Abstract
Overexpression of both cellular Src (c-Src) and the epidermal growth factor receptor (EGFR) occurs in many of the same human tumors, suggesting that they may functionally interact and contribute to the progression of cancer. Indeed, in murine fibroblasts, overexpression of c-Src has been shown to potentiate the mitogenic and tumorigenic capacity of the overexpressed EGFR. Potentiation correlated with the ability of c-Src to physically associate with the activated EGFR and the appearance of two unique in vivo phosphorylations on the receptor (Tyr-845 and Tyr-1101). Using stable cell lines of C3H10T½ murine fibroblasts that contain kinase-deficient (K−) c-Src and overexpressed wild-type EGFR, we show that the kinase activity of c-Src is required for both the biological synergy with the receptor and the phosphorylations on the receptor, but not for the association of c-Src with the receptor. In transient transfection assays, not only epidermal growth factor but also serum- and lysophosphatidic acid-induced DNA synthesis was ablated in a dominant-negative fashion by a Y845F mutant of the EGFR, indicating that c-Src-induced phosphorylation of Y845 is critical for the mitogenic response to both the EGFR and a G protein-coupled receptor (lysophosphatidic acid receptor). Unexpectedly, the Y845F mutant EGFR was found to retain its full kinase activity and its ability to activate the adapter protein SHC and extracellular signal-regulated kinase ERK2 in response to EGF, demonstrating that the mitogenic pathway involving phosphorylation of Y845 is independent of ERK2-activation. The application of these findings to the development of novel therapeutics for human cancers that overexpress c-Src and EGFR is discussed.
Considerable evidence has accumulated in recent years to suggest that cellular Src (c-Src) and members of the epidermal growth factor (EGF) receptor (EGFR) family are critical elements in the etiology of multiple human cancers. Both kinases are found overexpressed in many of the same types of tumors, including glioblastomas and carcinomas of the colon, breast, and lung (1–4), raising the question of whether they functionally interact to promote the growth of these malignancies. In breast cancer, overexpression of EGFR family members is estimated to occur in 60% or more of the cases (5), and overexpression of the family member HER2/NEU, has been associated with a poor prognosis for the disease (6). Recent reports have also described overexpression of c-Src in a significant majority of patients with breast cancer, a frequency that approaches 100% (1). Studies to assess the oncogenic potential of each kinase have shown that the EGFR is tumorigenic when overexpressed in cultured fibroblasts and activated by ligand (7, 8), but overexpression of c-Src alone is insufficient for malignant transformation (9, 10).
A possible role for c-Src in tumorigenesis was revealed when it was demonstrated in C3H10T½ murine fibroblasts that co-overexpression of c-Src and the EGFR resulted in a synergistic increase in EGF-induced DNA synthesis, growth in soft agar, and tumorigenesis, as compared with cells overexpressing either the EGFR or c-Src alone (11). This cooperation correlated with the EGF-dependent formation of a physical complex between c-Src and the EGFR (11), the appearance of two unique sites of tyrosine phosphorylation (Y845 and Y1101) on the c-Src-associated EGFR, and increased phosphorylation of receptor substrates (11). These results suggest that one mechanism by which c-Src could augment the mitogenic/tumorigenic activity of the receptor is by associating with and hyperactivating the receptor by phosphorylation of novel tyrosine residues. Co-overexpression, co-association, and phosphorylation of Y845 and Y1101 have also been observed in human tumor cells (12–15), suggesting that synergism between c-Src and the EGFR may occur in a subset of human tumors as well as in murine fibroblasts.
To determine whether phosphorylation of Y845 or Y1101 is critical to the biological synergy between c-Src and the EGFR and to determine whether c-Src is responsible for mediating the phosphorylations, we analyzed a panel of murine fibroblasts that overexpressed either wild-type (wt) c-Src (K+ c-Src) or kinase-defective c-Src (K− c-Src) alone or together with the EGFR for growth properties and the presence of a stable complex containing the EGFR and c-Src. We found that K− c-Src inhibits EGF-dependent growth in soft agar and tumorigenesis in nude mice even though it is still capable of associating with the receptor. However, K− c-Src was unable to mediate the phosphorylation of Y845 on the receptor. As a direct test of the requirement of this phosphorylation for receptor function, we engineered a variant receptor harboring a Y845F mutation in the EGFR and observed that this mutated receptor ablated EGF, serum, and lysophosphatidic acid (LPA)-induced DNA synthesis without inhibiting receptor kinase activity or activation of the extracellular signal-regulated kinase ERK2. The data support a model wherein phosphorylation of Y845 on the EGFR by c-Src is required for EGF-induced mitogenesis and tumorigenesis in a manner that appears to be independent of ERK2.
MATERIALS AND METHODS
Cell Lines.
The derivation, characterization, and maintenance of the clonal C3H10T½ murine fibroblast cell lines Neo (control), K+ (wt chicken c-Src overexpressors), K− (A430V kinase-deficient, chicken c-Src overexpressors), EGFR (wt human EGFR overexpressors), and EGFR/K+ (wt EGFR/wt c-Src double overexpressors) have been described previously (10, 11, 16). EGFR/K− (wt EGFR overexpressors/kinase-deficient c-Src) cell lines were derived by infection of K− cells with a recombinant amphotropic retrovirus encoding the human EGFR (8), cloning by limiting dilution, and screening for overexpression of the receptor and maintenance of K− c-Src by Western immunoblotting. Clonal cell lines used in this study were estimated to express 25,000–60,000 human EGFRs per cell, based on comparative Western blotting analysis that used as a standard a C3H10T½ cell line that by Scatchard analysis was shown to express approximately 200,000 receptors per cell (5HR11 cells) (11). Clones included EGFR5, EGFR8, EGFR27, EGFR/K+8, EGFR/K+9, EGFR/K+10, and EGFR/K−2, EGFR/K−5, EGFR/K−41, and EGFR/K−56. K+ and K− c-Src overexpression was estimated to be 20- to 25-fold over endogenous.
Western Immunoblotting.
Western blot analysis was performed as previously described (11, 16), using Ab-4 rabbit polyclonal antibody (Calbiochem) or F-4 mouse monoclonal antibody (mAb)(Sigma) to detect the EGFR, purified 2–17 mAb (Quality Biologicals; Gaithersburg, MD) or EC10 ascites fluid mAb (prepared in our laboratory and used at a 1:10,000 dilution) to identify c-Src, polyclonal anti-SHC antibody (Upstate Biotechnology; Lake Placid, NY) to visualize SHC, B3B9 mAb (17) to detect MAPK, polyclonal anti-phospho-MAPK antibody (Promega), or 4G10 anti-phosphotyrosine antibody (Upstate Biotechnology) to detect tyrosine-phosphorylated SHC. 125I-labeled protein A (ICN) or 125I-labeled goat anti-mouse or anti-rabbit Ig (New England Nuclear) and autoradiography were employed to localize binding of primary antibodies.
Colony Formation in Soft Agar and Tumorigenicity.
Anchorage-independent growth was measured as previously described (11). Colonies were stained for 20 hr at 37°C in a solution of iodonitrotetrazolium violet (1 μg/ml; Sigma) in water and counted by using EagleSight analysis software (Stratagene). The soft agar colony data include analysis of three separate clones for each cell type, EGFR5, EGFR8, EGFR27, EGFR/K+8, EGFR/K+9, EGFR/K+10, EGFR/K−2, EGFR/K−5, and EGFR/K−56. Assessment of tumor formation in Taconic nu/nu mice was performed as previously described (11).
In Vitro Kinase Assay, Metabolic Labeling with 32Pi, and Two-Dimensional Tryptic Phosphopeptide Analysis.
Methods for immunoprecipitation, in vitro kinase assay, metabolic 32Pi labeling, and two-dimensional phosphopeptide analysis have been described (11, 15). In the metabolic labeling experiments, 5 μM pervanadate and 3 mM H2O2 were added to cells simultaneously with 100 ng/ml EGF and incubated for 5 min before harvesting.
5-Bromodeoxyuridine (BrdUrd) Incorporation.
A pcDNA3 vector (Invitrogen) encoding human EGFR with a Y845F mutation was constructed by inserting a DraIII–BstEII fragment containing the Y845F mutation (from plasmid pCO11, gift of L. Beguinot, Laboratory of Molecular Oncology, Milan, Italy) into the corresponding DraIII–BstEII site of pcDNA3 encoding wt EGFR (gift of S. Decker, Parke–Davis, Ann Arbor, MI). K+ cells were transiently transfected with 4 μg of vector, wt EGFR, or Y845F EGFR plasmid DNA by using 30 μg of Superfect (Qiagen; Chatsworth, CA) according to manufacturer’s directions and incubated in a humidified, 37°C, 5% CO2/95% air atmosphere for 48 hr to allow a confluent monolayer to form. Transfected cells were then serum-starved for 30 hr prior to addition of 100 μM BrdUrd and 40 ng/ml EGF, 10% fetal bovine serum (FBS) in growth medium, or 10 μM LPA, at which time they were incubated for an additional 18 hr and costained for human EGFR expression and BrdUrd incorporation as described by the manufacturer of the BrdUrd-specific mAb (Boehringer Mannheim). Specifically, fixed cells were treated with 2 M HCl for 1 hr at 37°C and incubated with a mixture of primary antibodies (1:100 dilution of EGFR-specific Ab-4 and a 1:15 dilution of anti-BrdUrd mAb), followed by incubation with a mixture of secondary antibodies (75 μg/ml fluorescein isothiocyanate-conjugated goat anti-rabbit IgG and 4 μg/ml Texas red-conjugated goat anti-mouse IgG, both from Jackson ImmunoResearch).
Transient Transfections.
COS-7 cells were transiently transfected with plasmids encoding SHC and ERK2, by using Superfect as described above. HA-SHC (gift of K. Ravichandran; Univ. of Virginia; HA indicates influenza virus hemagglutinin) or Flag-ERK2 (gift of M. Weber; Univ. of Virginia) was transfected at a 1:5 ratio with or without either wt EGFR or Y845F mutant EGFR and incubated in a humidified, 37°C, 5% CO2/95% air atmosphere for 48 hr to allow a confluent monolayer to form. Transfected cells were then serum-starved overnight and either stimulated with 100 ng/ml EGF for 10 min or left untreated. Extracts were immunoprecipitated with either 12CA5 anti-HA antibody (Babco: Richmond, CA) or anti-Flag M2 affinity gel (Kodak) and resolved by SDS/PAGE.
RESULTS AND DISCUSSION
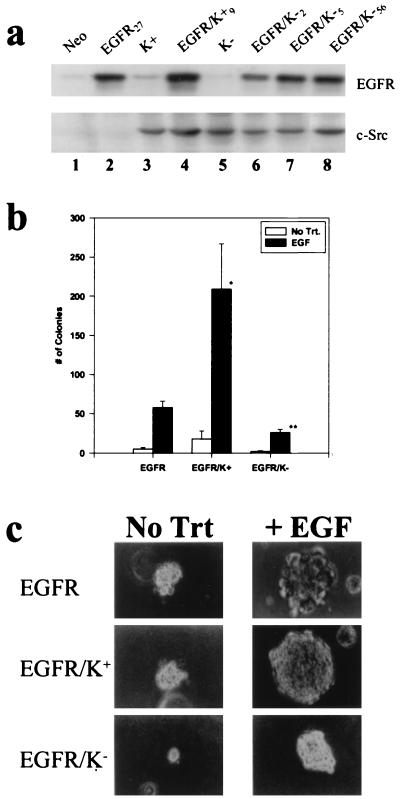
To determine whether phosphorylation of Y845 or Y1101 was dependent on c-Src catalytic activity, clonal C3H10T½ fibroblasts that stably overexpress wt EGFR and K− chicken c-Src were created from a stable K− c-Src expressing line, as described in Materials and Methods. Fig. 1a shows the levels of receptor and c-Src that are expressed in the various cell lines used in this study. EGFR/K− clones (lanes 6–8) expressed levels of receptor comparable to those in EGFR/K+ (lane 4) and EGFR (lane 2) lines, whereas all clones expressing K− c-Src (lanes 5–8) contained amounts of c-Src comparable to those of clones expressing K+ c-Src (lanes 3 and 4). Fig. 1b shows that the EGFR/K− clones exhibited diminished anchorage-independent growth in the presence of EGF compared with EGFR/K+ double overexpressors, demonstrating a requirement for the kinase activity of c-Src for potentiation of EGF-induced soft agar growth. Moreover, relative to cells overexpressing EGFR alone, the EGFR/K− clones also showed reduced soft agar growth, indicating that K− c-Src can function in a dominant-negative fashion for EGFR-induced colony formation. The dominant-negative effect was manifested by both reduced number (Fig. 1b) and significantly smaller average size (Fig. 1c) of the EGFR/K− colonies as compared with those of EGFR/K+ or EGFR cells. As previously reported, Neo control and K+ c-Src cells produced no or significantly fewer colonies than EGFR cells (11). K− c-Src cells also gave no colonies (data not shown). Table 1 shows that the growth of tumors in vivo was completely ablated in mice injected with EGFR/K− cells compared with EGFR or EGFR/K+ cells, demonstrating that K− c-Src has an even stronger dominant-negative effect on tumor growth in vivo than on growth in soft agar. Together these results underline the requirement for c-Src kinase activity in both the potentiating effect of overexpressed wt c-Src and the ability of overexpressed EGFR alone to induce oncogenic growth.
Figure 1.
Dominant repressive effect of K− c-Src on EGF-induced soft agar colony formation. (a) Western immunoblot analysis of C3H10T½ murine fibroblast clonal cell lines stably overexpressing EGFR and either wt (K+) or kinase-deficient (K−) c-Src. (b) Values for number of colonies are the mean ± SEM of at least six experiments in which 105 cells of each clone were seeded per plate in triplicate. Three clones of each cell type were averaged. ∗, P < 0.04 and ∗∗, P < 0.002 compared with EGFR. (c) Photomicrographs of representative fields of soft agar colonies formed from the indicated cell lines were taken after 2 weeks of growth. Trt, treatment. (×200.)
Table 1.
K− c-Src completely ablates tumor formation in nude mice in 10T½ clones
Mean tumor volume ± SEM of eight individual sites was measured at day 52 after subcutaneous injection.
EGFR/K− represents the mean tumor volume of two individual clones, EGFR/K−41 and EGFR/K−56.
To determine whether K− c-Src might be eliciting its biological effects through the receptor, we examined the association between the two kinases, using an immune complex in vitro kinase assay as previously described (11). c-Src was immunoprecipitated from the C3H10T½ clones with a chicken c-Src-specific antibody, EC10, to minimize recognition of endogenous c-Src and to determine whether the exogenously expressed K− c-Src could interact with the EGFR. An EGF-sensitive in vitro phosphorylation of an ≈170-kDa protein was observed in the c-Src immunoprecipitates prepared from EGFR/K− (Fig. 2a, lanes 20, 23, and 26) as well as from EGFR/K+ cells (lanes 11 and 14). These results demonstrate that c-Src kinase activity is not required for association and suggest that K− c-Src may be eliciting its dominant negative effects (at least in part) directly through the receptor, since the association is still intact.
Figure 2.
K− c-Src associates with the EGFR. Confluent, serum-starved cells were untreated or treated with EGF (100 ng/ml) for 15 min, and extracts were immunoprecipitated with either chicken c-Src-specific mAb EC10 (designated +) or a negative control mouse IgG (−). (a) Immunocomplexes were subjected to an in vitro kinase assay using [γ-32P]ATP, and phosphorylated products were analyzed by SDS/PAGE and autoradiography. The region of the autoradiograph around the 170-kDa products is shown. (b) The amount of c-Src in each precipitate was visualized by Western immunoblot analysis with EC10 mAb. K− c-Src in EGFR/K− clones was verified to be catalytically inactive in the immune complex kinase assay (data not shown).
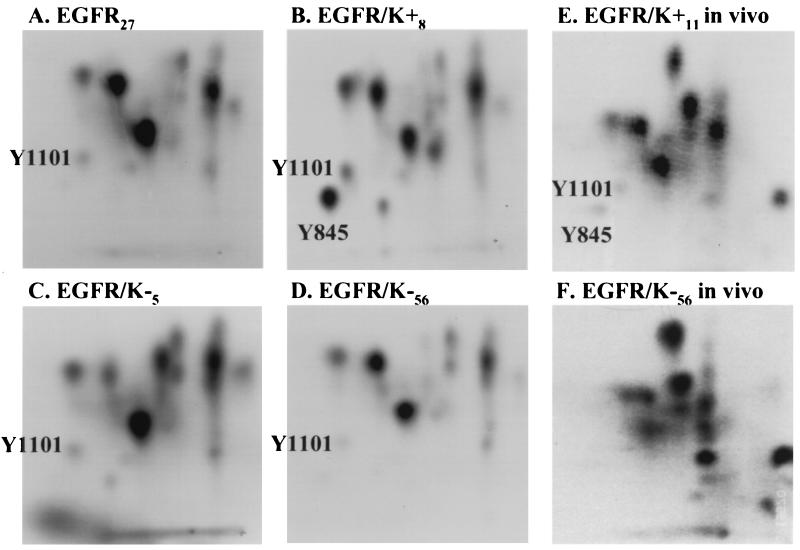
As described before (11), two tryptic phosphopeptides appear in the map of in vitro phosphorylated receptor associated with K+ c-Src (Fig. 3B) that are either absent or present in reduced amounts in the map of “free” activated receptor (Fig. 3A). These peptides contain Y845 and Y1101, whose identification is described in ref. 15. In contrast to the receptor associated with K+ c-Src, phosphorylation of Y845 was undetectable in receptor associated with K− c-Src (Fig. 3 C and D), while the level of Y1101 phosphorylation was visible but reduced. Similar results were observed in 32Pi metabolic labeling experiments (Fig. 3 E and F). Phosphorylation on Y845 was also observed in cells expressing endogenous levels of c-Src, but only after treatment with pervanadate (15), suggesting that this site is phosphorylated in the absence of overexpression of c-Src and that it is rapidly turned over. These results indicate that phosphorylation of Y845, and to a lesser extent of Y1101, depends on the kinase activity of c-Src, both in vitro and in vivo.
Figure 3.
Y845 is not phosphorylated in EGFR complexed with K− c-Src. (A–D) The 170-kDa bands that were phosphorylated in vitro (as in Fig. 2) in c-Src (B–D) or receptor (A) immunocomplexes prepared from the indicated cell lines were excised, digested with trypsin, resolved by two-dimensional electrophoresis/chromatography, and subjected to autoradiography. The positions of peptides containing Y845 and Y1101, which were identified previously (15), are indicated. (E and F) Receptor immunoprecipitates from the indicated cell lines that had been metabolically labeled with 32Pi were analyzed as in A–D. Equal cpm were loaded in A–D and in E vs. F. The apparent increase in tyrosine phosphorylation in F is due to a slightly darker exposure compared with E to emphasize the complete ablation of Y845 phosphorylation. The appearance of darker or novel spots in F was not reproduced in repeated experiments.
The position corresponding to Y845 is highly conserved among serine/threonine and tyrosine kinases and is situated in the activation loop between subdomains VII and VIII (18). Three-dimensional structural studies of several kinases have pointed to the importance of phosphorylation of this residue in stabilizing the activation loop in a conformation favorable for substrate and ATP binding (19–21). In agreement with the structural data, mutational analysis of the corresponding residue in tyrosine kinase receptors, including p185neu, a highly conserved family member, has shown a requirement for phosphorylation of this residue for full biological function in response to ligand (22–27). Y845 homologues in other tyrosine kinase receptors have all been shown to be autophosphorylation sites. In contrast, Y845 of the EGFR has not been identified as such, and its importance to EGFR function has not been ascertained. The failure to identify Y845 as a site of autophosphorylation may reflect either the highly labile nature of the phosphorylation or the c-Src dependency of the phosphorylation (15, 28).
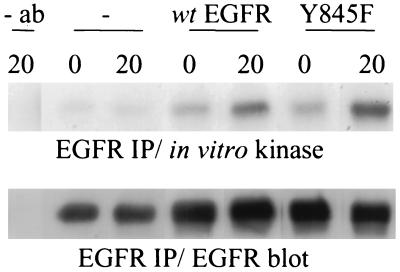
To determine whether phosphorylation of Y845 is required for receptor kinase activity, we compared wt EGFR autokinase activity with that of a mutant Y845F EGFR. Similar amounts of autophosphorylation were observed in an in vitro kinase assay of Y845F or wt EGFR immunoprecipitated from transiently transfected and EGF-stimulated COS-7 cells (Fig. 4). Further evidence that the EGFR, unphosphorylated on Y845F, retains its ability to autophosphorylate is provided by a comparison of the tryptic phosphopeptide maps of wt EGFR from EGFR cells (Fig. 3A) and wt EGFR from EGFR/K− cells (Fig. 3 C and D), where in the latter instance, autophosphorylation is maintained in the absence of detectable Y845 phosphorylation. These data suggest that, unlike other receptor tyrosine kinases mutated at the Y845 homologue, the Y845 mutant EGFR maintains its ability to autophosphorylate.
Figure 4.
Phosphorylation of Y845 is not essential for EGFR autokinase activity. COS-7 cells were transiently transfected with either vector alone (−), or plasmids encoding Y845F or wt EGFR and stimulated with EGF for 5 min. The EGFR was immunoprecipitated from extracts and subjected to an in vitro kinase assay for 0 or 20 min. The reaction was stopped with the addition of sample buffer, and the products were resolved by SDS/PAGE and transferred to a membrane. After autoradiography, EGFR was detected by Western immunoblotting and visualized by using enhanced chemiluminescence (ECL; Amersham).
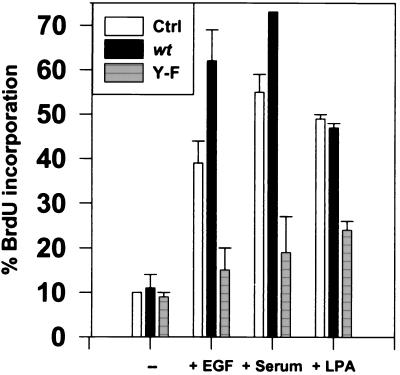
To test whether phosphorylation on Y845 is important for the mitogenic function of the EGFR independent of its autokinase activity, we transiently transfected a Y845F mutant or wt receptor into K+ cells and assessed mitogenesis by measuring EGF-induced BrdUrd incorporation into newly synthesized DNA. In contrast to the wt receptor, the Y845F mutant was unable to stimulate DNA synthesis upon EGF treatment (Fig. 5). Indeed, the reduced level of BrdUrd incorporation, which approached that of serum-starved cells, indicated that Y845F EGFR is capable of interfering with signaling through endogenous receptors, thereby acting in a dominant-negative fashion. These data support the hypothesis that phosphorylation of Y845 is required for the EGF-induced mitogenic function of the receptor.
Figure 5.
Phosphorylation of Y845 is essential for EGFR function. K+ cells were transfected with plasmid DNA encoding Y845F (Y-F) or wt EGFR, cultured for 2 days, serum starved for 30 hr, and left untreated or treated with 40 ng/ml EGF, 10% fetal bovine serum, or 10 μM LPA for 18 hr. Results are expressed as the mean percent ± SEM of cells expressing EGFR that were positive for BrdUrd incorporation. Thirty-five to 100 cells were analyzed for each variable in three independent experiments.
Surprisingly, the Y845F variant of the EGFR also inhibited serum-induced DNA synthesis in a dominant-negative manner (Fig. 5). The mechanism of this inhibition is unclear at the present time. However, the EGFR has recently been shown to play an essential role in signaling and growth stimulation through G protein-coupled receptors (GPCR) (29), and the Src family of tyrosine kinases has also been directly implicated in GPCR-mediated mitogen-activated protein kinase (MAPK) activation (30, 31). c-Src is thought to be responsible for phosphorylating the EGFR in response to GPCR activation (32), leading to the generation of docking sites. The major mitogenic component of serum is LPA, a ligand for GPCR (33). Therefore, one possible mechanism by which the Y845F EGFR could prevent serum-induced BrdUrd incorporation might be the inability to phosphorylate Y845 via a GPCR route. Indeed, Y845F mutant EGFR was able to reduce (but not ablate) induction of DNA synthesis by LPA, demonstrating an involvement of EGFR signaling in the GPCR pathway (Fig. 5).
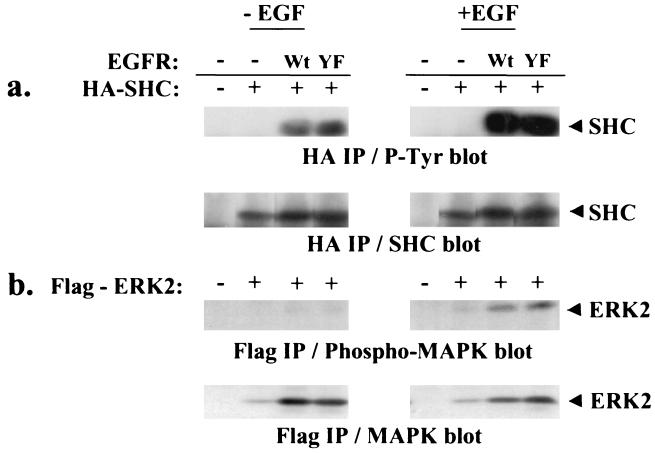
Because the EGFR is known to signal to MAPK via a SHC-Grb2-SOS-Ras pathway upon both EGF and G protein stimulation, we also tested the ability of the mutant EGFR to phosphorylate the direct substrate SHC and to activate ERK2. The presence of the Y845F mutation in the EGFR did not alter the EGF-induced increase in tyrosine phosphorylation of cotransfected SHC or ERK2, compared with wt EGFR (Fig. 6). Minor differences in the relative phosphorylations of either SHC or ERK2 between mutant and wt EGFR were not significant when multiple experiments were quantitated (data not shown). These results suggest that the EGFR stimulates mitogenesis through an ERK2-independent pathway.
Figure 6.
Y845F mutant receptor retains its ability to phosphorylate SHC and activate MAPK. COS-7 cells were transfected with plasmid DNA encoding HA-SHC or Flag-ERK2 and either Y845F or wt EGFR, cultured for 2 days, serum starved overnight, and left untreated or treated with 100 ng/ml EGF for 10 min. Extracts were immunoprecipitated with 12CA5 anti-HA antibody or anti-Flag M2 affinity gel and resolved by SDS/PAGE. The amount of tyrosine phosphorylated HA-SHC (a) and Flag-ERK2 (b) was observed by Western immunoblot analysis. The amount of wt EGFR or Y845F EGFR expressed in the populations of cells was the same as that shown for Fig. 4.
The data presented here provide a mechanism for c-Src’s role in EGF- and GPCR-mediated DNA synthesis and tumorigenesis. We propose that phosphorylation of Y845 on the EGFR by a c-Src-mediated event is required for EGF- and LPA-induced DNA synthesis. On the basis of the findings that Y845 is not phosphorylated by the wt receptor alone (Fig. 3) and that the kinase activity of c-Src is required for phosphorylation of Y845, we conclude that c-Src is the most likely kinase to phosphorylate the receptor. Interruption of this phosphorylation by overexpressing a kinase-deficient c-Src or a Y845F mutant of the EGFR blocks signaling and thus growth. Furthermore, the block of DNA synthesis by Y845F mutant EGFR is not dependent on its ability to autophosphorylate or to signal to MAPK, suggesting a mechanism of activation in which the activation loop tyrosine does not need to be phosphorylated for kinase activity, but is required for stimulation of a mitogenic pathway not involving ERK2.
These findings have direct implications for the etiology of human cancers. In tumor cells that overexpress both c-Src and the EGF receptor, we postulate that the probability of Y845 phosphorylation increases, an event that results in promotion of growth and anchorage independence. Since phosphorylation of Y845 has been shown to occur in cultured human tumor cells that overexpress c-Src (15), the above paradigm may have relevance for the disease in situ. Development of methods to inhibit the ability of c-Src to phosphorylate Y845 may result in a novel, more “tumor-specific” treatment for cancers such as carcinomas of the colon, breast, and lung.
Acknowledgments
We thank Drs. S. Decker and L. Beguinot for wt and Y845F EGFR cDNA, respectively, D. Lowy and his lab for retroviral stocks, and members of the S.J.P. lab and the Parsons–Weber–Parsons group for helpful discussions. This work was supported by research grants CA71449 and CA39438 from the National Cancer Institute and 4621 from the Council for Tobacco Research (S.J.P.), a graduate fellowship (DAMD 17-96-6126) (D.A.T.), and a postdoctoral fellowship (DAMD 17-97-7329) (J.S.B.), both from the Department of Defense.
ABBREVIATIONS
- c-Src
cellular Src
- EGF
epidermal growth factor
- EGFR
EGF receptor
- K+
wild-type c-Src
- K−
kinase-deficient c-Src
- wt
wild- type
- LPA
lysophosphatidic acid
- BrdUrd
5-bromodeoxyuridine
- HA
hemagglutinin
- MAPK
mitogen-activated protein kinase
- GPCR
G protein-coupled receptor
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Ottenhoff-Kalff A E, Rijksen G, van Beurden E A, Hennipman A, Michels A A, Staal G E. Cancer Res. 1992;52:4773–4778. [PubMed] [Google Scholar]
- 2.Khazaie K, Schirrmacher V, Lichtner R B. Cancer Metastasis Rev. 1993;12:255–274. doi: 10.1007/BF00665957. [DOI] [PubMed] [Google Scholar]
- 3.Banker N, Evers B M, Hellmich M R, Townsend C J. Surg Oncol. 1996;5:201–210. doi: 10.1016/s0960-7404(96)80023-5. [DOI] [PubMed] [Google Scholar]
- 4.Biscardi, J. S., Tice, D. A. & Parsons, S. J. (1999) Adv. Cancer Res. 76, in press. [DOI] [PubMed]
- 5.Harris J R, Lippmann M E, Veronesi U, Willett W. N Engl J Med. 1992;327:473–480. doi: 10.1056/NEJM199208133270706. [DOI] [PubMed] [Google Scholar]
- 6.Slamon D J, Clark G M, Wong S G, Levin W J, Ullrich A, McGuire W L. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 7.DiFiore P P, Pierce J H, Fleming T P, Hazan R, Ullrich A, King C R, Schlessinger J, Aaronson S A. Cell. 1987;51:1063–1070. doi: 10.1016/0092-8674(87)90592-7. [DOI] [PubMed] [Google Scholar]
- 8.Velu T J, Beguinot L, Vass W C, Willingham M C, Merlino G T, Pastan I, Lowy D R. Science. 1987;238:1408–1410. doi: 10.1126/science.3500513. [DOI] [PubMed] [Google Scholar]
- 9.Shalloway D, Coussens P M, Yaciuk P. Proc Natl Acad Sci USA. 1984;81:7071–7075. doi: 10.1073/pnas.81.22.7071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luttrell D K, Luttrell L M, Parsons S J. Mol Cell Biol. 1988;8:497–501. doi: 10.1128/mcb.8.1.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maa M-C, Leu T-H, McCarley D J, Schatzman R C, Parsons S J. Proc Natl Acad Sci USA. 1995;92:6981–6985. doi: 10.1073/pnas.92.15.6981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luttrell D K, Lee A, Lansing T J, Crosby R M, Jung K D, Willard D, Luther M, Rodriguez J, Berman J, Gilmer T M. Proc Natl Acad Sci USA. 1994;91:83–87. doi: 10.1073/pnas.91.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stover D R, Becker M, Liebetanz J, Lydon N B. J Biol Chem. 1995;270:15591–15597. doi: 10.1074/jbc.270.26.15591. [DOI] [PubMed] [Google Scholar]
- 14.Biscardi J S, Belsches A P, Parsons S J. Mol Carcinog. 1998;21:261–272. doi: 10.1002/(sici)1098-2744(199804)21:4<261::aid-mc5>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 15.Biscardi, J. S., Maa, M.-C., Tice, D. A., Cox, M. E., Leu, T.-H. & Parsons, S. J. (1999) J. Biol. Chem. 274, in press. [DOI] [PubMed]
- 16.Wilson L K, Luttrell D K, Parsons J T, Parsons S J. Mol Cell Biol. 1989;9:1536–1544. doi: 10.1128/mcb.9.4.1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reuter C W, Catling A D, Weber M J. Methods Enzymol. 1995;255:245–256. doi: 10.1016/s0076-6879(95)55027-5. [DOI] [PubMed] [Google Scholar]
- 18.Hanks S J, Quinn A M, Hunter T. Science. 1988;241:42–52. doi: 10.1126/science.3291115. [DOI] [PubMed] [Google Scholar]
- 19.Yamaguchi H, Hendrickson W A. Nature (London) 1996;384:484–489. doi: 10.1038/384484a0. [DOI] [PubMed] [Google Scholar]
- 20.Hubbard S R. EMBO J. 1997;16:5572–5581. doi: 10.1093/emboj/16.18.5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Russo A A, Jeffrey P D, Pavletich N P. Nat Struct Biol. 1996;3:696–700. doi: 10.1038/nsb0896-696. [DOI] [PubMed] [Google Scholar]
- 22.Ellis L, Clauser E, Morgan D O, Edery M, Roth R A, Rutter W J. Cell. 1986;45:721–732. doi: 10.1016/0092-8674(86)90786-5. [DOI] [PubMed] [Google Scholar]
- 23.Fantl W J, Escobedo J A, Williams L T. Mol Cell Biol. 1989;9:4473–4478. doi: 10.1128/mcb.9.10.4473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van der Geer P, Hunter T. Mol Cell Biol. 1991;11:4698–4709. doi: 10.1128/mcb.11.9.4698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vigna E, Naldini L, Tamagnone L, Longati P, Bardelli A, Maina F, Ponzetto C, Comoglio P M. Cell Mol Biol. 1994;40:597–604. [PubMed] [Google Scholar]
- 26.Mohammadi M, Dikic I, Sorokin A, Burgess W H, Jaye M, Schlessinger J. Mol Cell Biol. 1996;16:977–989. doi: 10.1128/mcb.16.3.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang H-T, O’Rourke D M, Zhao H, Murali R, Mikami Y, Davis J G, Greene M I, Qian X. Oncogene. 1998;16:2835–2842. doi: 10.1038/sj.onc.1201820. [DOI] [PubMed] [Google Scholar]
- 28.Sato K I, Sato A, Aoto M, Fukami Y. Biochem Biophys Res Commun. 1995;215:1078–1087. doi: 10.1006/bbrc.1995.2574. [DOI] [PubMed] [Google Scholar]
- 29.Daub H, Weiss F U, Wallasch C, Ullrich A. Nature (London) 1996;379:557–560. doi: 10.1038/379557a0. [DOI] [PubMed] [Google Scholar]
- 30.Dikic I, Tokiwa G, Lev S, Courtneidge S A, Schlessinger J. Nature (London) 1996;383:547–550. doi: 10.1038/383547a0. [DOI] [PubMed] [Google Scholar]
- 31.Luttrell L M, Hawes B E, van Biesen T, Luttrell D K, Lansing T J, Lefkowitz R J. J Biol Chem. 1996;271:19443–19450. doi: 10.1074/jbc.271.32.19443. [DOI] [PubMed] [Google Scholar]
- 32.Luttrell L M, Della Roca G J, van Biesen T, Luttrell D K, Lefkowitz R J. J Biol Chem. 1997;272:4637–4644. doi: 10.1074/jbc.272.7.4637. [DOI] [PubMed] [Google Scholar]
- 33.Moolenaar W, Kranenburg O, Postma F R, Zondag C M. Curr Opin Cell Biol. 1997;9:168–173. doi: 10.1016/s0955-0674(97)80059-2. [DOI] [PubMed] [Google Scholar]