Abstract
Nineteen oropharyngeal Candida albicans isolates from six children and seven adults living with AIDS at the Russia AIDS Centre, Moscow, from 1990 to 1998 were selected for molecular typing. Two fluconazole-resistant C. albicans genotypes were identified from a child who contracted human immunodeficiency virus infection during the Elista Hospital outbreak in the Kalmyk Republic in 1989. Highly related strains were observed 4 years later in the oral lesions and colonization of two patients and a health care worker. There may be a tendency for persons who are living with AIDS in a long-term care facility and who receive fluconazole therapy for oropharyngeal candidiasis to harbor and spread fluconazole-resistant C. albicans strains.
Oropharyngeal candidiasis (OPC) was a common opportunistic infection in the early and later stages of human immunodeficiency virus (HIV) infection and afflicted between 20 and 50% of HIV-infected patients before the advent of highly active antiretroviral therapy (4, 16, 20, 23, 29; O. Yurin, T. Irova, E. Sundukova, and V. V. Pokrovsky, Abstr. VII Int. AIDS Conf., abstr. 2048, 1991). OPC may progress to esophagitis (20). Coincident with the availability of highly active antiretroviral therapy, the incidence of OPC declined in the United States (2, 3, 15); however, in the Russian Federation, highly active antiretroviral agents are not generally available (8). The first HIV epidemic in Russia, in which 250 children became infected after the accidental reuse of nonsterile syringes in the Elista Hospital in the Kalmyk Republic from May 1988 to January 1989, was described previously (13, 22; T. Irova, O. Yurin, I. Eramova, I. Mazankova, and V. V. Pokrovsky, Abstr.VI Int. AIDS Conf., abstr. FB 462, 1990; N. Makarova and V. V. Pokrovsky, Abstr. VIII Int. AIDS Conf., abstr. 7329, 1992). The Russia AIDS Centre is a long-term care hospital in Moscow created in 1990 to accommodate those children. The majority (54%) of the HIV-infected children were admitted to the Russia AIDS Centre with bacterial and fungal infections. The high prevalence of OPC in about half of them contributed to the decision to use azole drugs in their treatment (13). Fluconazole has been successful in treating OPC and esophageal candidiasis in HIV-infected patients (14, 20, 23), but its long-term use raises the possibility of the acquisition of resistance by the yeast Candida albicans (7, 12, 17, 25) and the emergence of other Candida species, such as C. krusei and C. glabrata, with innately reduced susceptibilities to fluconazole (5, 27).
The transmission of oral Candida species between cohabiting HIV-seropositive persons has been demonstrated (6, 21), so it was of interest to know whether horizontal transmission of the yeast could be occurring in a long-term care facility for persons living with AIDS and whether health care workers (HCWs) might be asymptomatic carriers of similar strains of C. albicans. Routine bacterial and fungal surveillance was initiated at the Russia AIDS Centre in 1990 by obtaining samples for culture from patients and HCWs at various time intervals. Since patients may reside in the Russia AIDS Centre for months or years, the aim of the present research was to use molecular typing to determine the relatedness of C. albicans strains from individual patients and HCWs and among patients and HCWs.
MATERIALS AND METHODS
Patients.
Clinical microbiologic testing data were reviewed for 45 HIV-seropositive patients (26 adults and 19 children) from whom yeasts had been isolated and maintained. Patients either were treated for OPC with fluconazole or became colonized with oral yeasts during their stay in the Russia AIDS Centre hospital and received fluconazole therapy. The demographic and clinical information collected for patients relevant to the selection of their oral Candida strains for molecular typing were sex, age, risk factors for HIV infection, date of admission to the Russia AIDS Centre hospital, duration of hospital stay, number of clinic visits for OPC and/or oral colonization with Candida species, the in vitro susceptibilities of the yeast strains to fluconazole, and, for deceased patients, histologically confirmed postmortem signs of invasive or disseminated candidiasis. The mean duration of the stay in the Russia AIDS Centre hospital for the 19 children was 54.5 days, whereas that for the 26 adults was 32.7 days.
Among the isolates from this group of subjects, C. albicans from 13 patients and 1 HCW were selected for molecular typing for the following reasons: the oropharyngeal C. albicans isolates from eight patients (including two children involved in the Elista Hospital outbreak of HIV infection) demonstrated decreased in vitro susceptibilities to fluconazole, two male patients shared a room in the Russia AIDS Centre hospital for 3 months, a female patient who had died was found at postmortem evaluation to have invasive or disseminated candidiasis, a female patient received prolonged fluconazole treatment for OPC, and a pediatric patient had resided at the Russia AIDS Centre hospital since birth. The HCW, a physician, had been in continuous employment at the Russia AIDS Centre hospital from 1990 to 1998.
Culture methods and antifungal susceptibility testing.
Cultures of yeasts from oropharyngeal samples of patients and the hands of four HCWs were sent to the Mycotic Diseases Branch of the Centers for Disease Control and Prevention, where they were subcultured on CHROMagar Candida (Hardy Diagnostics, Santa-Maria, Calif.) and identified by standard morphological and biochemical methods (30). Samples for culture were obtained from the hands of HCWs by swabbing the hands with cotton swabs soaked in peptone broth. The swabs were then streaked onto Sabouraud-glucose agar. The fluconazole MICs for the C. albicans isolates were determined by the microdilution broth method according to the guidelines outlined in NCCLS document M27-A (19).
Molecular typing.
Genomic DNA from 25 C. albicans isolates was purified by lysis of spheroplasts and phenol-chloroform extraction (9). Purified genomic DNA (3 μg) was digested with 10 U of EcoRI (Roche Diagnostics Corp., Indianapolis, Ind.) per μg for 6 to 8 h at 37°C, and the restriction fragments were then electrophoresed overnight on 0.7% agarose gels at 2 V/cm. DNA fragments were transferred by capillary action to nylon membranes (Roche) by using 20× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate [pH 7.0]). DNA was bound to the filter by UV cross-linking. Ca3 DNA was generously provided by David Soll, University of Iowa (26). Ca3 DNA was labeled with digoxigenin, as described by the manufacturer (DIG DNA Labeling and Detection kit; Roche). The membranes were hybridized overnight at 65°C and then washed twice at 65°C with 0.2× SSC. Bands containing Ca3 DNA were detected by the immuno-alkaline phosphatase method according to instructions provided with the same kit.
Hybridization patterns were photographed and then digitized (Fluor S Imager; Bio-Rad, Hercules, Calif.). Similarity was computed by use of the Jaccard coefficient [s(J)], and dendrograms were prepared by using unweighted pair group analysis with arithmetic means with a band tolerance of ±1.3% (Molecular Analyst software; Bio-Rad). Strains with s(J) values ≥0.90 were considered highly related, whereas strains with s(J) values <0.90 were considered distinct genotypes and were assigned unique letter designations (9, 10). C. albicans isolates were also typed by polymorphic microsatellite analysis with respect to protein kinase (ERK1) or Candida translation elongation factor (CEF3) or by determination of the presence or absence of the insertion element IS1 in the 28S ribosomal subunit (11).
RESULTS
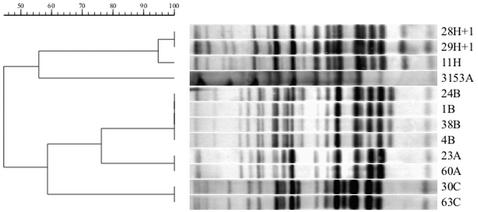
The results of molecular typing of 19 C. albicans isolates (7 isolates from pediatric patients, 11 isolates from adult patients, and 1 isolate from an HCW) by Southern blotting by restriction fragment length polymorphism (RFLP) analysis with the Ca3 probe and the PCR polymorphism method are shown in Table 1. Southern blots comparing 11 C. albicans isolates from six patients and one HCW representing four epidemiologically related groups are presented in Fig. 1. C. albicans isolates 23 and 24, isolated from a 3-year-old patient (Elista Hospital outbreak patient 1), had s(J) values of 0.76. These isolates were obtained at two different clinic visits in 1993 and 1994; both strains were resistant to fluconazole in vitro. Isolate 60, which colonized the oropharynx of patient 7, a 1-year-old girl who had resided at the Russia AIDS Centre since birth in 1997, was resistant to fluconazole in vitro and was identical to C. albicans 23, which was isolated from Elista Hospital outbreak patient 1 in 1993, by both Ca3 profiling and microsatellite analysis. C. albicans 1 and 38 were isolated at two successive clinic visits in 1998 from patient 8, a 28-year-old man. The isolate responsible for this patient's OPC was clinically resistant to fluconazole therapy, but he was infected with an isolate demonstrating dose-dependent susceptibility. The genotypes of these two isolates were identical (genotype B) by both Ca3 probe and microsatellite analysis to that obtained from C. albicans 24 from patient 1, who was involved in the Elista Hospital outbreak, and isolate 4, a fluconazole-susceptible dose-dependent C. albicans strain carried on the hands of a physician who had worked at the Russia AIDS Centre hospital for more than 6 years. These six isolates represented epidemiologic genotypes A and B, traceable to two strains obtained from a child who contracted HIV infection in the original Elista Hospital outbreak. Two additional pairs of highly related C. albicans strains (genotype C) were isolated from two men sharing a hospital room (patients 2 and 4) and 1 month apart from a woman and an unrelated infant (patients 12 and 13, genotype L). Evidence for microevolution was provided by the three oral C. albicans isolates successively recovered from patient 10: over a period of 3 months, the MICs for the isolates declined from 4 to 1 μg of fluconazole per ml, concomitant with the appearance of an additional Ca3-binding fragment [s(J) = 0.94]. These minor variants retained the same PCR fingerprints and the same microsatellite sizes. The genotypes based on Ca3 hybridization profiles were all confirmed by PCR analysis.
TABLE 1.
Genotypes of oropharyngeal C. albicans isolates from persons living with AIDS and an HCW at the Russia AIDS Centre determined by Southern blotting with the Ca3 probe and, for selected strains,a PCR polymorphisms at three nuclear loci
| Patient no. | Sex,b age (yr) | No. of clinic visitsc | Date of visit (mo/yr) | OPCd | Fluconazole MIC (μg/ml) | C. albicans isolate no. | Genotype
|
|||
|---|---|---|---|---|---|---|---|---|---|---|
| RFLP analysis with Ca3 probee | Insertion element IS1f | Length of ampliconsf,g
|
||||||||
| ERK 1 | CEF 3 | |||||||||
| 1 | M, 3 | 2 | 11/93 | Y | >64 | 23 | A | + | 209/233 | 144/168 |
| 5/94 | N | >64 | 24 | B | − | 209/239 | 168/168 | |||
| 2 | M, 18 | 1 | 5/94 | N | 0.25 | 63 | C | − | 256/292 | 174/174 |
| 3 | M, 3 | 3 | 6/94 | Y | >64 | 6H | D | NDh | ND | ND |
| 4 | M, 21 | 1 | 3/95 | N | 0.125 | 30 | C | − | 256/292 | 174/174 |
| 5 | F, 17 | 1 | 9/95 | Y | >64 | 37 | E | ND | ND | ND |
| 6 | F, 2 | 1 | 11/96 | N | 8 | 35 | F | ND | ND | ND |
| 7 | F, 1 | 1 | 12/97 | N | >64 | 60 | A | + | 209/233 | 144/168 |
| 8 | M, 28 | 2 | 2/98 | Y | 16 | 1 | B | − | 209/239 | 168/168 |
| 3/98 | N | 16 | 38 | B | − | 209/239 | 168/168 | |||
| 9 | M, 44 | 5 | 2/98 | Y | >64 | 44 | G | ND | ND | ND |
| 10 | M, 23 | 3 | 2/98 | Y | 4 | 11 | H | + | 236/238 | 168/171 |
| 5/98 | N | 1 | 28 | H + 1i | + | 236/238 | 168/171 | |||
| 7/98 | Y | 1 | 29 | H + 1i | + | 236/238 | 168/171 | |||
| HCWj | M, 28 | NAk | 4/98 | N | 32 | 4 | B | − | 209/239 | 168/168 |
| 11 | F, 29 | 2 | 4/98 | Y | 0.25 | 49 | J | ND | ND | ND |
| 4/98 | N | 0.25 | 59 | K | ND | ND | ND | |||
| 12 | F, <1 | 1 | 6/98 | Y | 0.25 | 93 | L | − | 256/292 | 168/174 |
| 13 | F, 24 | 1 | 7/98 | Y | 0.125 | 5 | L | − | 256/292 | 168/174 |
The strains analyzed for PCR polymorphisms are those shown in Fig. 1.
M, male; F, female.
Clinic visits for oral examination and culture of yeasts.
Y, yes; N, no.
Genotypes were determined by RFLP analysis with the Ca3 probe (see Materials and Methods and reference 27).
For details, see Materials and Methods and reference 12.
The amplicon length is given as the number of nucleotides/number of nucleotides per haploid genotype (C. albicans is diploid).
ND, not determined.
H + 1, a minor genotypic variant that contained an additional Ca3-binding fragment (Fig. 1).
A physician who had been employed at the Russia AIDS Centre hospital since 1990.
NA, not applicable.
FIG. 1.
Ca3 hybridization patterns for 11 oropharyngeal C. albicans isolates obtained from six patients at successive clinic visits and one isolate from the hands of an HCW. The designation for each row indicates the strain number followed by a letter designating the genotype. H + 1 is a minor genotype variant of genotype H. C. albicans 3153A is a standard test strain from the United States. The dendrogram indicates the s(J) values for C. albicans isolates according to their Ca3 hybridization patterns. Further strain information is presented in Table 1.
Not shown in Table 1 are data for yeasts obtained from the hands of HCWs: three nurses and a physician. Two nurses each were colonized with C. parapsilosis; the hand of the remaining nurse yielded C. krusei. The physician was colonized with C. albicans 4H, which was significant because of its genotype (genotype B), which was shared with two patients, and its resistance to fluconazole in vitro.
DISCUSSION
At its inception in 1990 the Russia AIDS Centre received children from the Elista Hospital in the Kalmyk Republic of the former Soviet Union. A distinctive feature of the Russia AIDS Centre is its policy of long-term care. The population of adult HIV-infected patents admitted to the Russia AIDS Centre has grown since 1991. Their poor socioeconomic status and serious clinical conditions obliged the majority of children, their mothers, and unrelated adult patients to remain in the hospital for more than 1 year. From 1991 to 1993 regional AIDS clinics were created in Russia, and the majority of children from the Elista Hospital outbreak were gradually relocated to these centers. The recent increase in rates of HIV infection among injection drug users in Russia and the growing number of children born to HIV-infected mothers who could not provide adequate care for them contributed to a need to accommodate a new population of children at the hospital.
The C. albicans isolates from two children (patients 1 and 3) from the Elista Hospital outbreak that we studied were resistant in vitro to fluconazole. Before their transfer to the Russia AIDS Centre, children at the Elista Hospital received antibacterial therapy and had close contact with different patients and HCWs (24). This environment may have contributed to their becoming a source of potentially drug-resistant hospital-acquired oral microflora. There is no definitive evidence, however, that drug-resistant yeast strains have an advantage in nosocomial transmission, and the persistence of such strains with highly related genotypes in a long-term care facility for 4 years is remarkable, suggesting that fluconazole-resistant strains are not destined to be restricted to their original human host or to revert to a fluconazole-susceptible state.
We observed patients with OPC at the Russia AIDS Centre among whom potential nosocomial transmission of C. albicans was supported by strain typing by RFLP analysis with the Ca3 probe and confirmed by polymorphic microsatellite analysis. A noteworthy example was patient 1, a child involved in the Elista Hospital outbreak, whose oral C. albicans strains consisted of isolates of genotypes A and B. Although these strains had distinct genotypes [s(J) = 0.76], they may have diverged from a common clone through microevolution (10). Alternatively, this patient may have been infected with two different strains. Genotype A was observed 4 years later and maintained a fluconazole-resistant phenotype, colonizing the oropharynx of an HIV-seropositive child who had resided at the Russia AIDS Centre since birth (Fig. 1). Meanwhile, a C. albicans genotype B strain was also recovered 4 years later from a patient with OPC and was clinically resistant to fluconazole, and a C. albicans genotype B strain was recovered from the hands of a 28-year-old physician who had been employed at the Centre since 1990. Not only were the strain types persistent, but they also maintained decreased susceptibilities to fluconazole, although the MICs for the genotype B strain from patient 8 declined from 64 to 16 μg/ml and the MIC for the strain from the HCW was 32 μg/ml.
Although both genotypes A and B originated from a single child involved in the Elista Hospital outbreak, we cannot exclude the possibility that genotype A was originally transmitted to the child from the HCW. HCWs have been implicated in the transmission of C. albicans in hospital intensive care units, as determined by molecular typing (1, 28). The hands of the HCW may have served as a reservoir for the persistence and potential transmission of the C. albicans isolate with reduced susceptibility to fluconazole.
The present findings are believed to describe the longest period of time (4 years) between the isolation of two C. albicans genotypes with reduced fluconazole susceptibilities from a single patient and their reemergence in other patients and from the hands of an attending HCW. The clonal spread of C. albicans strains demonstrating decreased susceptibilities to fluconazole was reported previously among persons with AIDS in a single geographic area (31). A C. albicans strain with reduced susceptibility to fluconazole was isolated over a 5-year period from an HIV-seropositive child with recurrent OPC, and highly related oral strains were isolated over a 2-year period from his HIV-seropositive family members (18).
Other examples of presumed nosocomial transmission were uncovered in the present study. Oral C. albicans isolates (genotype C) were isolated from two men sharing the same hospital room whose risk factor for HIV infection was men having sex with men. C. albicans transmission between HIV-infected couples has been described previously (6). We also isolated two identical [s(J) = 1] oropharyngeal C. albicans strains (genotype L) 1 month apart from a 7-month-old child (patient 12) and an unrelated 24-year-old female patient (patient 13) (Table 1).
In the United States, exogenously acquired infection is considered less common than endogenous infection with the host's own commensal C. albicans flora, as has been reported for another cohort of persons living with AIDS (9). C. albicans strains appeared to persist for months or years in the Russia AIDS Centre patient population, contributing to infection and colonization. These strains of known genotype provide benchmarks for future surveillance in the Russia AIDS Centre. Whether C. albicans strains tend to accumulate among persons with AIDS in other long-term care facilities deserves further study.
Acknowledgments
This research was supported by the appointment of N.U.M. to the International Emerging Infectious Diseases Fellowship Program, endowed by Eli Lilly & Co., Inc., and administered by the Association of Public Health Laboratories and the Centers for Disease Control and Prevention.
We thank Timothy J. Lott for consultation on microsatellite polymorphisms and Susanna Schmink for assistance with image analysis.
REFERENCES
- 1.Burnie, J. P., F. C. Odds, W. Lee, C. Webster, and J. D. Williams. 1985. Outbreak of systemic Candida albicans in intensive care unit caused by cross infection. Br. Med. J. 290:746-748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carpenter, C. C., M. A. Fischl, S. M. Hammer, M. S. Hirsch, D. M. Jacobsen, D. A. Katzenstein, J. S. Montaner, D. D. Richman, M. S. Saag, R. T. Schooley, M. A. Thompson, S. Vella, P. G. Yeni, and P. A. Volberding. 1997. Antiretroviral therapy for HIV infection in 1997. Updated recommendations of the International AIDS Society—USA panel. JAMA 277:1962-1969. [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. 1997. HIV/AIDS surveillance report. HIV/AIDS Surveillance Rep. 9(2):18.
- 4.Coleman, D. C., D. E. Bennett, D. J. Sullivan, P. J. Gallagher, M. C. Henman, D. B. Shanley, and R. J. Russell. 1993. Oral Candida in HIV infection and AIDS: new perspectives/new approaches. Crit. Rev. Microbiol. 19:61-82. [DOI] [PubMed] [Google Scholar]
- 5.Coppola, S., G. Angarano, M. T. Montagna, P. Congedo, L. Monno, A. Bellisario, and G. Pastore. 1997. Efficacy of itraconazole in treating AIDS-associated infections due to Candida krusei. Eur. J. Epidemiol. 11:243-244. [DOI] [PubMed] [Google Scholar]
- 6.Dromer, F., L. Improvisi, B. Dupont, M. Eliaszewicz, G. Pialoux, S. Fournier, and V. Feuillie. 1997. Oral transmission of Candida albicans between partners in HIV-infected couples could contribute to dissemination of fluconazole-resistant isolates. AIDS 11:1095-1101. [DOI] [PubMed] [Google Scholar]
- 7.Johnson, E. M., D. W. Warnock, J. Luker, S. R. Porter, and C. Scully. 1995. Emergence of azole drug resistance in Candida species from HIV-infected patients receiving prolonged fluconazole therapy for oral candidosis. J. Antimicrob. Chemother. 35:103-114. [DOI] [PubMed] [Google Scholar]
- 8.Kalichman, S. C., J. A. Kelly, K. J. Sikkema, A. P. Koslov, A. Shaboltas, and J. Granskaya. 2000. The emerging AIDS crisis in Russia: review of enabling factors and prevention needs. Int. J. STD AIDS 11:71-75. [DOI] [PubMed] [Google Scholar]
- 9.Lasker, B. A., C. M. Elie, T. J. Lott, A. Espinel-Ingroff, L. Gallagher, R. J. Kuykendall, M. E. Kellum, W. R. Pruitt, D. W. Warnock, D. Rimland, M. M. McNeil, and E. Reiss. 2001. Molecular epidemiology of Candida albicans strains isolated from the oropharynx of HIV-positive patients at successive clinic visits. Med. Mycol. 39:341-352. [DOI] [PubMed] [Google Scholar]
- 10.Lockhart, S. R., J. J. Fritch, A. S. Meier, K. Schroppel, T. Srikantha, R. Galask, and D. R. Soll. 1995. Colonizing populations of Candida albicans are clonal in origin but undergo microevolution through C1 fragment reorganization as demonstrated by DNA fingerprinting and C1 sequencing. J. Clin. Microbiol. 33:1501-1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lott, T. J., B. P. Holloway, D. A. Logan, R. Fundyga, and J. Arnold. 1999. Towards understanding the evolution of the human commensal yeast Candida albicans. Microbiology 145:1137-1143. [DOI] [PubMed] [Google Scholar]
- 12.Maenza, J. R., W. G. Merz, M. J. Romagnoli, J. C. Keruly, R. D. Moore, and J. E. Gallant. 1997. Infection due to fluconazole-resistant Candida in patients with AIDS: prevalence and microbiology. Clin. Infect. Dis. 24:28-34. [DOI] [PubMed] [Google Scholar]
- 13.Makarova, N., O. Yurin, V. Pokrovsky, and A. R. Tappuni. 1997. Infections in hospitalised Russian children with AIDS. Oral Dis. 3(Suppl. 1):S119-S121. [DOI] [PubMed] [Google Scholar]
- 14.Manfredi, R., A. Mastroianni, O. V. Coronado, and F. Chiodo. 1997. Fluconazole as prophylaxis against fungal infection in patients with advanced HIV infection. Arch. Intern. Med. 157:64-69. [PubMed] [Google Scholar]
- 15.Martins, M. D., M. Lozano-Chiu, and J. H. Rex. 1998. Declining rates of oropharyngeal candidiasis and carriage of Candida albicans associated with trends toward reduced rates of carriage of fluconazole-resistant C. albicans in human immunodeficiency virus-infected patients. Clin. Infect. Dis. 27:1291-1294. [DOI] [PubMed] [Google Scholar]
- 16.McCarthy, G. M. 1992. Host factors associated with HIV-related oral candidiasis. A review. Oral Surg. Oral Med. Oral Pathol. 73:181-186. [DOI] [PubMed] [Google Scholar]
- 17.Millon, L., A. Manteaux, G. Reboux, C. Drobacheff, M. Monod, T. Barale, and Y. Michel-Briand. 1994. Fluconazole-resistant recurrent oral candidiasis in human immunodeficiency virus-positive patients: persistence of Candida albicans strains with the same genotype. J. Clin. Microbiol. 32:1115-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mäuller, F. M., M. Kasai, A. Francesconi, B. Brillante, M. Roden, J. Peter, S. J. Chanock, and T. J. Walsh. 1999. Transmission of an azole-resistant isogenic strain of Candida albicans among human immunodeficiency virus-infected family members with oropharyngeal candidiasis. J. Clin. Microbiol. 37:3405-3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Committee for Clinical Laboratory Standards. 1997. Reference method for broth dilution antifungal susceptibility testing of yeast. Approved standard M27-A. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 20.Parente, F., S. Ardizzone, M. Cernuschi, S. Antinori, R. Esposito, M. Moroni, A. Lazzarin, G. B. Porro, and F. M. de Benedictis. 1994. Prevention of symptomatic recurrences of esophageal candidiasis in AIDS patients after the first episode: a prospective open study. Am. J. Gastroenterol. 89:416-420. [PubMed] [Google Scholar]
- 21.Peng, G., J. D. Sobel, L. Steele-Moore, P. Schuman, W. Holloway, J. D. Neaton, and F. Barchiesi. 2001. Transmission of fluconazole-resistant Candida albicans between patients with AIDS and oropharyngeal candidiasis documented by pulsed-field gel electrophoresis. Clin. Infect. Dis. 33:1069-1075. [DOI] [PubMed] [Google Scholar]
- 22.Pokrovskii, V. V., I. Eramova, M. O. Deulina, V. V. Lipetikov, K. B. Iashkulov, L. A. Sliusareva, N. M. Chemizova, and S. P. Savchenko. 1990. An intrahospital outbreak of HIV infection in Elista. Zh. Mikrobiol. Epidemiol. Immunobiol. 1990:17-23. (In Russian.) [PubMed]
- 23.Pons, V., D. Greenspan, and M. Debruin. 1993. Therapy for oropharyngeal candidiasis in HIV-infected patients: a randomized, prospective multicenter study of oral fluconazole versus clotrimazole troches. J. Acquir. Immune Defic. Syndr. 6:1311-1316. [PubMed] [Google Scholar]
- 24.Popova, I. A., N. V. Burova, A. Fomin Iu, A. G. Rakhmanova, E. E. Voronin, and M. V. Galkina. 1999. The clinical course of HIV infection in children who were parenterally infected. Zh. Mikrobiol. Epidemiol. Immunobiol. 1999:75-78. (In Russian.) [PubMed]
- 25.Sangeorzan, J. A., S. F. Bradley, X. He, L. T. Zarins, G. L. Ridenour, R. N. Tiballi, and C. A. Kauffman. 1994. Epidemiology of oral candidiasis in HIV-infected patients: colonization, infection, treatment, and emergence of fluconazole resistance. Am. J. Med. 97:339-346. [DOI] [PubMed] [Google Scholar]
- 26.Soll, D. R., C. J. Langtimm, J. McDowell, J. Hicks, and R. Galask. 1987. High-frequency switching in Candida strains isolated from vaginitis patients. J. Clin. Microbiol. 25:1611-1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vazquez, J. A., J. D. Sobel, G. Peng, L. Steele-Moore, P. Schuman, W. Holloway, and J. D. Neaton. 1999. Evolution of vaginal Candida species recovered from human immunodeficiency virus-infected women receiving fluconazole prophylaxis: the emergence of Candida glabrata? Clin. Infect. Dis. 28:1025-1031. [DOI] [PubMed] [Google Scholar]
- 28.Voss, A., M. A. Pfaller, R. J. Hollis, J. Rhine-Chalberg, and B. N. Doebbeling. 1995. Investigation of Candida albicans transmission in a surgical intensive care unit cluster by using genomic DNA typing methods. J. Clin. Microbiol. 33:576-580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walsh, T. J., and K. M. Butler. 1991. Fungal infections complicating pediatric AIDS, p. 225-244. In P. Pizzo and C. M. Wilfert (ed.), Pediatric AIDS: the challenge of HIV infection in infants, children, and adolescents. The Williams & Wilkins Co., Baltimore, Md.
- 30.Warren, N. G., and K. C. Hazen. 1999. Candida, Cryptococcus, and other yeasts of medical importance, p. 1184-1199. In P. R. Murray, E. J. Baron, M. A. Pfaller, F. C. Tenover, and R. H. Yolken (ed.), Manual of clinical microbiology, 7th ed. American Society for Microbiology, Washington, D.C.
- 31.Xu, J., A. R. Ramos, R. Vilgalys, and T. G. Mitchell. 2000. Clonal and spontaneous origins of fluconazole resistance in Candida albicans. J. Clin. Microbiol. 38:1214-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]