Abstract
This is the first reported case of endocarditis due to the Lactobacillus-like vancomycin-resistant gram-positive bacillus Weissella confusa. Full identification and susceptibility testing of Lactobacillus-like organisms recovered in blood culture should be performed for patients with clinical presentations that suggest endocarditis.
CASE REPORT
A 49-year-old man presented with a 100-lb weight loss over the preceding year and weakness, memory loss, and rash but no fever during the previous 6 months. Three months prior to admission he experienced sudden loss of vision in his right eye. A year prevoiusly he had been treated with oral corticosteroids for transverse myelitis. He had a history of chronic alcohol abuse. Recently his diet contained large quantities of milk. Clinically, the protracted and atypical course of this patient's disease, evidenced by weight loss and weakness over many months without fever, initially suggested an occult malignancy rather than endocarditis. On examination, his temperature was 37.0°C. A petechial rash was present on his abdomen and all four extremities, including the palms and soles, and the rash was confluent with palpable purpura over his shins. His oral cavity had palatal petechiae, and there were multiple carious and missing teeth. He had right eye blindness except for slight nasal sparing. His right pupil did not constrict to light and was larger than the left, and his right fundus appeared pale, consistent with central retinal artery thrombotic occlusion. A loud holosystolic murmur was present over the entire precordium, and his spleen was palpable. Laboratory examination showed a hemoglobin level of 6.3 g/dl, an erythrocyte sedimentation rate of 55 mm/h, and a serum creatinine level of 4.0 mg/dl. Human immunodeficiency virus type 1 antibody tests were negative. A skin biopsy showed leukocytoclastic vasculitis. A catalase-negative gram-positive coccobacillus that demonstrated alpha hemolysis on sheep blood agar was recovered from both the aerobic and anaerobic bottles in three sets of blood cultures obtained at different times. The vancomycin MIC for this organism in cation-supplemented Mueller-Hinton broth with lysed horse blood was 512 μg/ml. Transthoracic echocardiography revealed moderate-to-severe mitral insufficiency and a nodular echogenicity on the mitral valve. He refused antibiotic therapy and further care despite intensive counseling and was found dead approximately 4 days after leaving the hospital. Autopsy examination revealed cardiomegaly (720 g) and hepatosplenomegaly (2,400 and 1,200 g, respectively). Microscopically there were septic emboli with bacterial colonies in multiple organs, most notably the heart, but also the brain, lungs, liver, kidneys, spleen, adrenals, and skin. Multiple microscopic abscesses were noted in the myocardium, with bacterial colonies in central regions of the abscesses. There were large areas of acute coagulative necrosis of the myocardium despite minimal coronary atherosclerosis. His mitral valve was sclerotic and calcified, with microscopic infiltrates of polymorphonuclear leukocytes, as well as deposits and fragments of fibrin consistent with a detached vegetation.
The blood culture isolate was a nonmotile, pleomorphic gram-positive coccobacillus that produced small (1- to 2-mm) alpha-hemolytic colonies on rabbit blood agar after 2 days of incubation in air at 35°C. There was no growth on MacConkey agar. The organism was negative for catalase and cytochrome oxidase activity and was unable to reduce nitrate. Tests for esculin and arginine hydrolysis were positive, and a test for gelatin hydrolysis was negative. Acid was produced from cellobiose, galactose, maltose, sucrose, and xylose, but not from l-arabinose, melibiose, raffinose, or trehalose. The Special Bacteriology Reference Laboratory of the Centers for Disease Control and Prevention identified this organism as Weissella confusa on the basis of these key reactions (6, 17).
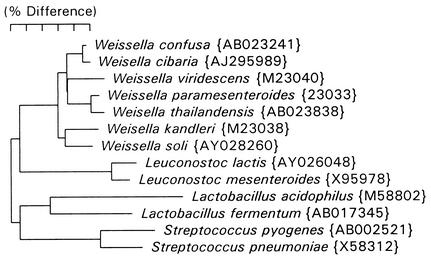
The 16S rRNA cistrons of this isolate were amplified with the bacterial universal primers F24 (9 to 27, forward; 5′-GAGTTTGATYMTGGCTCAG-3′) and F25 (1525 to 1541, reverse; 5′-AAGGAGGTGWTCCARCC-3′). Purified DNA from PCR was sequenced with an ABI Prism cycle sequencing kit (BigDye terminator cycle sequencing kit with AmpliTaq DNA polymerase FS; Perkin-Elmer). The primer F15 (519 to 533, reverse; 5′-TTACCGCGGCTGCTG-3′) was used for sequencing. Sequence data were entered into RNA, a program set for data entry, editing, sequence alignment, secondary structure comparison, similarity matrix generation, and dendrogram construction for 16S rRNA in Microsoft QuickBasic for use with personal computers and were aligned as previously described (14). The database contains over 2,000 sequences obtained by the laboratory of one of us (F. E. Dewhirst) and over 1,000 from GenBank. Sequences were first checked by BLAST analysis versus all entries in GenBank (1), and sequences for related organisms not already in the database were downloaded and added. Approximately 500 bases of sequence were determined and, when searched against the database, differed by only a single base from W. confusa entry AB023241 and by only 9 bases from the closely related Weissella cibaria entry AJ295989. A phylogenetic tree including Weissella species and representatives of three neighboring lactic acid-producing genera was constructed by the neighbor-joining method (15) and is shown in Fig. 1.
FIG. 1.
Phylogenetic tree based on 16S rRNA sequence comparison. Marker bar, 5% difference. Distance is measured by adding the horizontal distances connecting any two species. GenBank accession numbers are listed in parentheses.
Based on a comparative 16S rRNA phylogenetic analysis of Leuconostoc-like organisms from dry fermented Greek sausage, it was proposed in 1993 that Leuconostoc paramesenteroides and related species (including Lactobacillus confusus) be reclassified in a new genus Weissella (6). Species of Weissella including W. confusa constitute a distinct phylogenetic group separate from those of other genera of lactic acid bacteria, including Leuconostoc, Lactobacillus, and Streptococcus (Fig. 1). Production of gas from carbohydrates distinguishes Weissella from homofermentative lactobacilli and other lactic acid bacteria, while the presence within the cell wall of lysine and alanine joined with an intrapeptide bond distinguishes Weissella from heterofermentative lactobacilli (6). W. confusa can be differentiated from the genus Leuconostoc by the hydrolysis of arginine and the formation of d,l-lactate. W. cibaria is most closely related taxonomically to W. confusa (Fig. 1). Both species have the ability to produce NH3 from arginine. However, unlike W. confusa, W. cibaria is negative for the fermentation of galactose and xylose and positive for the fermentation of arabinose (5). Diverse habitats for W. confusa have been detected: fermented meats, sourdough, acidic high-carbohydrate foods, sugar cane, carrot juice, milk, canary liver, an otitis sample from a dog, sewage, and human feces (5, 6, 7, 9, 12). The large quantity of milk in our patient's diet combined with multiple carious and missing teeth could have provided an exposure to and portal of entry for infection by W. confusa.
In a survey of vancomycin-resistant gram-positive bacteria in the feces of 48 children, W. confusa was detected in stool specimens of 4 (9). One child was a multivisceral transplant recipient treated with an oral selective-decontamination regimen including vancomycin who developed W. confusa bacteremia. Subsequent to this survey, two additional episodes of W. confusa bacteremia were reported at the same hospital, and W. confusa was detected in stool and throat specimens as well (9). The clinical significance and outcome of W. confusa bacteremia were not described for these three episodes (9). An encapsulated abscess of the right thumb has been reported for an otherwise healthy 49-year-old man (2); purulent material obtained from the abscess by aspiration yielded a pure culture of W. confusa. A case of a 46-year-old man with a history of abdominal aortic aneurysm repair and aortic root homograft replacement due to Enterococcus faecalis endocarditis has recently been reported (13). This man developed fever and chills associated with multiple blood cultures positive for Klebsiella pneumoniae and W. confusa. Imaging studies of his abdomen and chest and a transesophageal echocardiogram failed to reveal a source of the bacteremia. With combined treatment by piperacillin-tazobactam and gentamicin, blood cultures became negative. Our patient is, as far as we know, the first reported case of monomicrobial bacteremia due to W. confusa unequivocally associated with invasive infection in which W. confusa has been identified by extensive phenotypic testing and rRNA gene sequencing. Features of our case are strongly reminiscent of Lactobacillus endocarditis, with systemic arterial embolization; an association with carious teeth and milk ingestion; predisposing factors including alcohol abuse, steroid therapy, and cardiac valve abnormalities; and a high mortality especially in the absence of antibiotic treatment (3, 10, 11, 16). Endocarditis is predominantly associated with three species of Lactobacillus, L. plantarum, L. casei, and L. acidophilus (3, 4, 8, 10, 11, 16). Lactobacillus and W. confusa demonstrate constitutive high-level resistance to vancomycin, and, for patients with continuous bacteremia accompanied by clinical signs of endocarditis, poor oral hygiene, predisposing factors for endocarditis, and/or embolic manifestations, Lactobacillus-like organisms detected in blood culture should be fully identified and tested for antimicrobial susceptibility.
REFERENCES
- 1.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bantar, C. E., S. Relloso, F. R. Castell, J. Smayevsky, and H. M. Bianchini. 1991. Abscess caused by vancomycin-resistant Lactobacillus confusus. J. Clin. Microbiol. 29:2063-2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bayer, A. S., A. W. Chow, D. Betts, and L. B. Guze. 1978. Lactobacillemia—report of nine cases. Am. J. Med. 64:808-813. [DOI] [PubMed] [Google Scholar]
- 4.Bessis, D., A. Le Quellec, A. Sotto, C. Perez, and A.-J. Ciurana. 1995. Lactobacillus acidophilus endocarditis after an appendectomy. Clin. Infect. Dis. 20:724-725. [DOI] [PubMed] [Google Scholar]
- 5.Bjoerkroth, K. J., U. Schillinger, R. Geisen, N. Weiss, B. Hoste, W. H. Holzapfel, H. J. Korkeala, and P. Vandamme. 2002. Taxonomic study of Weissella confusa and description of Weissella cibaria sp. nov., detected in food and clinical samples. Int. J. Syst. E vol. Microbiol. 52:141-148. [DOI] [PubMed] [Google Scholar]
- 6.Collins, M. D., J. Samelis, J. Metaxopoulus, and S. Wallbanks. 1993. Taxonomic studies on some leuconostoc-like organisms from fermented sausages: description of a new genus Weissella for the Leuconostoc paramesenteroides group of species. J. Appl. Bacteriol. 75:595-603. [DOI] [PubMed] [Google Scholar]
- 7.Corsetti, A., P. Lavermicocca, M. Morea, F. Baruzzi, N. Tosti, and M. Gobbetti. 2001. Phenotypic and molecular identification and clustering of lactic acid bacteria and yeasts from wheat (species Triticum durum and Triticum aestivum) sourdoughs of Southern Italy. Int. J. Food Microbiol. 64:95-104. [DOI] [PubMed] [Google Scholar]
- 8.Davies, A. J., P. A. James, and P. M. Hawkey. 1986. Lactobacillus endocarditis. J. Infect. 12:169-174. [DOI] [PubMed] [Google Scholar]
- 9.Green, M., R. M. Wadowsky, and K. Barbadora. 1990. Recovery of vancomycin-resistant gram-positive cocci from children. J. Clin. Microbiol. 28:484-488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Griffiths, J. K., J. S. Daly, and R. A. Dodge. 1992. Two cases of endocarditis due to Lactobacillus species: antimicrobial susceptibility, review, and discussion of therapy. Clin. Infect. Dis. 15:250-255. [DOI] [PubMed] [Google Scholar]
- 11.Husni, R. N., S. M. Gordon, J. A. Washington, and D. L. Longworth. 1997. Lactobacillus bacteremia and endocarditis: review of 45 cases. Clin. Infect. Dis. 25:1048-1055. [DOI] [PubMed] [Google Scholar]
- 12.Leisner, J. J., B. Pot, H. Christensen, G. Rusul, J. E. Olson, B. W. Wee, K. Muhamad, and H. M. Ghazali. 1999. Identification of lactic acid bacteria from chili bo, a Malaysian food ingredient. Appl. Environ. Microbiol. 65:599-605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olano, A., J. Chua, S. Schroeder, A. Minari, M. La Salvia, and G. Hall. 2001. Weissella confusa (basonym: Lactobacillus confusus) bacteremia: a case report. J. Clin. Microbiol. 39:1604-1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paster, B. J., and F. E. Dewhirst. 1988. Phylogeny of Campylobacter wolinellas, Bacteroides gracilis, and Bacteroides ureolyticus by 16S ribosomal ribonucleic acid sequencing. Int. J. Syst. Bacteriol. 38:56-62. [Google Scholar]
- 15.Saitou, N., and M. Nei. 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4:406-425. [DOI] [PubMed] [Google Scholar]
- 16.Sussman, J. I., E. J. Baron, S. M. Goldberg, M. H. Kaplan, and R. A. Pizzarello. 1986. Clinical manifestations and therapy of Lactobacillus endocarditis: report of a case and review of the literature. Rev. Infect. Dis. 8:771-776. [DOI] [PubMed] [Google Scholar]
- 17.Weyant, R. S., C. W. Moss, R. E. Weaver, D. G. Hollis, J. G. Jordan, E. C. Cook, and M. I. Daneshvar. 1995. Identification of unusual pathogenic gram-negative aerobic and facultatively anaerobic bacteria, 2nd ed. Williams and Wilkins, Baltimore, Md.