Abstract
An indigenous freshwater bacterium (Sphingomonas sp. strain B18) from Lake Pluβsee (Schleswig-Holstein, Germany) was used to isolate 44 phages from 13 very different freshwater and brackish habitats in distant geographic areas. This bacterial strain was very sensitive to a broad spectrum of phages from different aquatic environments. Phages isolated from geographically distant aquatic habitats, but also those from the same sample, were diverse with respect to morphology and restriction pattern. Some phages were widely distributed, while different types coexisted in the same sample. It was concluded that phages could be a major factor in shaping the structure of bacterial communities and maintaining a high bacterial diversity.
Counts by transmission electron microscopy have shown that the total number of viruses in coastal ocean and freshwaters is about 107 to 108 ml−1 (5-7, 22) and often exceeds the concentration of bacteria by a factor of around 10. Similar results have been obtained by epifluorescence microscopic counting by using fluorochromes like Yo-Pro 1 (12) or SYBR Green I (18).
Host-specific phage concentrations for heterotrophic bacteria vary widely in coastal waters from undetectable levels to 1,500 to 8,100 PFU ml−1 (4, 16) and were assumed to be usually as low as 1 to 10 PFU ml−1 (15, 25). Many further investigations into the contribution of viruses to microbial food webs and their function in shaping the structure of microbial communities have been initiated since then (summarized in reference 30).
Most of these studies were done in estuarine and marine environments. Almost no information is available about phages that infect autochthonous heterotrophic bacteria in freshwater habitats. However, here, as in estuarine and marine environments, the abundance of viruses mostly exceeded that of bacteria (12, 14, 29, 30). Therefore, similar PFU concentrations can be expected when a suitable host bacterium is used. Here we describe bacteriophages that have been isolated from distant aquatic habitats and that are lysing the same host strain, which is a major component of the culturable bacteria of Lake Pluβsee in northern Germany.
Host strain.
The bacterial host strain B18 was isolated by inoculating a water sample taken from a 1-m depth of Lake Pluβsee on 26 August 1985 on solid casein-peptone-starch medium (CPS medium) (9) and stored as frozen stock culture in liquid nitrogen. Bacteria of this type are regularly detected in Pluβsee as yellow, round, and smooth colonies with a size of 1 to 3 mm on CPS agar after 1 week of incubation at 28°C. They have been identified as strain B18 with polyclonal antibodies. Based on 16S rDNA nucleotide sequence (GenBank accession number AF410927), strain B18 is affiliated with a recently constructed genus (27, 31) that comprises many species formerly grouped as Pseudomonas or Flavobacterium within the alpha subgroup of the class proteobacteria. Sphingomonas adhaesiva (GenBank accession number D13722) is the closest known relative, with a sequence similarity of 95.5%, found with BLASTN on the NCBI homepage (http://www.ncbi.nlm.nih.gov/BLAST/). Bacteria of this genus usually have a great metabolic versatility and have the potential to colonize a variety of different habitats (20, 21, 24).
Detection of phages.
Phages were counted and isolated from water samples collected near shore in about 0.3 m depth with heat-sterilized 500-ml glass bottles from the habitats characterized in Table 1. Either the top agar method (3) or the phage enrichment in liquid cultures of the host strain was used. PFU were counted on CPS-agar plates after 3 days of incubation at 28°C. The detection limit of this procedure was about 1 PFU ml−1. Phage enrichment cultures were incubated for at least 3 days at 28°C and 100 rpm shaking and had a significantly lower detection limit. Phages with different plaque types were selected from each habitat and purified three times by repetitive isolation of single plaques. Phage suspensions were prepared from plates with confluent lysis and stored in SM buffer (23) at 4°C.
TABLE 1.
Concentrations of phages lysing the host Sphingomonas sp. strain B18
| Habitat type | Habitat no. and name | Coordinates | Distance (km)a | Trophic state | Sampling date (day.mo.yr) | PFU ml−1 |
|---|---|---|---|---|---|---|
| Freshwater lakes | 1, Bautzener Stausee | 51°12′N 14°26′E | 427 | Polytrophic | 08.06.92 | 3,000 |
| 2, Satower See | 53°59′N 11°53′E | 97 | Polytrophic | 24.05.92 | 8,000 | |
| 3, Kellersee | 54°11′N 10°37′E | 11 | Eutrophic | 23.04.92 | 10 | |
| 4, Krakower See | 53°37′N 12°16′E | 133 | Eutrophic | 31.05.92 | 1,000 | |
| 5, Kleiner Plöner See | 54°09′N 10°22′E | 5 | Eutrophic | 11.09.90 | 3,500 | |
| 04.06.92 | 300 | |||||
| 6, Groβer Plöner See | 54°09′N 10°25′E | 3 | Eutrophic | 12.12.91 | 51 | |
| 04.06.92 | 200 | |||||
| 7, Belauer See | 54°05′N 10°15′E | 16 | Eutrophic | 03.12.91 | 4 | |
| 16.06.92 | 1b | |||||
| 0, Pluβsee | 54°11′N 10°26′E | 0 | Eutrophic | 26.02.90 | 1,600 | |
| 18.06.92 | 2,000 | |||||
| 8, Schöhsee | 54°09′N 10°26′E | 2 | Mesotrophic | 11.06.92 | <1b | |
| 9, Lake Kinneret | 32°47′N 34°23′E | 3,085 | Meso-eutrophic | 12.04.91 | <1b | |
| Bog lake | 10, Groβer Teufelssee | 54°03′N 12°25′E | 130 | Dystrophic | 13.07.92 | None detected |
| River | 11, Danube (Vienna) | 48°19′N 16°20′E | 770 | 21.10.92 | 100,000 | |
| Brackish waters | 12, Salzhaff (12 PSUc) | 54°02′N 11°34′E | 75 | Eu-polytrophic | 24.05.92 | 7 |
| 13, Zingster Strom (7 PSU) | 54°25′N 12°40′E | 149 | Eu-polytrophic | 28.06.92 | 200 |
Distance from Lake Pluβsee, from which the host strain was isolated.
Phages detectable only after enrichment culture.
PSU, practical salinity units.
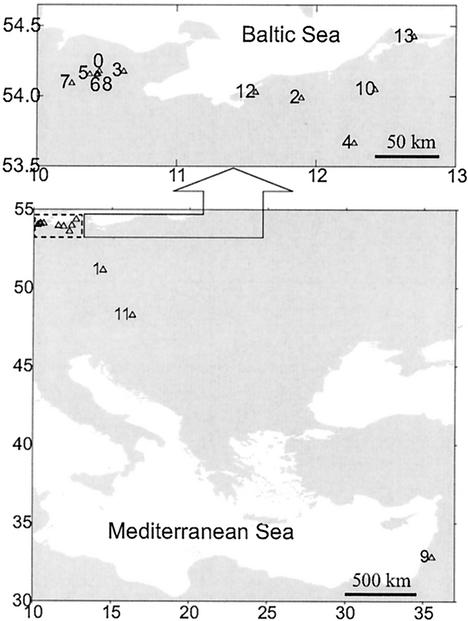
Lytic phages of Sphingomonas sp. strain B18 were found in all investigated aquatic habitats except the dystrophic Lake Teufelssee (Mecklenburg) (Fig. 1 and Table 1). The highest concentration of PFU, about 105 ml−1, was found in the water sample from the River Danube (near Vienna). In lakes, the highest concentrations were observed in Satower See (Mecklenburg) and in Bautzener Stausee (Saxony), which were the most eutrophic within the selected habitats. In samples of the mesotrophic Lake Schöhsee and the eutrophic Lake Belauer See, phages could be isolated only after an enrichment culture. Phage concentration was not determined for the Lake Kinneret sample, which was used for enrichment culture only.
FIG. 1.
Locations of sampling sites (for the numbers of the habitats, see Table 1).
While host-specific phage concentrations in the marine environment have been estimated with autochthonous host bacteria (4, 15-17, 25), comparable data for freshwater habitats are not available. The earlier studies (15, 25) revealed phage concentrations in the range of 1 to 10 PFU ml−1 or even lower. The phage counts reported here are in the same order of magnitude as those from more recent studies where 200 to 700 PFU ml−1 (17) and 1,500 PFU ml−1 (16) were detected in the North Sea around the island Helgoland.
Frank and Moebus (11) concluded that the highest concentrations of specific phages could be found with host strains that had been isolated from the same water body shortly beforehand. Using hosts that had been isolated several years ago or from geographically distant locations resulted in lower efficiency or no phage detection at all. Our results are not in accordance with these findings. The host strain used in this study was isolated in 1985, and most of the phages were isolated about seven years later. Moreover, the water samples covered a range of different trophic states as well as fresh- and brackish water sites (see Table 1). Due to the longer time needed for transportation of the samples from Mecklenburg and Saxony (about 24 h at 4°C), and even more so from the River Danube and Lake Kinneret (1 day at ambient temperature), the estimated numbers show at least that phages lysing the host bacterium were distributed over quite distant geographical locations.
The sample from the dystrophic bog lake Teufelssee (Mecklenburg), with a slightly acidic pH of 6 to 7, was the only one where phages specific for this host were not detectable, even with the more sensitive enrichment cultures. Most obviously, this could be due to either the absence of appropriate host bacteria or unfavorable conditions for multiplication or survival of phages.
Diversity of the phages.
A total of 44 lytic phages were isolated from 13 of the 14 investigated habitats (see Table 2). The phages were selected on the basis of plaque type, which included size, clarity of the center, and shape of the edges. Plaque size varied between <1 and 5 mm. A total of 32 from the 44 isolated phages produced plaques which were smaller than 1 mm in diameter. The phage Dan18/9, isolated from the River Danube, formed the largest plaques (5 mm). Some phages isolated from the same habitat had the same plaque type but differed in morphology (Gps18/13, Gps18/14, and Gps18/16) and vice versa (Gps18/13 and Gps18/18).
TABLE 2.
Sources and characteristics of selected lytic phages isolated with the host Sphingomonas sp. strain B18
| Phage isolatea | Method of isolationb | Date of isolation (day.mo.yr) | Plaque typec | Morphologyd | Genome size (kb) |
|---|---|---|---|---|---|
| Pls18/1 | D | 05.10.89 | 3a | B1 | 51.8 |
| Kin18/3 | D | 12.04.91 | 1 | B1 | |
| Bel18/8 | E | 16.06.92 | 2 | A1 | |
| Dan18/9 | D | 21.10.92 | 1 | C1 | 43.8 |
| Dan18/11 | D | 21.10.92 | 3a | C1 | |
| Gps18/13 | E | 04.06.92 | 2 | C1 | |
| Gps18/14 | E | 04.06.92 | 2 | C1 | 59.5 |
| Gps18/15 | E | 04.06.92 | 2 | C1 | |
| Gps18/16 | E | 04.06.92 | 2 | A1 | |
| Gps18/17 | E | 04.06.92 | 2 | C1 | |
| Gps18/18 | E | 04.06.92 | 3b | C1 | 38.6 |
| Kps18/22 | E | 04.06.92 | 3a | C1 | |
| Kra18/26 | E | 31.05.92 | 3b | 38.3 | |
| Pls18/29 | D | 18.06.92 | 3b | C1 | 31.4 |
| Sal18/31 | D | 24.05.92 | 3a | C1 | |
| Sal18/32 | D | 24.05.92 | 3b | ||
| Sal18/35 | E | 24.05.92 | 3a | C1 | 31.4 |
| Sch18/42 | E | 11.06.92 | 3a | C1 |
The first three letters of the phage isolate name indicate the isolate source. Bel, Belauer See; Dan, Danube near Vienna; Gps, Groβer Plöner See; Kin, Lake Kinneret; Kps, Kleiner Plöner See; Kra, Krakower See; Pls, Pluβsee; Sal, Salzhaff; Sch, Schöhsee.
D, direct isolation; E, enrichment culture.
1, large (up to 5 mm), clear; 2, medium (1 to 4 mm), clear; 3, small (up to 1 mm), clear, with (3a) or without (3b) a turbid area around a clear center.
Morphological classification according to reference 1. A1, Myoviridae; B1, Siphoviridae; C1, Podoviridae.
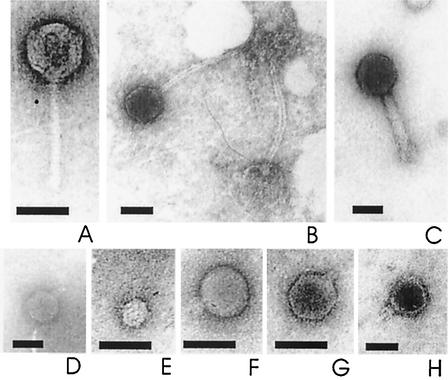
For electron microscopy (10), phages were adsorbed onto a carbon-coated grid, negatively stained with uranyl acetate, and examined with a BS 500 TESLA electron microscope (Brno, Czech Republic) at 60 kV and a final magnification between 110,000× and 190,000×. All investigated phages (Fig. 2) were tailed phages of the Myoviridae, Siphoviridae, and Podoviridae families, which represent the largest part (96%) of all phage isolates in culture (1). Most of the phages in this study had a short tail and belonged to the Podoviridae, similar to observations from the Pacific Ocean (Yaquina Bay, Oregon) (28), the northern Atlantic Ocean and the North Sea (11), and Lake Pluβsee (10). All phage head structures were isometric icosahedra (subtypes A1, B1, and C1) (2), but they differed in head size and tail morphology (Table 2).
FIG. 2.
Transmission electron microscopy pictures of selected phages. Shown are Siphoviridae phages Pls18/1 (A) and Kin18/3 (B), Myoviridae phage Gps18/16 (C), and Podoviridae phages Gps18/13 (D), Gps18/14 (E), Gps18/15 (F), Gps18/17 (G), and Sal18/35 (H). Bars, 50 nm.
For a molecular characterization of the phages by restriction enzyme digestion, 1 to 17.5 μl of phage DNA (isolated and purified in accordance with reference 23) at a final concentration of 10 to 30 ng μl−1 was incubated for 3 h at 60°C in a mixture of 2 μl of restriction buffer, 0.5 μl of restriction enzyme (BstEII), and deionized water (final volume, 20 μl). Fragments were visualized after electrophoresis in 0.8% agarose (23). The size of phage genomes was calculated with the software BioProfile 1D, version 6.3, from the density of each band in relation to the corresponding molecular weight.
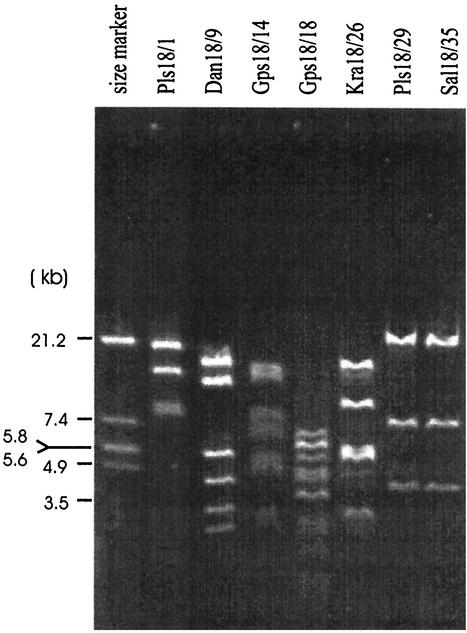
While Kellogg et al. (13) found that phages for the same host that were distributed over a wide geographic distance all had the same morphology and were genetically related, our results revealed a significant variability of the phages among the different freshwater systems. Restriction patterns from seven selected phages revealed that all except two (Pls18/29 and Sal18/35) were clearly distinct (Fig. 3). The genome sizes, spanning a range from 31.4 (Pls18/29 and Sal18/35) to 59.5 kb (Gps18/14) (Table 2), were in the ranges set by the two main groups for marine virus-like DNA of 31 to 36 kb and 58 to 63 kb (26) and were without obvious preference for a certain size. Phages Pls18/29 and Sal18/35 had been isolated from two fairly different habitats but showed the same restriction pattern and similar genome sizes, indicating that they might be identical. Likewise, phages that differed in plaque type, morphology, restriction pattern, and genome size were isolated from the same sample. A good example to illustrate this is the phages Gps18/13 (Fig. 2D), Gps18/14 (Fig. 2E), Gps18/16 (Fig. 2C), Gps18/17 (Fig. 2G), and Gps18/18. They all were isolated from the same sample of Lake Groβer Plöner See. It is not unusual that phages of different morphology can infect the same host strain (8, 19). But the coexistence of different phages for the same host in the same sample should require a fine-tuned interaction of defense and counterdefense strategies of phage and host, like reproductive efficiency, host range, abundance of potential host cells, and many other factors that we do not understand well as yet.
FIG. 3.
Restriction patterns of total genomic DNA from selected phages and size standard (EcoRI digest of phage λ).
Acknowledgments
We are grateful to V. L. Jonas and his staff of the Electron Microscopy Center at the Institute of Pathology of Rostock University for support with the transmission electron microscopy investigations. We thank B. Velimirov, University of Vienna, who kindly provided the sample from River Danube. We gratefully acknowledge Curtis Suttle for his helpful comments on an earlier version of this manuscript.
REFERENCES
- 1.Ackermann, H. W. 1992. Frequency of morphological phage descriptions. Arch. Virol. 124:201-209. [DOI] [PubMed] [Google Scholar]
- 2.Ackermann, H. W., and A. Eisenstark. 1974. The present state of phage taxonomy. Intervirology 3:201-219. [DOI] [PubMed] [Google Scholar]
- 3.Adams, M. H. 1959. Bacteriophages. Interscience Publishers, Inc., New York, N.Y.
- 4.Ahrens, R. 1971. Untersuchungen zur Verbreitung von Phagen der Gattung Agrobacterium in der Ostsee. Kieler Meeresforsch. 27:102-112. [Google Scholar]
- 5.Bergh, Ø., K. Y. Børsheim, G. Bratbak, and M. Heldal. 1989. High abundance of viruses found in aquatic environments. Nature 340:467-468. [DOI] [PubMed] [Google Scholar]
- 6.Børsheim, K. Y., G. Bratbak, and M. Heldal. 1990. Enumeration and biomass estimation of planktonic bacteria and viruses by transmission electron microscopy. Appl. Environ. Microbiol. 56:352-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bratbak, G., M. Heldal, S. Norland, and T. F. Thingstad. 1990. Viruses as partners in spring bloom microbial trophodynamics. Appl. Environ. Microbiol. 56:1400-1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chakrabarti, A. K., A. N. Ghosh, G. B. Nair, S. K. Niyogi, S. K. Bhattacharya, and B. L. Sarkar. 2000. Development and evaluation of a phage typing scheme for Vibrio cholerae O139. J. Clin. Microbiol. 38:44-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Collins, V. G., and L. G. Willoughby. 1962. The distribution of bacteria and fungal spores in Blelham Tarn with particular reference to an experimental overturn. Arch. Mikrobiol. 43:294-307. [DOI] [PubMed] [Google Scholar]
- 10.Demuth, J., H. Neve, and K. P. Witzel. 1993. Direct electron microscopy study on the morphological diversity of bacteriophage populations in Lake Plussee. Appl. Environ. Microbiol. 59:3378-3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frank, H., and K. Moebus. 1987. An electron microscopic study of bacteriophages from marine waters. Helgol. Meeresunters. 41:385-414. [Google Scholar]
- 12.Hennes, K., and M. Simon. 1995. Significance of bacteriophages for controlling bacterioplankton growth in a mesotrophic lake. Appl. Environ. Microbiol. 61:333-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kellogg, C. A., J. B. Rose, S. C. Jiang, J. M. Thurmond, and J. H. Paul. 1995. Genetic diversity of related vibriophages isolated from marine environments around Florida and Hawaii, USA. Mar. Ecol. Prog. Ser. 120:1-3. [Google Scholar]
- 14.Maranger, R., and D. F. Bird. 1995. Viral abundance in aquatic systems: a comparison between marine and fresh waters. Mar. Ecol. Prog. Ser. 121:217-226. [Google Scholar]
- 15.Moebus, K. 1987. Ecology of marine bacteriophages, p. 137-156. In S. M. Goyal, C. P. Gerba, and G. Bitton (ed.), Phage ecology. Wiley, New York, N.Y.
- 16.Moebus, K. 1992. Further investigations on the concentration of marine bacteriophages in the water around Helgoland, with reference to the phage-host systems encountered. Helgol. Meeresunters. 46:275-292. [Google Scholar]
- 17.Moebus, K. 1992. Preliminary observations on the concentration of marine bacteriophages in the water around Helgoland. Helgol. Meeresunters. 45:411-422. [Google Scholar]
- 18.Noble, R. T., and J. A. Fuhrman. 1998. Use of SYBR Green I for rapid epifluorescence counts of marine viruses and bacteria. Aquat. Microb. Ecol. 14:113-118. [Google Scholar]
- 19.Park, S. C., I. Shimamura, M. Fukunaga, K. I. Mori, and T. Nakai. 2000. Isolation of bacteriophages specific to a fish pathogen, Pseudomonas plecoglossicida, as a candidate for disease control. Appl. Environ. Microbiol. 66:1416-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pernthaler, J., F. O. Glockner, S. Unterholzner, A. Alfreider, R. Psenner, and R. Amann. 1998. Seasonal community and population dynamics of pelagic bacteria and archaea in a high mountain lake. Appl. Environ. Microbiol. 64:4299-4306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pinhassi, J., and Å. Hagström. 2000. Seasonal succession in marine bacterioplankton. Aquat. Microb. Ecol. 21:245-256. [Google Scholar]
- 22.Proctor, L. M., and J. A. Fuhrman. 1990. Viral mortality of marine bacteria and cyanobacteria. Nature 343:60-62. [Google Scholar]
- 23.Sambrook, J., and E. F. Fritsch. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 24.Schut, F., R. A. Prins, and J. C. Gottschal. 1997. Oligotrophy and pelagic marine bacteria: facts and fiction. Aquat. Microb. Ecol. 12:177-202. [Google Scholar]
- 25.Spencer, R. 1963. Bacterial viruses in the sea, p. 350-365. In C. D. Oppenheimer (ed.), Symposium on marine microbiology. Charles C. Thomas, Springfield, Ill.
- 26.Steward, G. F., J. L. Montiel, and F. Azam. 2000. Genome size distributions indicate variability and similarities among marine viral assemblages from diverse environments. Limnol. Oceanogr. 45:1697-1706. [Google Scholar]
- 27.Takeuchi, M., K. Hamana, and A. Hiraishi. 2001. Proposal of the genus Sphingomonas sensu stricto and three new genera, Sphingobium, Novosphingobium and Sphingopyxis, on the basis of phylogenetic and chemotaxonomic analyses. Int. J. Syst. E vol. Microbiol. 51:1405-1417. [DOI] [PubMed] [Google Scholar]
- 28.Torrella, F., and R. Y. Morita. 1979. Evidence by electron micrographs for a high incidence of bacteriophage particles in the waters of Yaquina Bay, Oregon: ecological and taxonomical implications. Appl. Environ. Microbiol. 37:774-778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weinbauer, M. G., and M. G. Höfle. 1998. Significance of viral lysis and flagellate grazing as factors controlling bacterioplankton production in a eutrophic lake. Appl. Environ. Microbiol. 64:431-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wommack, K. E., and R. R. Colwell. 2000. Virioplankton: viruses in aquatic ecosystems. Microbiol. Mol. Biol. Rev. 64:69-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yabuuchi, E., I. Yano, H. Oyaizu, Y. Hashimoto, T. Ezaki, and H. Yamamoto. 1990. Proposals of Sphingomonas paucimobilis gen. nov. and comb. nov., Sphingomonas parapaucimobilis sp. nov., Sphingomonas yanoikuyae sp. nov., Sphingomonas adhaesiva sp. nov., Sphingomonas capsulata comb. nov., and two genospecies of the genus Sphingomonas. Microbiol. Immunol. 34:99-119. [DOI] [PubMed] [Google Scholar]