zone intercepted the scale on the strip. Any growth such as microcolonies throughout a discernible inhibition ellipse was ignored.
Disk diffusion testing of fluconazole and voriconazole was performed as described by Barry et al. (3) and Meis et al. (5). Fluconazole (25-μg) and voriconazole (1-μg) disks were obtained from Becton Dickinson (Sparks, Md.). For disk diffusion testing, 90-mm-diameter plates containing MBE agar at a depth of 4.0 mm were used. The agar surface was inoculated by using a swab dipped in a cell suspension adjusted to the turbidity of a 0.5 McFarland standard. The inoculum was allowed to dry, and both the disks and the Etest strips were placed on the same plates. The plates were incubated in air at 35°C, and the zone diameters surrounding the fluconazole and voriconazole disks were read at 24 h. Zone diameter endpoints were read at 80% growth inhibition by using the BIOMIC image analysis plate reader system (version 5.9; Giles Scientific, Santa Barbara, Calif.) (5).
MIC interpretive criteria for fluconazole were those published by Rex et al. (13) and the NCCLS (17): S, ≤ 8 μg/ml; S-DD, 16 to 32 μg/ml; R, ≥ 64 μg/ml. The interpretive criteria for the fluconazole disk test were those published by Barry et al. (3): S, ≥19 mm; S-DD, 15 to 18 mm; R, ≤14 mm. Although interpretive breakpoints have not yet been established for voriconazole, we have elected to use the following criteria for purposes of comparison in this study (10-12): S, ≤1 μg/ml (zone diameter, ≥ 14 mm); R, ≥2 μg/ml (zone diameter, ≤13 mm).
QC.
Quality control (QC) was performed for the BMD and Etest methods in accordance with NCCLS document M27-A by using C. krusei ATCC 6258 and C. parapsilosis ATCC 22019 (2, 7). QC determinations made on each day of testing were within the control limits for fluconazole and voriconazole described by Barry et al. (2). QC for disk diffusion testing was performed by using C. albicans ATCC 90028 and C. parapsilosis ATCC 22019 (3, 5).
Analysis of results.
The Etest MICs of fluconazole and voriconazole on both RPG and MBE agars were read at 48 h and were compared to the reference BMD MICs read at 48 h. The Etest MICs were rounded up to the next even log2 concentration in order to simplify analysis (3, 8, 9). Discrepancies of no more than 2 dilutions were used to calculate the percent agreement.
The diameters of the zones of inhibition surrounding the fluconazole and voriconazole disks at 24 h of incubation were plotted against the respective BMD MICs read at 48 h (3). The method of least squares was used to calculate a regression line for each comparison.
The interpretive breakpoints described above were used to determine the categorical agreement between the results of the agar-based tests (the disk diffusion and Etest methods) and the results of the reference BMD method for fluconazole and voriconazole. Major errors were identified as a classification of R by the disk or Etest methods and a classification of S by BMD, very major errors were identified as a result of S by the disk diffusion or Etest method and a result of R by the BMD method, and minor errors were identified as a result of S or R by one of the tests and a result of S-DD by the other method.
RESULTS AND DISCUSSION
In vitro susceptibility testing by both the reference BMD method and the Etest with either RPG or MBE agar demonstrated the relatively high MICs of fluconazole for C. glabrata (Table 1). The MICs obtained by the BMD method tended to cluster at the upper end of the S category (4 to 8 μg/ml) and in the S-DD category. A similar distribution was observed by the Etest, although on both media the Etest MICs tended to be slightly higher than the BMD MICs. The overall levels of agreement (within 2 dilutions) between the Etest and the BMD method were 91% with RPG agar and 96% with MBE agar, consistent with those reported previously (3, 8).
TABLE 1.
In vitro activities of fluconazole and voriconazole against 235 clinical isolates of C. glabrata as determined by the reference BMD method and the Etest with two different media
| Antifungal agent | Test methoda | MIC (μg/ml)b
|
% Agree- mentc | ||
|---|---|---|---|---|---|
| Range | 50% | 90% | |||
| Fluconazole | BMD | 1->128 | 8 | 32 | |
| ET-RPG | 1->256 | 16 | 64 | 91 | |
| ET-MBE | 0.5->256 | 16 | 64 | 96 | |
| Voriconazole | BMD | 0.03-8 | 0.25 | 1 | |
| ET-RPG | 0.012-64 | 0.25 | 1 | 93 | |
| ET-MBE | 0.012-64 | 0.25 | 1 | 95 | |
The BMD method was performed according to the guidelines for the NCCLS M27-A method (7); ET-RPG, Etest with RPG agar; ET-MBE, Etest with MBE agar.
50 and 90%, MICs at which 50 and 90% of isolates tested, respectively, are inhibited.
Percentage of Etest MICs (read at 48 h) that are within 2 log2 dilutions of the reference BMD MICs.
The MIC results obtained by both the BMD and the Etest methods demonstrated that voriconazole is very active against the vast majority of C. glabrata BSI isolates (Table 1). Overall, 92 to 93% of isolates were inhibited by ≤1 μg of voriconazole per ml, as determined by the Etest and the BMD methods. Similar to the results obtained with fluconazole, the level of agreement between the results of the BMD method and those of the Etest was good (93 to 95% agreement within 2 dilutions). Again, these results are similar to those reported previously (9). Voriconazole MICs were ≥2 μg/ml for 14 of the 235 C. glabrata isolates tested. Among these 14 isolates, 2 were S-DD and 12 were R to fluconazole, confirming their lack of susceptibility to azole antifungal agents.
When susceptibility testing methods are compared, it is generally useful to determine the more qualitative categorical agreement of the investigational methods and the established reference method. Despite the availability of interpretive breakpoints for fluconazole MIC and disk testing of Candida spp., very few of the published evaluations of these methods provide data on overall categorical agreement and error rates (3, 6, 8). As seen in Table 2, despite reasonably good quantitative agreement between the Etest and the BMD MICs for fluconazole and C. glabrata, the overall categorical agreements were rather poor: 52.3% for the Etest with RPG agar and 61.7% for the Etest with MBE agar. This is almost entirely due to minor errors consisting of shifts between the S and S-DD categories. Etest results tended to be slightly higher (usually 1 dilution) than the BMD results, with a higher percentage of isolates being in the S-DD category. Small numbers of major errors (false-positive resistance) were seen by the Etest, but no very major errors were observed.
TABLE 2.
Overall interpretive agreement between results of fluconazole and voriconazole agar-based susceptibility tests and of standard 48-h BMD reference tests for 235 C. glabrata isolates
| Antifungal agent | Method and mediuma | % of isolates by categoryb
|
% of discrepant resultsc
|
% Categorical agreementd | ||||
|---|---|---|---|---|---|---|---|---|
| S | S-DD | R | Minor | Major | Very major | |||
| Fluconazole | BMD | 57 | 36 | 7 | ||||
| ET-RPG | 32 | 54 | 14 | 46.0 | 1.7 | 0 | 52.3 | |
| ET-MBE | 44 | 44 | 12 | 36.6 | 1.7 | 0 | 61.7 | |
| Disk | 90 | 2 | 8 | 34.9 | 0 | 0.4 | 64.7 | |
| Voriconazole | BMD | 93 | 7 | |||||
| ET-RPG | 92 | 8 | 2.6 | 2.6 | 94.8 | |||
| ET-MBE | 93 | 7 | 1.7 | 2.1 | 96.2 | |||
| Disk | 94 | 6 | 0.9 | 1.7 | 97.4 | |||
See footnote a of Table 1 for definitions of BMD, ET-RPG, ET-MBE; disk, disk diffusion test with fluconazole (25-μg disk) and voriconazole (1-μg disk).
Percentage of isolates classified in the different susceptibility categories. See Materials and Methods for definitions.
Percentage of test results with minor, major, or very major discrepancies compared to the results of the reference BMD method at 48 h. See Materials and Methods for definitions.
Agreement rates reflect the percentage of isolates classified in the same category by both the agar-based (Etest and disk) and the reference BMD methods.
By using the putative breakpoints for voriconazole of S being an MIC ≤1 μg/ml and R being an MIC ≥2 μg/ml, with no intermediate or S-DD category, the categorical agreements by the Etest were 94.8% with RPG agar and 96.2% with MBE agar. There were <3% major or very major errors, indicating that the Etest with either medium may be useful in determining the in vitro susceptibilities of C. glabrata isolates to voriconazole.
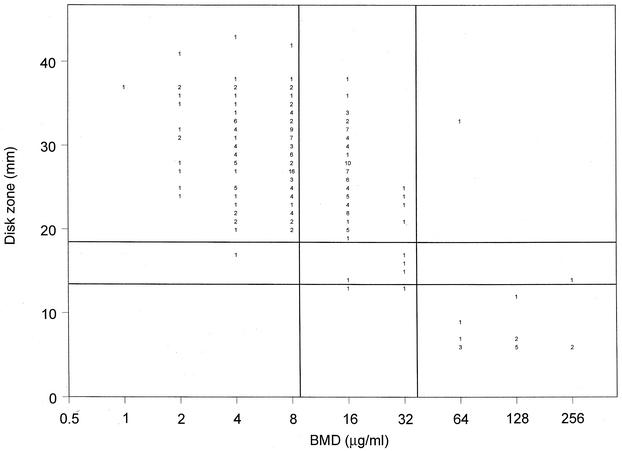
Although disk diffusion testing with MBE agar is relatively new, it is clear that this medium formulation supports the growth of C. glabrata and allows the measurement of zone diameters surrounding both fluconazole and voriconazole disks within 24 h (3, 6, 12). Figure 1 shows the correlation of the 25-μg fluconazole disk zone diameters read at 24 h with the BMD MIC results. The clustering of the results for the isolates around the breakpoint values is evident from the scattergram, with most of the isolates for which the fluconazole MICs were 16 μg/ml (S-DD) appearing to be susceptible by the disk method, with zone diameters of >19 mm. This shift toward larger numbers of susceptible isolates by disk testing resulted in an overall categorical agreement of 64.7%, with 34.9% minor errors, no major errors, and only one very major error (Table 2). Thus, although the fluconazole disk test was unable to distinguish S versus S-DD isolates, it did reliably detect those strains with resistance (MICs, ≥64 μg/ml) to fluconazole.
FIG. 1.
Zones of inhibition around 25-μg fluconazole disks on MBE agar plotted against the MICs determined at 48 h by the reference BMD method for 235 C. glabrata isolates. The method of least squares was used to calculate a regression line (y = 71.1 − 3.6x; R = 0.7). The horizontal lines indicate the S (≥19 mm) and R (≤14 mm) zone diameter breakpoints for the fluconazole disk test. The vertical lines indicate the S (≤8 μg/ml) and R (≥64 μg/ml) MIC breakpoints for fluconazole. The numbers inside the graph indicate numbers of isolates.
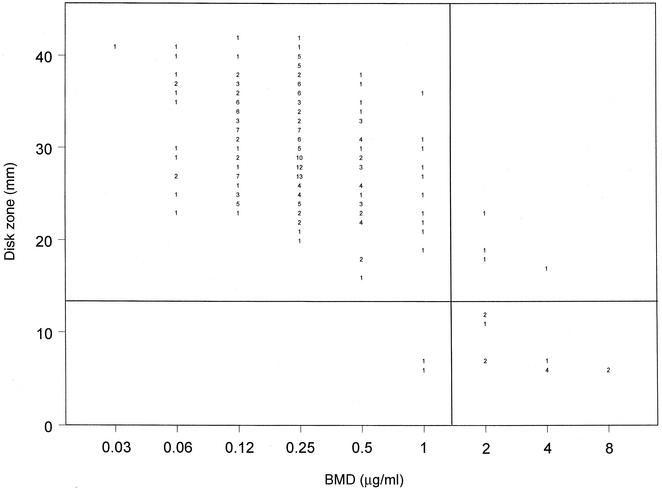
The results of testing with the 1-μg voriconazole disk are shown in Fig. 2 and Table 2. The regression statistics (y = 55.8 − 3.8x; R = 0.7) show a good level of agreement between the two methods. Zone diameters of ≤13 mm identified those isolates with decreased susceptibility to voriconazole (MICs, ≥2 μg/ml), and the resulting categorical agreement was 97.4%, with <2% very major and major errors. Again, those isolates with smaller inhibition zone diameters and for which voriconazole MICs were higher also exhibited decreased susceptibility to fluconazole.
FIG. 2.
Zones of inhibition around 1-μg voriconazole disks plotted against the MICs determined at 48 h by the reference BMD method for 235 C. glabrata isolates. The method of least squares was used to calculate a regression line (y = 55.8 − 3.8x; R = 0.7). The horizontal line indicates the putative S zone diameter breakpoint (≥14 mm) for the voriconazole disk test. The vertical line indicates the putative S MIC breakpoint (≤1 μg/ml) for voriconazole. The numbers inside the graph indicate numbers of isolates.
The results of this study complement the previous observations of Barry et al. (3) and Morace et al. (6) regarding the use of Etest and disk diffusion testing to determine the susceptibilities of C. glabrata isolates to azole antifungal agents. Barry et al. (3) found that both the Etest and the disk diffusion methods were accurate, precise, and reproducible for the testing of susceptibility to fluconazole, although they included only 37 C. glabrata isolates among the 495 isolates tested and did not analyze the data for this species separately. Those investigators did examine the performance of the Etest on MBE agar and found that its performance was comparable to but not superior to that on RPG agar.
More recently, Morace et al. (6) reported the results of a multicenter evaluation of the Etest and disk diffusion methods, as well as other commercially available methods, for the testing of the fluconazole susceptibilities of 793 clinical Candida isolates, including 133 C. glabrata isolates. Their conclusions were similar to those of Barry et al. (3), stating that both the Etest and the disk diffusion methods were accurate, reproducible, and potentially useful for determining susceptibility to fluconazole in the clinical microbiology laboratory. However, when the data for C. glabrata were analyzed separately, those investigators found categorical agreements of 68% for the Etest and 50% for disk diffusion testing. The vast majority of the discrepancies by both of these agar-based tests were minor errors involving changes from S to S-DD.
On the basis of the results of the previously published studies described above plus the data presented herein, it appears that both the Etest and the disk diffusion test methods may be useful in identifying isolates of C. glabrata expressing resistance to fluconazole but are not reliable in differentiating isolates that are S versus those that are S-DD. Given the fact that the fluconazole dosing recommendations for the treatment of C. glabrata BSIs (800 mg/day) are intended to account for those isolates in the S-DD category, the inability to differentiate S from S-DD isolates may not be a critical failing of these tests (14). It is far more important to identify those strains with resistance (15), and these tests appear to perform that function reasonably well.
Although there are very few data regarding agar-based tests for voriconazole, it does appear that the results of both the Etest and the disk diffusion test methods provide good agreement with those of the BMD method for the testing of Candida spp., including C. glabrata (4, 9, 12). Previously, we evaluated the voriconazole Etest and demonstrated overall levels of agreement with the BMD method of 98% for Candida spp. and 91% for C. glabrata; however, we did not determine categorical agreement in that study (9). More recently, we have demonstrated (12) a good overall correlation between BMD and disk diffusion testing for voriconazole and a categorical agreement of 99% when a collection of 1,586 isolates of Candida was tested by using the putative interpretive criteria described herein. The data presented in Fig. 2 and Table 2 demonstrate that both the Etest and the disk diffusion test with voriconazole perform comparably to the BMD method in identifying C. glabrata strains with decreased susceptibility to this agent. The fact that those C. glabrata isolates that appear to be less susceptible to voriconazole by all three methods also exhibit decreased susceptibility to fluconazole suggests that these approaches may be useful clinically to guide therapeutic decision making for infections due to C. glabrata. Establishment of clinical correlates is essential; however, these results are very promising.
In summary, we have performed an extensive analysis of agar-based testing methods for determination of the in vitro susceptibilities of C. glabrata isolates to fluconazole and voriconazole. We have demonstrated the usefulness of MBE agar for the performance of both the Etest and the disk diffusion methods to determine the susceptibilities of this relatively fastidious species of Candida to both of these antifungal agents. We have shown that both Etest and disk diffusion testing may reliably identify C. glabrata isolates that express resistance to fluconazole but that neither agar-based method can differentiate fluconazole-S from fluconazole-S-DD isolates. Likewise, both agar-based methods can identify those few C. glabrata isolates with decreased susceptibility to voriconazole. C. glabrata isolates that are S-DD to fluconazole are virtually all “susceptible” to voriconazole as determined by the BMD and Etest methods (MICs, ≤1 μg/ml) and disk diffusion testing (zone diameters, ≥14 mm).
From the standpoint of antifungal resistance, C. glabrata is clearly the Candida species with the greatest potential to acquire resistance to fluconazole and other azoles (14, 15). The availability of test methods to reliably identify those C. glabrata strains that express resistance to fluconazole, voriconazole, and other azoles will allow clinicians to optimize their therapeutic approaches to infections due to this important species (14, 15). We have demonstrated that the relatively simple agar-based methods for the testing of susceptibility to fluconazole and voriconazole may be used for this purpose. The commercial availability of broth- and agar-based antifungal susceptibility testing methods should bring this technology into the mainstream of clinical microbiology and infectious disease practice.
Acknowledgments
This study was supported in part by unrestricted research grants from Pfizer Pharmaceuticals (ARTEMIS Program).
Linda Elliott provided excellent support in the preparation of the manuscript. We express our appreciation to all ARTEMIS Program participants. Participants contributing isolates to the study included Hershey Medical Center, Hershey, Pa. (P. Appelbaum); Stanford Hospitals and Clinics, Stanford, Calif. (E. J. Baron); University of California, Los Angeles, Medical Center (D. Brucker); New York State Department of Health, Albany (V. Chaturvedi); Wishard Health Services, Indianapolis, Ind. (T. Davis); Summa Health System, Akron, Ohio (J. DiPersio); Case Western Reserve University Hospital, Cleveland, Ohio (M. Ghannoum); Cleveland Clinic Foundation, Cleveland, Ohio (G. Hall); University of Rochester Medical Center, Rochester, N.Y. (D. Hardy); University of Virginia Health System, Charlottesville (K. Hazen); Veterans Affairs Medical Center, Ann Arbor, Mich. (C. Kauffman); New York Presbyterian Hospital, Cornell Medical Center, New York, N.Y. (D. Larone); Harper Hospital, Division of Infectious Diseases, Detroit, Mich. (J. Sobel); Christiana Care ID Lab, Wilmington, Del. (L. Steele Moore); Temple University School of Medicine, Philadelphia, Pa. (B. Suh); Good Samaritan Medical Regional Center, Phoenix, Ariz. (D. Sussland); Westchester Medical Center, Valhalla, N.Y. (K. Van Horn); Scaolabrini Ortiz, Buenos Aires, Argentina (J. Finquelievich); Arabe Seria, Buenos Aires, Argentina (I. N. Tiraboschi); Rua Diogo de Faria, San Paulo, Brazil (A. Lopes Colombo); Laboratorio de Micologia, Medellin, Columbia (A. Restrepo); Hospital Militar Central, Bogota, Columbia (R. Vega); Hospital Militar, Quito, Ecuador (J. Ayabaca); Instituto de Patologia Infecciosa y Experimental, Guadalajara, Mexico (E. Rodriquez Noriega); Hospital General O'Horan, Merida, Mexico (M. Zaidi); SAIMR, Johannesburg, South Africa (H. H. Crewe-Brown); Department of Medical Microbiology, Medunsa, South Africa (A. Hoosen); Groote Schuur Hospital, Cape Town, South Africa (D. Roditi); Johannesburg Hospital, Parktown, South Africa (T. Towindo); Pelanomi Hospital, UOFS, Bloemfontein, South Africa (J. van Rensburg); Women's and Children's Hospital, North Adelaide, Australia (D. Ellis); Department of Medical Microbiology, Kuala Lumpur, Malaysia (N. K. Peng); Chiang Mai University, Chiang Mai, Thailand (P. Tharavichitkul); China Medical College Hospital, Taichung, Taiwan (J.-H. Wang); National Taiwan University Hospital, Taipei, Taiwan (K.-W. Yu); Vzhongshan Hospital, Shanghai, China (H. Bijie); Peking Union Medical College Hospital, Beijing, China (Y. Xu); Yonsei University College of Medicine, Seoul, Korea (Y. Chong, K. Lee); St. Cyril and Metod Hospital, Bratislava, Slovakia (H. Hupkova); National Cancer Institute, Bratislava, Slovakia (J. Trupl); Derer University Hospital, Bratislava, Slovakia (A. Vaculikova); Nernocnice C. Budejovice, Ceske Budejovice, Czech Republic (N. Mallatova); Odd. Klinicke Myckkologie, Ostrava, Czech Republic (D. Stanislava); Children's Memorial Health Institute, Warsaw, Poland (D. Dzierzanowska); Pescara Civil Hospital, Pescara, Italy (D. D'Antonio); Ospedale di Novara, Novara, Italy (G. Fortina); Departimento di Biotechnologie Cellulari ed Ematologia, Rome, Italy (P. Martino); Azienda Ospedaliera Umbertol, Torrett, Italy (G. Scalise); Ospedale di Genova, Genoa, Italy (G. C. Schito); University of Rome “Tor Vergata,” Rome, Italy (G. P. Testore); Universita degli Studi di Torino, Torino, Italy (V. Tullio); Hopital Universitario 12 de Octobre, Madrid, Spain (A. del Palacio); Canisius-Wilhelmina Ziekerhuis Megishe microbiologie C70, Nijmegen, The Netherlands (J. F. G. M. Meis); Friarage Hospital, Northallerton, United Kingdom (N. Weightman); Children's Hospital, Ankara, Turkey (D. Gur); Mamara Medical School Hospital, Istanbul, Turkey (V. Korten); University of Alberta Hospitals, Edmonton, Alberta, Canada (R. Rennie); Department of Microbiology and Immunology, Saskatoon, Saskatchewan, Canada (K. Mochoruk); NHS Trust, Aberdeen Royal Infirmary, Foresterhill, United Kingdom (I. Gould); and Roswell Park Cancer Institute, Buffalo, N.Y. (B. Segal).
REFERENCES
- 1.Arendrup, M., B. Lundgren, I. M. Jensen, B. S. Hansen, and N. Frimondt-Møfller. 2001. Comparison of Etest and a tablet diffusion test with the NCCLS broth microdilution method for fluconazole and amphotericin B susceptibility testing of Candida isolates. J. Antimicrob. Chemother. 47:521-526. [DOI] [PubMed] [Google Scholar]
- 2.Barry, A. L., M. A. Pfaller, S. D. Brown, A. Espinel-Ingroff, M. A. Ghannoum, C. Knapp, R. P. Rennie, J. H. Rex, and M. G. Rinaldi. 2000. Quality control limits for broth microdilution susceptibility tests of ten antifungal agents. J. Clin. Microbiol. 38:3457-3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barry, A. L., M. A. Pfaller, R. P. Rennie, P. C. Fuchs, and S. D. Brown. 2002. Precision and accuracy of fluconazole susceptibility tests by broth microdilution, Etest, and disk diffusion methods. Antimicrob. Agents Chemother. 46:1781-1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kronvall, G., and I. Karlsson. 2001. Fluconazole and voriconazole multidisk testing of Candida species for disk calibration and MIC estimation. J. Clin. Microbiol. 39:1422-1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meis, J., M. Petrou, J. Bille, D. Ellis, D. Gibbs, and the Global Antifungal Surveillance Group. 2000. A global evaluation of the susceptibility of Candida species to fluconazole by disk diffusion. Diagn. Microbiol. Infect. Dis. 36:215-223. [DOI] [PubMed] [Google Scholar]
- 6.Morace, G., G. Amato, F. Bistoni, G. Fadda, P. Marone, M. T. Montagna, S. Oliveri, L. Polonelli, R. Rigoli, I. Mancuso, S. La Face, L. Masucci, L. Romano, C. Napoli, D. Tato, M. G. Buscema, C. M. C. Belli, M. M. Picirillo, S. Coni, S. Covan, F. Fanti, C. Cavanna, F. D'Alo, and L. Pitzurra. 2002. Multicenter comparative evaluation of six commercial systems and the National Committee for Clinical Laboratory Standards M27-A broth microdilution method for fluconazole susceptibility testing of Candida species. J. Clin. Microbiol. 40:2953-2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.National Committee for Clinical Laboratory Standards. 1997. Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard M27-A. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 8.Pfaller, M. A., S. A. Messer, A. Karlsson, and A. Bolmström. 1998. Evaluation of the Etest method for determining fluconazole susceptibilities of 402 clinical yeast isolates by using three different agar media. J. Clin. Microbiol. 36:2586-2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pfaller, M. A., S. A. Messer, A. Houston, K. Mills, A. Bolmström, and R. N. Jones. 2000. Evaluation of the Etest method for determining voriconazole susceptibilities of 312 clinical isolates of Candida species by using three different media. J. Clin. Microbiol. 38:3715-3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pfaller, M. A., D. J. Diekema, R. N. Jones, H. S. Sader, A. C. Fluit, R. J. Hollis, S. A. Messer, and the SENTRY Participant Group. 2001. International surveillance of bloodstream infections due to Candida species: frequency of occurrence and in vitro susceptibilities to fluconazole, ravuconazole, and voriconazole of isolates collected from 1997 through 1999 in the SENTRY Antimicrobial Surveillance Program. J. Clin. Microbiol. 39:3254-3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pfaller, M. A., S. A. Messer, R. J. Hollis, R. N. Jones, and D. J. Diekema. 2002. In vitro activities of ravuconazole and voriconazole compared with those of four approved systemic antifungal agents against 6,970 clinical isolates of Candida spp. Antimicrob. Agents Chemother. 46:1723-1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pfaller, M. A., D. J. Diekema, S. A. Messer, L. Boyken, and R. J. Hollis for the ARTEMIS Global Antifungal Susceptibility Program Participants Group. Activity of fluconazole and voriconazole determined by broth microdilution, disk diffusion, and Etest methods against 1,586 recent clinical isolates of Candida species: report from the ARTEMIS Global Antifungal Susceptibility Program, 2001. J. Clin. Microbiol., in press. [DOI] [PMC free article] [PubMed]
- 13.Rex, J. H., M. A. Pfaller, J. N. Galgiani, M. S. Bartlett, A. Espinel-Ingroff, M. A. Ghannoum, M. Lancaster, M. G. Rinaldi, T. J. Walsh, and A. L. Barry. 1997. Development of interpretive breakpoints for antifungal susceptibility testing: conceptual framework and analysis of in vitro-in vivo correlation data for fluconazole, itraconazole, and Candida infections. Clin. Infect. Dis. 24:235-247. [DOI] [PubMed] [Google Scholar]
- 14.Rex, J. H., T. J. Walsh, J. D. Sobel, S. G. Filler, P. G. Pappas, W. E. Dismukes, and J. E. Edwards. 2000. Practice guidelines for the treatment of candidiasis. Clin. Infect. Dis. 30:662-678. [DOI] [PubMed] [Google Scholar]
- 15.Rex, J. H., and M. A. Pfaller. 2002. Has antifungal susceptibility testing come of age? Clin. Infect. Dis. 35:982-989. [DOI] [PubMed] [Google Scholar]
- 16.Simor, A. E., G. Goswell, L. Louie, M. Lee, and M. Louie. 1997. Antifungal susceptibility testing of yeast isolates from blood cultures by microbroth dilution and the Etest. Eur. J. Clin. Microbiol. Infect. Dis. 16:693-697. [DOI] [PubMed] [Google Scholar]
- 17.Warnock, D. W., E. M. Johnson, T. R. Rogers, et al. 1998. Multi-centre evaluation of the Etest method for antifungal drug susceptibility testing of Candida spp. and Cryptococcus neoformans. J. Antimicrob. Chemother. 42:321-331. [DOI] [PubMed] [Google Scholar]
- 18.Warren, N. G., and K. C. Hazen. 1999. Candida, Cryptococcus, and other yeasts of medical importance, p. 1184-1199. In P. R. Murray, E. J. Baron, M. A. Pfaller, F. C. Tenover, and R. H. Yolken (ed.), Manual of clinical microbiology, 7th ed. ASM Press, Washington, D.C.