Abstract
Centrosomes are the main microtubule-organizing centers in animal cells. During meiosis and mitosis, two centrosomes form the poles that direct the assembly of a bipolar spindle, thus ensuring the accurate segregation of chromosomes. Cells cannot tolerate the presence of more than two active centrosomes during meiosis or mitosis because doing so results in the formation of multipolar spindles, infidelity in chromosome segregation, and aneuploidy. Here, we show that fertilization of Spisula solidissima oocytes results in cells that contain three active centrosomes, two maternal and one paternal. During meiosis I, the paternal centrosome’s ability to nucleate microtubules is selectively shut off while maternal centrosomes remain competent to nucleate microtubules and assemble asters in the same cytoplasm. We propose that embryos can identify paternal vs. maternal centrosomes and can control them differentially.
Centrosomes are the major microtubule (Mt)- organizing centers in animal cells. These organelles play an important role in directing the organization of the cell cytoplasm during interphase and the assembly of bipolar Mt arrays (spindles), which segregate chromosomes during meiosis/mitosis (1–3). In most animal cells, centrosomes are composed of two centrioles that are surrounded by pericentriolar material. The pericentriolar material is a protein complex that is responsible for the nucleation of Mts (1, 4–8) and contains γ-tubulin, a unique form of tubulin that is essential for centrosome-dependent Mt nucleation (6–12). Although bipolar Mt arrays can assemble in the absence of centrosomes (13–15), when centrosomes are present, they dominate by organizing Mts into astral arrays that form the poles of meiotic/mitotic spindles (13–18). Thus, the presence of more than two active centrosomes in a cell during meiosis or mitosis leads to the assembly of multipolar spindles and, consequently, abortive chromosome segregation (19). Therefore, controlling the number and the ability of centrosomes to nucleate Mts during meiosis/mitosis is essential for cell viability.
Fertilization and early development requires the control of maternal and paternal centrosomes to ensure that cells never contain more than two functional centrosomes. For example, both maternal and paternal gametes contain centrosomes during their developmental cycle. Thus, fertilization can pose a challenge because the resulting embryo could inherit too many centrosomes, one from the female and one from the male gamete. If both centrosomes are capable of replication and function in aster formation during mitotic cycles, this would result in the assembly of tetrapolar spindles and abortive development. To avoid this problem, it has been proposed that eggs destroy the maternal centrosome’s ability to replicate during meiosis I, and it is the paternal centrosome that continues to replicate and function during normal development (20, 21).
Typically, sperm centrosomes are not capable of nucleating and organizing astral Mt arrays before insemination (11, 22, 23). However, after fertilization, the sperm centrioles recruit maternally derived materials stored in the oocyte cytoplasm and assemble a functional centrosome that acquires the ability to nucleate Mts and assemble asters (11, 23). Of interest, recent evidence indicates that bovine and human infertility can result from defects in the embryo’s ability to activate sperm centrosome maturation and aster formation after fertilization (18, 24, 25), suggesting the possibility that specific regulatory mechanisms control paternal centrosome maturation. Given that a maternal centrosome’s replication potential can be destroyed during meiosis (20, 21), and paternal centrosome maturation can be activated after fertilization (18, 24, 25), an important question arises: Are maternal and paternal centrosomes differentially regulated?
The surf clam (Spisula solidissima) oocyte/embryo system offers an opportunity for detailed investigation of the fate of maternal and paternal centrosomes in the same cell after fertilization. Before fertilization, Spisula oocytes are naturally arrested at prophase of meiosis I and contain no asters (26–29). Artificial activation of oocytes induces the formation of two maternal centrosomes, which direct the assembly of the two asters needed for bipolar spindle assembly and the completion of meiosis (26–29). Similarly, fertilization induces the formation of two maternal centrosomes; however, sperm entry introduces a third Mt-organizing center, the paternal centrosome (27, 28). Given that the presence of three active centrosomes could lead to tripolar spindle assembly and aborted meiosis I, it was proposed that the sperm centrosome remains inactive until the end of meiosis, when it is finally activated to function as a Mt-organizing center during subsequent mitotic cycles (28). This unusual differential behavior of paternal vs. maternal centrosomes within the same cell led to the speculation that the Spisula sperm centrosome remains concealed and inaccessible, perhaps somehow masked and unable to recruit maternal components needed for paternal centrosome maturation until the completion of meiosis (18, 21).
In contrast to previous observations, we show that, after fertilization, the Spisula sperm centrosome does in fact acquire the ability to nucleate Mts and forms a sperm aster during meiosis I. Thus, fertilization of Spisula oocytes results in an unusual and potentially disruptive situation in which three centrosomes are present in the same cell, two maternal and one paternal, all of which are active in Mt nucleation. Surprisingly, by metaphase of meiosis I, sperm centrosomes selectively lose their ability to nucleate Mts, and sperm asters disappear whereas maternal centrosomes remain competent and retain Mts, and maternal asters persist. Further, we show that sperm centrosomes regain the ability to organize Mts into astral arrays by metaphase of meiosis II. Using in vitro reconstitution assays, we demonstrate that this gain and loss of paternal centrosome function does not depend on the location of the sperm centrosome within the embryo. Based on these results, we propose that Spisula embryos can differentially control maternal and paternal centrosome function, and we suggest the concept of centrosome identity based on parental inheritance.
MATERIALS AND METHODS
Obtainment of Organisms and Gametes.
Mature S. solidissima were ordered from the Marine Resources Department of the Marine Biological Laboratory (Woods Hole, MA). Ovaries were dissected from ripe females and were minced and filtered through cheesecloth into a large volume of artificial sea water (ASW). The oocytes were washed four times with ASW by cycles of resuspension/settling before fertilization (26). Testes were removed from male clams and were kept cool on ice. Released sperm was collected as “dry” sperm, was stored at 4°C, and used for fertilization within 5 hours.
Fertilization and Fixation of Sperm and Embryos.
Oocytes were suspended in ASW containing 10 mM of Tris base (pH 8.0). Dry sperm was diluted in ASW with the Tris base, test fertilizations were conducted, and final fertilization and development were allowed to proceed at room temperature according to the method of Allen (26).
At different time points after fertilization, 2–3 drops of the sperm/embryo/sea-water suspension were loaded onto poly-l-lysine coated, round, 12-mm coverslips (BellCo Glass). The sperm and embryos were allowed to attach for 4 min, and the coverslips were transferred into drops of 0.5 ml of extraction buffer (100 mM Pipes, pH 6.9/1 mM MgSO4/5 mM EGTA/1 mM DTT/10% glycerol/0.1% Nonidet P-40/20 mM phenylmethylsulfonyl fluoride) on a parafilm surface. After 6–9 min of extraction, the samples were fixed in 90% methanol/20 mM EGTA at −20°C for a minimum of 10 min and were processed for immunofluorescence.
In Vitro Assembly of Paternal and Maternal Asters.
Centrosome-free lysates were prepared from Spisula oocytes 10, 20, and 40 min after KCl activation, were snap-frozen in liquid nitrogen, and were stored at −80°C as described (29). Sperm heads with associated centrosomes were isolated as described (30), were frozen in liquid nitrogen, and were stored at −80°C. Frozen sperm heads were thawed and diluted in aster buffer (20 mM Pipes, pH 7.2/100 mM NaCl/5 mM MgSO4) to a concentration of 4 × 106/ml, which was determined by using a hemacytometer. Aliquots (10 μl) of lysates were thawed and mixed with 1 μl of the diluted sperm head solution (final concentration: 4 × 105/ml), and the mixtures were incubated at room temperature for 15 min. Samples then were diluted with 1.5 ml of cold reassembly buffer (100 mM Pipes/1 mM EGTA/5 mM MgSO4, pH 6.9), were placed on ice for 15 min to depolymerize Mts that might have formed in the lysates, and were centrifuged for 5 min at 4°C (6,000 × g) with a Beckman JS13.1 swinging bucket rotor to pellet. The supernatant was aspirated carefully, leaving 20–30 μl of buffer overlaying the sperm head pellet. The pellet was resuspended, and samples were diluted in equal volume of ice-cold Mt reassembly buffer containing 1 mg/ml sea urchin Mt protein and GTP (8, 31) and were incubated at room temperature for 15 min to allow aster formation. The samples were fixed (29, 33), and sperm-heads and associated asters were immobilized onto coverslips and were processed for immunofluorescence (8, 29, 33). For comparison, and as a control, the ability of isolated maternal Spisula centrosomes to assemble asters in 10, 20, and 40 min centrosome-free lysates was tested. Maternal centrosomes were isolated as described (8), were incubated in respective lysates at a final concentration of 4 × 105/ml for 15 min at room temperature, were placed on ice for 15 min to depolymerize Mts, were immobilized onto glass coverslips, were incubated with 0.5 mg/ml of sea urchin Mt protein, and were processed for immunofluorescence as described (8).
Immunofluorescence.
After fixation, coverslips with attached sperm and embryos, sperm heads and paternal asters, or maternal asters were processed for immunofluorescence. Coverslips first were washed with PBS and then with PBS containing 0.1% Tween-20 (PBST) for 5 min. Coverslips then were blocked with PBST containing 3% BSA (PBST/BSA) for 30 min at room temperature and were incubated with primary antibodies in PBST/BSA for 24–48 hours at 4°C. Rat-anti-α-tubulin (Serotec) was used to label Mts; polyclonal anti-γ-tubulin (EAD24; gift from Tim Stearns, Stanford University) was used to label the centrosomes. After incubation in primary antibody, samples were washed with PBST three times for 10-min intervals. The coverslips then were incubated with secondary antibodies, fluorescein isothiocyanate-conjugated goat-anti-rat IgG, and Cy5-conjugated goat-anti-rabbit IgG (Jackson ImmunoResearch), which had been diluted 1:100 in PBST/BSA for 60 min at 37°C. The coverslips were washed with PBS for 5 min and were incubated in PBS containing 2.5 μg/ml ethidium homodimer (Molecular Probes) for 20 min at room temperature in the dark to stain chromatin. After three 10-min washes with PBS, the coverslips were mounted on slides with elvanol-mounting medium (8, 29) and were observed with a Zeiss Axiovert inverted microscope or a laser-scanning confocal microscope (MRC-1000; Bio-Rad). Confocal microscope images were obtained with the comos program (Bio-Rad).
RESULTS
Maternal vs. Paternal Aster Formation in Spisula Embryos.
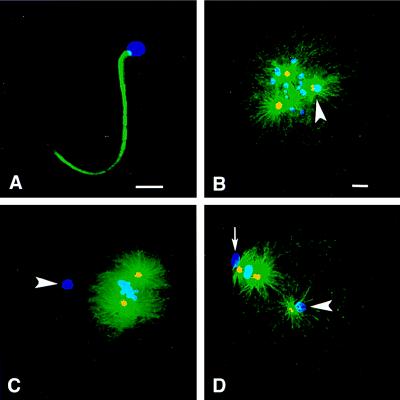
To track the ability of paternal and maternal centrosomes to nucleate microtubules and form asters, Spisula oocytes were fertilized, and embryos were prepared for immunofluorescence localization studies at various time points in development. Anti-α-tubulin antibody was used to assess aster formation, an indicator of a centrosome’s Mt nucleation potential. In addition, anti-γ-tubulin antibody was used to stain centrosomes within asters because γ-tubulin has been shown to be a component of Mt nucleation sites in the centrosome’s pericentriolar material and serves as an indicator of centrosome maturation (6–13). Because paternal centrosomes remain associated with the sperm nucleus during meiosis (27, 28, 30), the chromatin stain ethidium homodimer was used to determine the position of the sperm chromatin, which was used to identify the associated sperm aster and the expected position of the sperm centrosome (Fig. 1).
Figure 1.
Maternal vs. paternal aster formation in Spisula embryos. Shown is immunofluorescence of Spisula sperm (A) and embryos (B–D) during meiosis I and II by using anti-γ-tubulin antibody (yellow), antitubulin antibody (green), and ethidium homodimer to label chromatin (blue). (A) No γ-tubulin was detected in sperm before fertilization. (B) During prometaphase I, 10 min after fertilization, embryos contained two maternal asters and one sperm aster, and the paternal centrosome contained γ-tubulin and nucleated Mts (arrowhead). (C) During metaphase I, 20 min after fertilization, embryos contained two maternal asters but no sperm aster, and paternal centrosomes contained no γ-tubulin (arrowhead). (D) At metaphase of meiosis II, 40 min after fertilization, embryos again contained three asters, and the paternal centrosomes contained γ-tubulin and nucleated Mts (arrowhead). The small arrow in D indicates the first polar body. [Bars = 5 μm (A), and = 5 μm (B, for B–D).]
Consistent with previous studies in Xenopus (11), immunofluorescence analysis revealed that Spisula sperm did not contain γ-tubulin (Fig. 1A), and centrosomes associated with isolated sperm heads were not capable of forming asters when incubated in tubulin media (Fig. 3A). However, 10 min after fertilization (prometaphase of meiosis I), Spisula embryos contained three asters, two maternal and one paternal aster associated with the sperm nucleus (Fig. 1B). These results are in contrast to previous studies, which reported that Spisula sperm centrosomes do not form asters during meiosis I (27, 28). Of importance, at this time, all three centrosomes contained γ-tubulin (Fig. 1B). Thus, the sperm centrosome must have recruited γ-tubulin from the maternal cytoplasm and gained the ability to nucleate Mts and form asters after entry into the oocyte. Surprisingly, 20 min after fertilization, during metaphase of meiosis I, neither sperm asters nor γ-tubulin staining was found associated with sperm nuclei (Fig. 1C). However, in the same cells at this time point, both of the maternal centrosomes continued to nucleate Mts, and both showed obvious γ-tubulin staining (Fig. 1C). Later, during metaphase of meiosis II, 40 min after fertilization, sperm asters again were found associated with sperm nuclei, and again the paternal centrosomes contained γ-tubulin (Fig. 1D).
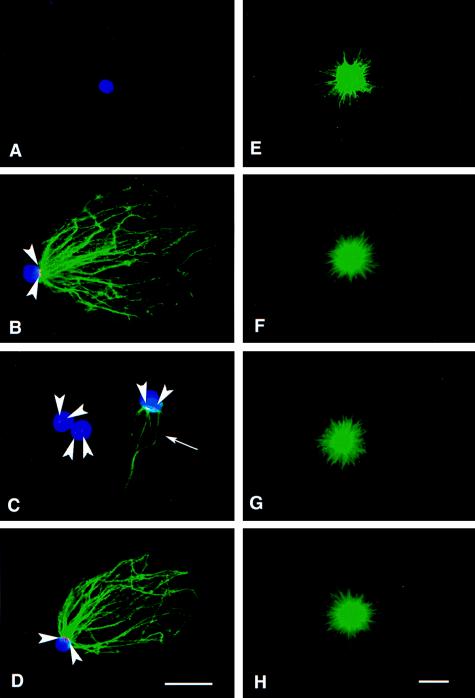
Figure 3.
In vitro assembly of paternal and maternal asters. Immunofluorescence analysis of isolated sperm heads (A–D) or isolated maternal centrosomes (E–H) incubated in buffer (A and E) or oocyte lysates (B–D and F–H) by using anti-γ-tubulin antibody (red), antitubulin antibody (green), and ethidium homodimer to stain chromatin (blue). (A) No γ-tubulin or Mts were found associated with sperm heads incubated in buffer. (B) Paternal centrosomes acquired both γ-tubulin staining (arrowheads) and the ability to nucleate Mts when incubated in 10-min lysate. (C) When incubated in 20-min lysate, the paternal centrosomes showed very weak γ-tubulin staining (arrowheads) and little or no Mt nucleation potential (arrow). (D) When treated with 40-min lysate, the paternal centrosomes nucleated Mts and displayed obvious γ-tubulin staining (arrowheads). (E–H) No obvious difference in the Mt nucleation potential of maternal centrosomes was observed as a function of buffer or lysate treatment. [Bars = 5 μm (D, for A–D, and H, for E–H).]
Quantitation of Maternal vs. Paternal Aster Formation in Fertilized Embryos.
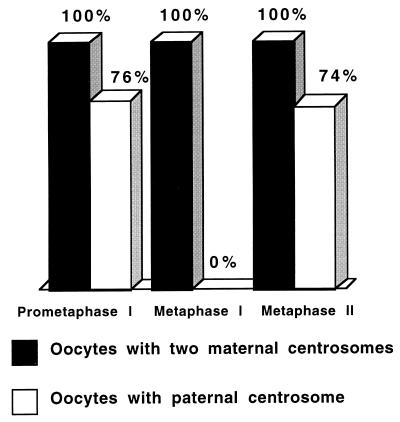
Embryos were fertilized and prepared for immunofluorescence analysis during prometaphase and metaphase of meiosis I and metaphase of meiosis II (10, 20, and 40 min after fertilization, respectively). Immunofluorescence analysis of >100 embryos for each time point (Fig. 2) revealed that, 10 min after fertilization, all embryos contained two maternal asters. Of importance, sperm asters were identified in 76% of these embryos. However, 20 min after fertilization, although all embryos contained two maternal asters, sperm asters were not identified in any of these. By 40 min after fertilization, sperm asters were identified in 74% of the embryos. In all cases, the presence and absence of γ-tubulin stain on the centrosomes correlated with the presence and absence of Mt nucleation potential, respectively. These results indicate that the ability of the sperm centrosome to recruit γ-tubulin and nucleate Mts during meiosis is transient: gained after fertilization during prometaphase of meiosis I, lost during metaphase of meiosis I, and regained by metaphase of meiosis II. Of importance, even though paternal centrosomes undergo this transient gain and loss of function, the maternal centrosomes remained competent to nucleate Mts at all stages analyzed (Figs. 1 B–D and 2).
Figure 2.
Quantitation of maternal and paternal aster formation in Spisula embryos during meiosis I and II. Oocytes were fertilized, were fixed at various times, and were processed for immunofluorescence analysis. Over 100 embryos were analyzed for maternal and paternal aster content at each stage. Ten minutes after fertilization (prometaphase I), all embryos contained two maternal asters, and 76% contained one paternal aster associated with the sperm nucleus. Twenty minutes after fertilization (metaphase I), all embryos contained two maternal asters, but no paternal asters were identified in any of the embryos analyzed at this time point. Forty minutes after fertilization (metaphase II), all embryos contained two maternal asters, and 74% contained one paternal aster.
In Vitro Formation of Paternal vs. Maternal Asters.
A possible explanation for the results described is that the relative position of the paternal centrosome within the maternal cytoplasm may have some affect on the paternal centrosome’s ability to acquire microtubule nucleation potential and form asters. To test this possibility, we disrupted oocytes, prepared centrosome-free oocyte lysates, and used an in vitro functional assay to test the ability of the paternal centrosome to mature and acquire function outside the confines of a living cell. Isolated Spisula sperm heads with associated centrioles (30) or isolated maternal centrosomes (8) were incubated in centrosome-free oocyte lysates (29) prepared from oocytes that had been parthenogenetically activated for 10, 20, and 40 min. After incubation in lysates, sperm heads were washed and incubated in defined media containing sea urchin Mt protein (8, 31) to allow aster formation. Samples then were fixed and processed for immunofluorescence analysis. Neither asters nor γ-tubulin were found associated with isolated sperm heads treated with buffer alone (Fig. 3A). However, when incubated in either 10-min (Fig. 3B) or 40-min (Fig. 3D) lysates, paternal centrosomes gained the ability to form asters and stained positive for γ-tubulin. Of importance, when incubated in 20-min lysate, few if any Mts were found associated with sperm heads, and <50% had any associated Mts (Fig. 3C). In addition, much weaker γ-tubulin staining was found associated with sperm heads incubated in the 20-min lysate (Fig. 3C). Although sperm centrosomes exhibited different Mt nucleation potentials depending on treatment with buffer or 10-, 20-, or 40-min lysates (Fig. 3 A–D), these same treatments had no obvious effect on the ability of isolated maternal centrosomes to nucleate Mts (Fig. 3 E–H) because these retained the ability to nucleate Mts regardless of treatment.
DISCUSSION
Centrosome proteins are stored in egg cytoplasm during oogenesis but are largely lost in sperm during spermatogenesis (11, 18, 22, 23). During fertilization, the sperm introduces a paternal centrosome that lacks the ability to nucleate Mts and form asters (11, 18, 22, 23) but is capable of reproduction (18, 20). Once in the egg, the sperm centrosome begins to recruit γ-tubulin and other centrosome components (10, 11, 18, 23), leading to the nucleation of Mts and the formation and enlargement of the sperm aster. Unlike Spisula sperm, immunofluorescence and Western blot analysis revealed the presence of at least some γ-tubulin in human sperm, even before fertilization (36). However, similar to Spisula and other systems (10, 11, 18, 23) after fertilization, human sperm centrosomes do recruit more γ-tubulin from the maternal stores, allowing for aster formation (36). The γ-tubulin present in human sperm appears to be cryptic because it cannot be detected unless sperm are first permeabilized and treated with disulfide reducing agents. Of importance, the ability of permeabilized human sperm to form asters in Xenopus egg lysates in vitro also requires pretreatment with disulfide reducing agents (36), suggesting a role for sulfhydral reduction in the activation of human sperm centrosome maturation. In contrast, γ-tubulin was not detected in Spisula sperm by the immunofluorescence analysis described here or by Western blot analysis (data not shown).
Although the time between sperm incorporation and the formation of the sperm aster is variable among different species (because of the differences in the length of the cell cycle and the stage at which fertilization takes place), it is believed that the sperm centrosome begins to recruit γ-tubulin and other centrosome components needed to form the paternal aster soon after fertilization (18, 20, 24, 25, 34, 35, 36). However, the behavior of the sperm centrosome during meiosis I in fertilized Spisula oocytes provides some additional insights regarding the regulation of centrosome function.
Spisula oocytes are fertilized at a relatively early developmental stage, during late prophase of meiosis I (26, 27, 28), before the segregation of meiotic chromatin and presumably before the loss of the maternal centrosome’s ability to replicate (20). Fertilization induces the assembly of a bipolar maternal meiotic spindle and results in the presence of three centrosomes, two maternal and one paternal within the same egg. During meiosis I, one of the two maternal centrosomes segregates with half of the maternal chromatin into the first polar body, leaving one maternal and one paternal centrosome within the egg. During meiosis II, the remaining maternal centrosome splits to form the two poles of the meiosis II spindle, followed by the segregation of another maternal centrosome (half-centrosome) to the second polar body, leaving one maternal (half-centrosome) and one paternal centrosome within the egg (ref. 28 and unpublished observations). The fate of the remaining maternal centrosome is not clear. However, it is apparently incapable of replication, because (i) artificially (KCl) activated eggs that enter mitosis after completion of meiosis can assemble a maternal monaster, but not a bipolar spindle, and (ii) fertilized eggs form bipolar spindles (ref. 28 and unpublished observations) but not the tetrapolar spindles that would be expected if both the paternal and maternal centrosomes that remain in the egg after meiosis II are capable of replication. Thus, it is reasonable to conclude that, during Spisula embryogenesis, as in other systems (18, 20, 21), the male centrosome continues to replicate and function while the maternal centrosome’s ability to replicate is lost during the completion of meiosis.
Previous ultrastructural (27) and tubulin immunofluorescence (28) analysis of fertilized Spisula embryos showed that, after entry, the sperm centrosome remains unable to nucleate Mts and organize an aster until the oocyte completes meiosis I and II. Based on these results, it was logically suggested that, during meiosis, the paternal centrosome is masked or hidden from cytoplasmic conditions that could activate processes leading to aster formation (28). However, immunofluorescence analysis presented here does not support these conclusions. The methods described here allowed us to carefully trace the position and the ability of maternal and paternal centrosomes to nucleate Mts and organize asters within the same cell during meiosis. This analysis clearly demonstrated that sperm centrosomes nucleate Mts and form asters during prometaphase of meiosis I within 10 min after fertilization. Of importance, the recruitment of the centrosomal protein γ-tubulin, which was not detected in the sperm before fertilization, to the paternal centrosome during this time verified that sperm centrosome maturation had indeed occurred. These results indicate that Spisula sperm centrosomes are not “masked” or blocked from responding to the cytoplasmic conditions within oocytes. Thus, γ-tubulin, and probably other maternal centrosomal components required for Mt nucleation, are recruited onto the paternal centrosome from the oocyte cytoplasm, and the paternal centrosome undergoes a transient maturation process, acquiring the ability to nucleate Mts during prometaphase of meiosis I.
Surprisingly, by metaphase of meiosis I, the sperm centrosome lost competence in Mt nucleation, indicated by the disappearance of the sperm aster at this time. Loss of the sperm centrosome’s ability to nucleate Mts removes the potential for tripolar spindle assembly, thus assuring the proper assembly of the bipolar maternal spindle needed for accurate segregation of the maternal chromosomes during meiosis I. Of importance, coincident with this loss in Mt nucleation potential was the loss of γ-tubulin localization to the sperm centrosome. In contrast, during this same time, the meiosis I spindle remained intact, and the two maternal centrosomes remained competent in Mt nucleation. Therefore, the Mt nucleation potential of the paternal centrosome was selectively lost while the maternal centrosomes remained competent during this time. Of importance, the loss of γ-tubulin stain from the paternal centrosome during this time strongly suggests that the loss of Mt nucleation potential is mediated by the selective disassembly of those proteins required for Mt nucleation from the pericentriolar material of the paternal centrosomes.
The results show that Spisula paternal centrosomes contain γ-tubulin and recover the ability to nucleate Mts by metaphase of meiosis II (≈40 min after insemination). Taken together, the data shows that the paternal centrosome recruits γ-tubulin and acquires the ability to nucleate Mts during prophase of meiosis I, loses γ-tubulin association and the Mt nucleation potential by metaphase of meiosis I, and again recruits γ-tubulin and regains Mt nucleation potential by metaphase of meiosis II. Although the paternal centrosome undergoes this gain and loss of function during meiosis I, in the same cell, the maternal centrosomes persist, retain γ-tubulin, and remain competent to nucleate Mts. Of importance, the results of the in vitro studies using cytoplasmic lysates and isolated sperm heads indicate that the ability to form sperm asters is independent of the presence of maternal centrosomes and/or the location of the sperm centrosome within the egg cytoplasm. Again, in vitro as in vivo, the recruitment of γ-tubulin to the paternal centrosome is coincident with the acquisition of Mt nucleation potential and the ability to form a sperm aster. Taken together, these results indicate that, in Spisula embryos, the paternal centrosome’s ability to nucleate Mts is selectively abolished during metaphase of meiosis I.
These results indicate that Spisula embryos can distinguish centrosomes based on parental origins and can differentially regulate the Mt nucleation potential of maternal and paternal centrosomes during meiosis I. The selective control of centrosome function, either the selective destruction of replication potential (20, 21) or the differential control of Mt nucleation potential shown here, requires mechanisms for distinguishing maternal from paternal centrosomes. Thus, we propose that either paternal or maternal centrosomes, or both, contain molecular markers that provide them with an identity based on their parental origin.
Acknowledgments
This work is dedicated to Dr. Paul Gross in recognition of his dedicated service to the Marine Biological Laboratory. We acknowledge B. J. Schnackenberg and A. Suddith for their assistance in manuscript preparation. This work was supported by grants from the National Institutes of Health (GM-43264) and the Robert Day Allen Fellowship of the Marine Biological Laboratory to R.E.P.
ABBREVIATIONS
- Mt
microtubule
- ASW
artificial sea water
- PBST
PBS with Tween 20
References
- 1.Wheatley D N. The Centriole: A Central Enigma in Cell Biology. New York: Elsevier; 1982. [Google Scholar]
- 2.Mazia D. Int Rev Cytol. 1987;100:49–92. doi: 10.1016/s0074-7696(08)61698-8. [DOI] [PubMed] [Google Scholar]
- 3.Kellog D R, Moritz M, Alberts B M. Annu Rev Biochem. 1994;63:639–674. doi: 10.1146/annurev.bi.63.070194.003231. [DOI] [PubMed] [Google Scholar]
- 4.Gould R R, Borisy G G. J Cell Biol. 1977;73:601–615. doi: 10.1083/jcb.73.3.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brinkley B R. Annu Rev Cell Biol. 1985;1:145–172. doi: 10.1146/annurev.cb.01.110185.001045. [DOI] [PubMed] [Google Scholar]
- 6.Moritz M, Braunfield M B, Sedat J W, Alberts B M, Agard D A. Nature (London) 1995;378:638–640. doi: 10.1038/378638a0. [DOI] [PubMed] [Google Scholar]
- 7.Zheng Y, Wong M L, Alberts B M, Mitchison T. Nature (London) 1995;378:578–583. doi: 10.1038/378578a0. [DOI] [PubMed] [Google Scholar]
- 8.Vogel J M, Stearns T, Rieder C L, Palazzo R E. J Cell Biol. 1997;137:193–202. doi: 10.1083/jcb.137.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oakley B R, Oakley C E, Yoon Y, Jung M K. Cell. 1990;61:1289–1301. doi: 10.1016/0092-8674(90)90693-9. [DOI] [PubMed] [Google Scholar]
- 10.Stearns T, Evans L, Kirschner M. Cell. 1991;65:825–836. doi: 10.1016/0092-8674(91)90390-k. [DOI] [PubMed] [Google Scholar]
- 11.Stearns T L, Kirschner M. Cell. 1994;76:623–637. doi: 10.1016/0092-8674(94)90503-7. [DOI] [PubMed] [Google Scholar]
- 12.Schnackenberg B J, Khodjakov A, Rieder C L, Palazzo R E. Proc Natl Acad Sci USA. 1998;95:9295–9300. doi: 10.1073/pnas.95.16.9295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Verde F, Berrez J M, Antony C, Karsenti E. J Cell Biol. 1991;112:1177–1187. doi: 10.1083/jcb.112.6.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heald R, Tournebize R, Blank T, Sandaltzopoulos R, Becker P, Hyman A, Karsenti E. Nature (London) 1996;382:420–425. doi: 10.1038/382420a0. [DOI] [PubMed] [Google Scholar]
- 15.Heald R, Tournebize R, Habermann A, Karsenti E, Hyman A. J Cell Biol. 1997;138:615–628. doi: 10.1083/jcb.138.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levine D S, Sanchez C A, Rabinovitch P S, Reid B J. Proc Natl Acad Sci USA. 1991;88:6427–6431. doi: 10.1073/pnas.88.15.6427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fukasawa K, Choi T, Kuriyama R, Rulong S, Vande Woude G F. Science. 1996;271:1744–1747. doi: 10.1126/science.271.5256.1744. [DOI] [PubMed] [Google Scholar]
- 18.Schatten G. Dev Biol. 1994;165:299–335. doi: 10.1006/dbio.1994.1256. [DOI] [PubMed] [Google Scholar]
- 19.Brinkley B R, Goepfert T M. Cell Motil Cytoskeleton. 1998;41:281–288. doi: 10.1002/(SICI)1097-0169(1998)41:4<281::AID-CM1>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 20.Sluder G, Miller F J, Lewis K E, Davison D, Rieder C L. Dev Biol. 1989;131:567–579. doi: 10.1016/s0012-1606(89)80027-2. [DOI] [PubMed] [Google Scholar]
- 21.Sluder G, Miller F J, Lewis K. Dev Biol. 1993;131:567–579. doi: 10.1016/s0012-1606(89)80027-2. [DOI] [PubMed] [Google Scholar]
- 22.Holy J, Schatten G. Dev Biol. 1991;147:343–353. doi: 10.1016/0012-1606(91)90292-b. [DOI] [PubMed] [Google Scholar]
- 23.Doxsey S K, Stein P, Evans L, Calarco P D, Kirschner M. Cell. 1994;76:639–650. doi: 10.1016/0092-8674(94)90504-5. [DOI] [PubMed] [Google Scholar]
- 24.Simerly C, Wu G, Zoran S, Ord T, Rawlins R, Jones J, Navara C, Gerrity M, Rinehart J, Binor Z, et al. Nat Med. 1995;1:47–52. doi: 10.1038/nm0195-47. [DOI] [PubMed] [Google Scholar]
- 25.Navara C S, First N L, Schatten G. Dev Biol. 1994;162:29–40. doi: 10.1006/dbio.1994.1064. [DOI] [PubMed] [Google Scholar]
- 26.Allen R D. Biol Bull. 1953;105:213–239. [Google Scholar]
- 27.Longo F J, Anderson E. J Vet Res. 1970;33:495–514. doi: 10.1016/s0022-5320(70)90177-2. [DOI] [PubMed] [Google Scholar]
- 28.Kuriyama R G, Borisy G, Masui Y. Dev Biol. 1986;114:151–160. doi: 10.1016/0012-1606(86)90391-x. [DOI] [PubMed] [Google Scholar]
- 29.Palazzo R E, Vaisberg E, Cole R W, Rieder C L. Science. 1992;256:149–280. doi: 10.1126/science.1566068. [DOI] [PubMed] [Google Scholar]
- 30.Longo F J, Mathews L, Palazzo R E. Dev Biol. 1994;62:245–258. doi: 10.1006/dbio.1994.1082. [DOI] [PubMed] [Google Scholar]
- 31.Suprenant K A, Marsh J C. J Cell Sci. 1987;87:71–84. doi: 10.1242/jcs.87.1.71. [DOI] [PubMed] [Google Scholar]
- 32.Palazzo R E, Brawley J B, Rebhun L I. Zool Sci. 1988;5:603–611. [Google Scholar]
- 33.Mitchision T, Kirschner M. Nature (London) 1984;312:232–237. doi: 10.1038/312232a0. [DOI] [PubMed] [Google Scholar]
- 34.Hollenbeck P J, Cande W Z. Eur J Cell Biol. 1985;37:140–148. [PubMed] [Google Scholar]
- 35.Schatten H, Walter M, Biessmann H, Schatten G. Cell Motil Cytoskeleton. 1988;11:248–259. doi: 10.1002/cm.970110404. [DOI] [PubMed] [Google Scholar]
- 36.Navara C S, Simerly C, Zoran S, Schatten G. Reprod Fertil Dev. 1995;7:747–754. doi: 10.1071/rd9950747. [DOI] [PubMed] [Google Scholar]