Abstract
Ozone is widely used to disinfect drinking water and wastewater due to its strong biocidal oxidizing properties. Recently, it was reported that hydroxyl radicals (·OH), resulting from ozone decomposition, play a significant role in microbial inactivation when Bacillus subtilis endospores were used as the test microorganisms in pH controlled distilled water. However, it is not yet known how natural organic matter (NOM), which is ubiquitous in sources of drinking water, affects this process of disinfection by ozone-initiated radical reactions. Two types of water matrix were considered for this study. One is water containing humic acid, which is commercially available. The other is water from the Han River. This study reported that hydroxyl radicals, initiated by the ozone chain reaction, were significantly effective at B. subtilis endospore inactivation in water containing NOM, as well as in pH-controlled distilled water. The type of NOM and the pH have a considerable effect on the percentage of disinfection by hydroxyl radicals, which ranged from 20 to 50%. In addition, the theoretical  T value of hydroxyl radicals for 2-log B. subtilis removal was estimated to be about 2.4 × 104 times smaller than that of ozone, assuming that there is no synergistic activity between ozone and hydroxyl radicals.
T value of hydroxyl radicals for 2-log B. subtilis removal was estimated to be about 2.4 × 104 times smaller than that of ozone, assuming that there is no synergistic activity between ozone and hydroxyl radicals.
Ozone is widely used to disinfect drinking water and wastewater due to its strong biocidal oxidizing properties. Many studies on ozone disinfection have focused on the effects of ozone on various microorganisms and the factors affecting the inactivation of these microorganisms such as pH, temperature, contact time, ozone dose, and ozone demand (1, 6, 9, 19). Among the factors, the role of pH in inactivating microorganisms is not well understood and is somewhat controversial (2, 5, 12, 19, 25). No pH requirement for inactivation of microroganisms or fast inactivation by low pH has been reported without much discussion of the exact duration of exposure to ozone or the coexistence of hydroxyl radicals. This is partly due to the limited approaches used which considered mainly the biocidal activity of molecular ozone (5, 11, 12) rather than the indirect effect of hydroxyl radicals resulting from ozone decomposition. On the other hand, several studies did emphasize the importance of hydroxyl radicals in microorganism inactivation (1, 3). Recently, Cho et al. (2) reported that under certain experimental conditions, the presence of hydroxyl radicals produced by ozone disinfection plays a significant role in the inactivation of Bacillus subtilis endospores in pH-controlled ozone-demand-free distilled water. In their study, the observed 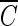 T values for achieving a 2-log inactivation were 40% lower at pH 8.2 than at pH 5.6 when time-dependent ozone decay as a function of the reaction time was considered in relation to the pH difference. However, in the presence of excess hydroxyl radical scavengers (t-butanol), where it is difficult for hydroxyl radical to exist, all the observed
T values for achieving a 2-log inactivation were 40% lower at pH 8.2 than at pH 5.6 when time-dependent ozone decay as a function of the reaction time was considered in relation to the pH difference. However, in the presence of excess hydroxyl radical scavengers (t-butanol), where it is difficult for hydroxyl radical to exist, all the observed 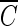 T values achieved parity, within a 10% margin of error, regardless of the pH difference, indicating that the difference in ozone disinfection was caused mainly by the presence of hydroxyl radicals, not by the change in pH.
T values achieved parity, within a 10% margin of error, regardless of the pH difference, indicating that the difference in ozone disinfection was caused mainly by the presence of hydroxyl radicals, not by the change in pH.
Although it is necessary to be cautious before extending this observation to other environmental conditions such as various water matrices containing different microorganisms, the implication of these results could be immense in terms of applying the advanced oxidation process to water treatment (2, 25). Therefore, whether similar findings would be obtained for drinking water containing natural organic matter (NOM), ubiquitously present in drinking-water sources, is an important question which needs to be elucidated.
The objective of this study was to investigate the process of disinfection by the ozone-initiated radical reaction in water containing NOM. Two types of water matrix were considered: (i) water containing commercially available humic acid and (ii) water from the Han River, which is the source of drinking water for the Seoul metropolitan area in Korea. A quantitative evaluation of the role of hydroxyl radicals in the inactivation of microorganisms was attempted by using 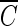 T values in water containing NOM.
T values in water containing NOM.
MATERIALS AND METHODS
The laboratory procedures used for the inactivation experiments in this study were similar to those used by Cho et al. (2). As shown in Table 1, the disinfection experiments were conducted at a constant temperature of 20°C and at controlled pHs of 5.6, 7.1, and 8.2 in distilled water, water containing humic acid, and river water. At each pH, two to six independent inactivation experiments were performed to determine the reproducibility of the data. The initial ozone dose and endospore population ranged approximately from 1.0 to 4.0 mg/liter, and from 104 to 106 CFU/ml, respectively, with or without the (30 mM) t-butanol hydroxyl radical scavenger. t-Butanol at this concentration had no effect on the inactivation of the B. subtilis endospores. All water was prepared using deionized-distilled water treated with a NANO pure system (Barnstead), and analytical reagent grade chemicals were used (Fisher Scientific, Itasca, Ill.). The pH was controlled with phosphate buffer solution. The resulting buffer solutions (of approximately 20 mM) were preozonated for 60 min to satisfy any ozone demand and left standing for 24 h. All glassware in these experiments was washed with presonicated distilled water and then autoclaved at 121°C for 15 min.
TABLE 1.
Experimental conditions of the B. subtilis spore inactivation study
| Water sample | pH | t-Butanol present | DOC (mg/liter) | No. of exp. seta | No. of samples | C0 (mg/liter)b | No. of microorganisms (104 CFU/ml) | k′ (s−1) (10−2)c |
|---|---|---|---|---|---|---|---|---|
| pH-controlled distilled water | 5.6 | No | 6 | 30 | 1.00-1.82 | 26-32 | 0.06 | |
| 7.1 | No | 5 | 20 | 1.38-1.50 | 26-32 | 0.18 | ||
| 8.2 | No | 6 | 33 | 0.91-1.65 | 26-32 | 0.26 | ||
| 5.6 | Yes | 3 | 12 | 1.30-1.63 | 25-39 | 0.06 | ||
| 7.1 | Yes | 3 | 12 | 1.32-1.55 | 26-39 | 0.18 | ||
| 8.2 | Yes | 3 | 12 | 1.55-1.75 | 26-39 | 0.26 | ||
| Water containing humic acid | 7.1 | No | 1.2 | 2 | 11 | 2.50-3.00 | 20-50 | 0.54 |
| 8.2 | No | 1.5 | 2 | 11 | 2.85-3.85 | 23-49 | 0.71 | |
| 7.1 | Yes | 1.2 | 2 | 9 | 2.15-3.20 | 22-45 | 0.54 | |
| 8.2 | Yes | 1.5 | 2 | 11 | 3.20-4.05 | 18-35 | 0.71 | |
| River water | 5.7 | No | 2.4 | 2 | 10 | 1.50-2.10 | 21-49 | 0.26 |
| 7.1 | No | 2.4 | 2 | 9 | 1.40-1.90 | 30-45 | 0.38 | |
| 8.2 | No | 2.4 | 2 | 10 | 1.00-2.10 | 20-50 | 0.53 | |
| 8.2 | Yes | 2.4 | 2 | 9 | 1.00-2.10 | 33-45 | 0.53 |
No. of exp. set, number of independent experimental set.
C0, initial ozone concentration.
k′, first-order ozone decay constant [found in C = C0 exp (−k′t)].
Endospore suspensions of B. subtilis (ATCC 6633) were prepared by the procedure described by Nakayama et al. (16) except for minor modifications such as 1/10 nutrient agar with an extended incubation time of 5 to 7 days. It should be noted that a different strain of B. subtilis (wild-type strain ATCC 6633 instead of mutant strain DB104 as in the previous study [2]) was used in this study. The stocks were diluted and treated at 80°C for 20 min just before each experiment. The numbers of viable endospores were measured by the spread plate method with nutrient agar grown at 37°C for 24 h. A 1-ml volume of solution was withdrawn in each sampling and was diluted to 1/10 and 1/100. Finally, 0.1 ml of the undiluted and diluted solutions were spread to count the number of B. subtilis endospores. Three replicate plates were used at each dilution.
The disinfection experiment with pH-controlled distilled water was repeated, as a preliminary study, to confirm the observation of the hydroxyl radical effect previously reported (2), since the Bacillus strain was different from that used in the previous work. In addition, two types of water samples were examined. The first water matrix consisted of water containing commercial humic acid (Aldrich Co.). The humic acid stock solution was made by dissolving 0.2 g of humic acid in 1 liter of distilled water at (pH 10.5) by the method proposed by Staelhelin and Hoigne (22). The solution was stirred for 80 min and then filtered through a membrane filter (0.45 μm pore size; Advantec MFS) to remove insoluble residues. The second water matrix consisted of Han River water. The characteristics of the Han River sample were as follows: dissolved-oxygen concentration (DOC) = 2.69 mg/liter; specific ultraviolet absorbance (SUVA) = 1.63 liters/cmmg; alkalinity = 20 mg/liter as CaCO3. The raw water sample was filtered off with a membrane filter (0.22 μm pore size, Advantec MFS) to remove all the indigenous endospores which might exist in the river water.
It should be noted that a significant amount of instantaneous ozone demand (IOD) was observed in both the water containing humic acid and the Han river water in comparison with the pH-controlled distilled water, which contained 30 to 50% of injected ozone. Larger ozone dosages (5 to 8 mg/liter) were injected to meet the IOD of water containing humic acid and Han River water. The C0 (initial ozone concentration) value shown in Table 1 represented the ozone concentration after subtraction of the IOD. The ozone decomposition rate was not affected by the presence of B. subtilis endospores or t-butanol.
A piston-type reactor (50 ml; Pyrex), which does not have free headspace, was used for the inactivation study. Concentrated ozone (>40 mg/liter) made with a CFS-1 ozone generator (Ozonia Co.) was introduced into the reactor and mixed immediately with a sample solution to attain an ozone concentration of approximately 1 to 4 mg/liter. The specially designed instrument, wherein the flow injection analysis technique was applied, was installed for continuous measurement of the accurate residual ozone concentration (2). Fixed quantities of small ozonated samples were taken continuously and mixed rapidly with an indigo solution to determine the ozone concentration. All materials used in this experimental apparatus were carefully selected so as not to have any undesirable reactivity with ozone. In general, four or five samplings were made to measure the Bacillus endospore concentrations for 5 to 8 min. Na2S2O3 (5 mM) was used to stop the reaction with residual ozone. All data under the detection limit were excluded from the data analysis.
The p-chlorobenzoic acid (pCBA; 300 μg/liter [1.92 μM]), used as a hydroxyl radical probe compound, was analyzed with a high-performance liquid chromatography system (Waters Co.). A C18 reverse-phase column (XTerra Rp-18 reverse-phase column; pore size of packed material, 5 μm; 150 by 2.1 mm) and a UV detector (Gilson 151 UV/VIS) at 230 nm were used for measuring the level of pCBA. A solvent mixture of 35% acetonitrile and 65% water containing 40 mM phosphate buffer was used as the mobile phase. The detection limit of pCBA was found be 5 μg/liter (0.032 μM).
With the purpose of comparing 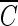 T values of hydroxyl radicals required for Bacillus endospore inactivation with those of chlorine, the chlorine disinfection experiments were performed using a 50-ml batch reactor. A chlorine stock solution (300 mg/liter) was prepared by dilution with 5% sodium hypochlorite solution (5 Junsei Co.). The chlorine dose ranged from 2 to 4 mg/liter as Cl2 at pH 8.2. Residual free chlorine levels were assayed with DPD (N,N-dimethyl-p-phenylenediamine) reagent at 530 nm (DR/2010 spectrophotometer; Hach Co.). In general, 5 to 10 samples were taken during the disinfection experiments (120 to 300 min) to measure the population of the microorganism.
T values of hydroxyl radicals required for Bacillus endospore inactivation with those of chlorine, the chlorine disinfection experiments were performed using a 50-ml batch reactor. A chlorine stock solution (300 mg/liter) was prepared by dilution with 5% sodium hypochlorite solution (5 Junsei Co.). The chlorine dose ranged from 2 to 4 mg/liter as Cl2 at pH 8.2. Residual free chlorine levels were assayed with DPD (N,N-dimethyl-p-phenylenediamine) reagent at 530 nm (DR/2010 spectrophotometer; Hach Co.). In general, 5 to 10 samples were taken during the disinfection experiments (120 to 300 min) to measure the population of the microorganism.
RESULTS
Determination of the disinfection kinetic model.
A disinfection kinetic model needs to be determined to compare the results obtained from different experimental conditions such as those involving disinfectant concentration, pH, and reaction time. The following four well-known disinfection kinetic models (2, 10, 18) were considered in order to find a best-fit model for the experimental results concerning the inactivation of the B. subtilis endospore:
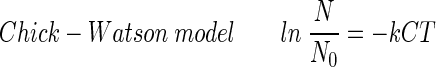 |
(1) |
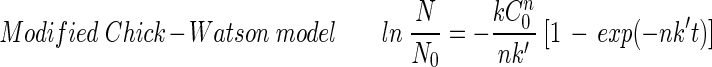 |
(2) |
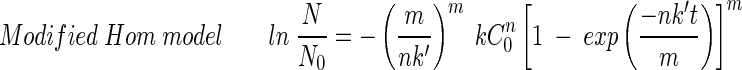 |
(3) |
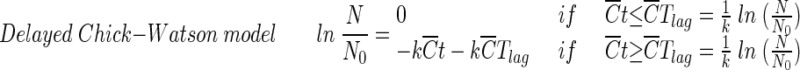 |
(4) |
where N0 is the initial B. subtilis endospore population (CFU per milliliter), N is the remaining B. subtilis endospore population at time t, C is the ozone concentration (milligrams per liter), 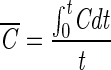 is the time-averaged ozone concentration (milligrams per liter), k is the inactivation rate constant with ozone (reciprocal minutes), k′ is the first-order ozone decay constant (reciprocal seconds), and m and n are model parameters of the modified Hom model.
is the time-averaged ozone concentration (milligrams per liter), k is the inactivation rate constant with ozone (reciprocal minutes), k′ is the first-order ozone decay constant (reciprocal seconds), and m and n are model parameters of the modified Hom model.
The Chick-Watson model (10) (equation 1) is simple and easy to apply. However, neither the shoulder nor the tailing off of the inactivation of the B. subtilis endospore can be explained using this model, due to its inability to explain the change in disinfectant concentration with time. On the other hand, the other three models (modified Chick-Watson model [10], modified Hom model [10], and delayed Chick-Watson model [2]) have the merit of explaining the inactivation kinetics including shoulder and disinfectant decay. The optimal parameters for each model (k in Chick-Watson, k and n in modified Chick-Watson, n, m, and k in modified Hom, and, k in delayed Chick-Watson) were obtained by minimizing the error sum of squares (ESS) (10) with the MATHLAB program using nonlinear regression. ESS was defined as the error sum of the squares of the logarithm of the predicted survival ratio subtracted from the logarithm of the observed survival ratio (equation 5). Although the ozone decay found in the inactivation experiments was well explained by first-order kinetics (k′ in Table 1, mostly R2 ≥ 0.96), time-averaged ozone exposure (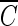 ) was used to determine the inactivation curves of the B. subtilis endospore. The slope (k, referred to as kI hereafter) and x-axis intercept (
) was used to determine the inactivation curves of the B. subtilis endospore. The slope (k, referred to as kI hereafter) and x-axis intercept (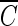 Tlag) of the delayed Chick-Watson model were estimated as average values (with the standard deviation) from the corresponding curves obtained from individual inactivation experiments.
Tlag) of the delayed Chick-Watson model were estimated as average values (with the standard deviation) from the corresponding curves obtained from individual inactivation experiments.
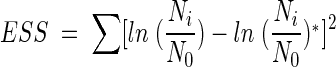 |
(5) |
where Ni/N0 is the observed survival ratio and (Ni/N0)∗ is the predicted survival ratio.
The ESS values for the four models are presented in Table 2. As shown in Table 2, the model which best fits the experimental results was found to be the delayed Chick-Watson model, which has the smallest ESS values among the four models. As shown in Table 2, the suitability of the delayed Chick-Watson model is further supported by a high correlation coefficient (R2 > 0.96). This observation is consistent with the previous study (2). The better fit of the delayed Chick-Watson model comes from its capacity to more accurately explain the lag phase of B. subtilis endospore inactivation by ozone. Table 2 shows that for pH-controlled water in the absence of t-butanol, relatively larger ESS values are obtained. This is because ESS is the total error sum of the squares obtained from the difference between measurements and model predictions. Larger ESS values are to be expected in the case of larger experimental measurements. Hereafter, all inactivation curves from the disinfection experiments were obtained by using the delayed Chick-Watson model.
TABLE 2.
Comparison of ESS among four disinfection kinetic models
| Water sample | pH | t-Butanol present | ESS ina:
|
R2b | |||
|---|---|---|---|---|---|---|---|
| CW | MCW | MHM | DCW | ||||
| pH-controlled distilled water | 5.6 | No | 3.56 | 2.74 | 2.58 | 2.36 | 0.96 |
| 7.1 | No | 4.49 | 3.90 | 2.24 | 2.11 | 0.97 | |
| 8.2 | No | 9.92 | 4.87 | 4.17 | 3.48 | 0.98 | |
| 5.6 | Yes | 3.54 | 1.34 | 0.56 | 0.45 | 0.97 | |
| 7.1 | Yes | 3.95 | 2.25 | 0.32 | 0.31 | 0.97 | |
| 8.2 | Yes | 4.12 | 1.85 | 0.38 | 0.48 | 0.97 | |
| Water containing humic acid | 7.1 | No | 0.45 | 0.97 | 0.09 | 0.09 | 0.98 |
| 8.2 | No | 2.40 | 1.36 | 0.26 | 0.23 | 0.98 | |
| 7.1 | Yes | 1.92 | 0.97 | 0.29 | 0.06 | 0.99 | |
| 8.2 | Yes | 2.22 | 1.02 | 0.18 | 0.07 | 0.97 | |
| River water | 5.7 | No | 4.10 | 0.97 | 0.21 | 0.13 | 0.98 |
| 7.1 | No | 2.45 | 1.12 | 0.32 | 0.31 | 0.99 | |
| 8.2 | No | 2.44 | 1.44 | 0.34 | 0.17 | 0.99 | |
| 8.2 | Yes | 3.15 | 1.57 | 0.44 | 0.21 | 0.98 | |
CW, Chick-Watson model; MCW, modified Chick-Watson model; MHM, modified Hom model; DCW, delayed Chick-Watson model.
Model fit of delayed Chick-Watson model.
Distilled water.
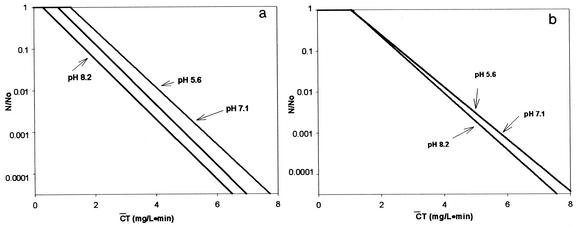
For pH-controlled distilled water, disinfection experiments were performed to confirm the previous observation (2) concerning the role of hydroxyl radicals in the inactivation of B. subtilis endospores; a different strain from that of the previous study was used. Inactivation curves of B. subtilis endospores in the presence and absence of the hydroxyl radical scavenger, expressed by means of the delayed Chick-Watson model, are presented in Fig. 1, as the pH was changed from 5.6 to 8.2. The inactivation curves in Fig. 1 are characterized by 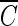 Tlag followed by a pseudo-first-order inactivation. In the absence of the hydroxyl radical scavenger, significant differences in the inactivation curves as a function of pH are observed in Fig. 1a. Bacillus endospore inactivation by ozone at pH 8.2 was approximately 25% more effective than that at pH 5.6. This observation of smaller
Tlag followed by a pseudo-first-order inactivation. In the absence of the hydroxyl radical scavenger, significant differences in the inactivation curves as a function of pH are observed in Fig. 1a. Bacillus endospore inactivation by ozone at pH 8.2 was approximately 25% more effective than that at pH 5.6. This observation of smaller 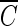 T values at higher pH is consistent with the previous study (2), although the observed slight difference in
T values at higher pH is consistent with the previous study (2), although the observed slight difference in 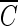 T values can probably be attributed to the difference in the Bacillus strains used. On the other hand, the three inactivation curves (Fig. 1b) became quite similar in the presence of the hydroxyl radical scavenger, regardless of pH, compared with those in Fig. 1a. This result indicates that the difference in
T values can probably be attributed to the difference in the Bacillus strains used. On the other hand, the three inactivation curves (Fig. 1b) became quite similar in the presence of the hydroxyl radical scavenger, regardless of pH, compared with those in Fig. 1a. This result indicates that the difference in 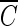 T values, brought about by the change in pH, was caused by the presence of hydroxyl radicals, as demonstrated in the previous study (2).
T values, brought about by the change in pH, was caused by the presence of hydroxyl radicals, as demonstrated in the previous study (2).
FIG. 1.
Inactivation of B. subtilis spores in pH-controlled distilled water. (a) Absence of t-butanol. (b) Presence of t-butanol.
Water containing humic acid.
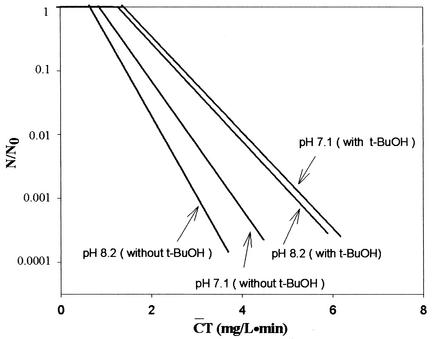
Humic acid is a NOM which is ubiquitous in surface water or in lakes used as a source of drinking water, although the amount present varies greatly. Therefore, it is important to examine the effect of NOM on disinfection resulting from the ozone-initiated radical reaction. Figure 2 presents the B. subtilis inactivation curves obtained with water containing humic acid (1.2 and 1.5 mg/liter). Figure 2 shows similar phenomena to those observed in Fig. 1 for pH-controlled distilled water. In the absence of t-butanol, 2-log Bacillus endospore inactivations by ozone at pH 7.1 and 8.2 were 35 and 50% more effective than those in the presence of t-butanol, respectively. However, the presence of t-butanol in water containing humic acid resulted in two very similar inactivation curves regardless of pH (pH 7.1 and 8.2).
FIG. 2.
Inactivation of B. subtilis spores in water containing humic acid. [DOC]0 = 1.2 mg/liter at pH 7.1 and 1.5 mg/liter at pH 8.2.
River water.
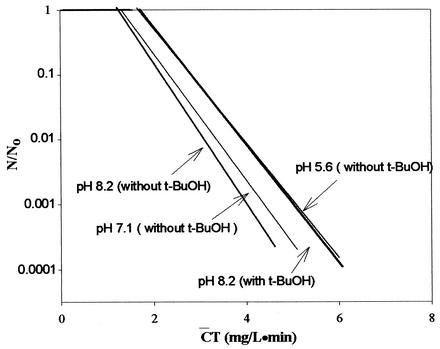
Figure 3 shows the inactivation curves of B. subtilis in the Han River sample. Again, Fig. 3 shows that lower 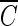 T values were found at a higher pH. These results are consistent with those observed in Fig. 1 and 2. As shown in Fig. 4, Bacillus endospore inactivation by ozone was approximately 20% more effective at pH 8.2 than at pH 5.6. The inactivation curves of B. subtilis in the Han River sample showed faster inactivation than those with pH-controlled distilled water but slower inactivation than those with water containing humic acid. The presence of t-butanol resulted in two very similar inactivation curves, with similar
T values were found at a higher pH. These results are consistent with those observed in Fig. 1 and 2. As shown in Fig. 4, Bacillus endospore inactivation by ozone was approximately 20% more effective at pH 8.2 than at pH 5.6. The inactivation curves of B. subtilis in the Han River sample showed faster inactivation than those with pH-controlled distilled water but slower inactivation than those with water containing humic acid. The presence of t-butanol resulted in two very similar inactivation curves, with similar 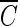 T values being observed at each pH within a margin of 10%.
T values being observed at each pH within a margin of 10%.
FIG. 3.
Inactivation curves of B. subtilis spores in Han River water. [DOC]0 = 2.4 mg/liter.
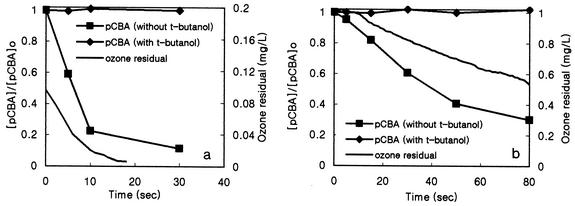
FIG. 4.
Formation of hydroxyl radicals in water containing NOM (pH 8.2, [pCBA]0 = 1.92 μM). (a) Water containing humic acid ([DOC]0 = 1.5 mg/liter). (b) River water ([DOC]0 = 2.4 mg/liter).
Formation of hydroxyl radicals in water containing NOM.
The change in the pCBA concentration was measured in water containing humic acid and Han River water as ozone dosages (0.8 mg/liter for water containing humic acid and 2 mg/liter for Han river water) were applied in the presence (10 mM) or absence of t-butanol at pH 8.2. pCBA was used as the hydroxyl radical probe compound, since it reacts quickly with hydroxyl radicals (5 × 109 M−1 s−1) but does not react well directly with ozone (0.15 M−1 s−1). Figure 4 shows the decrease in pCBA and residual ozone levels with time in water containing humic acid and Han River water.
From the results shown in Fig. 4, two interesting observations can be made. First, hydroxyl radicals produced by ozone decomposition were effectively scavenged in the presence of 100 mM t-butanol. Second, in the absence of t-butanol, the amount of hydroxyl radical formed in water containing humic acid as a result of ozone decomposition was larger than that in river water. This explanation is further supported by the results in Fig. 3, which show more rapid inactivation of the B. subtilis endospores in water containing humic acid when hydroxyl radical generation was increased by the presence of commercial humic acid (8). This occurred even though the residual ozone concentration in water containing humic acid was much lower than that in the river water, as shown in Fig. 4.
DISCUSSION
Similarity of 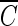 T values associated with the same integrated ozone exposure without hydroxyl radicals.
T values associated with the same integrated ozone exposure without hydroxyl radicals.
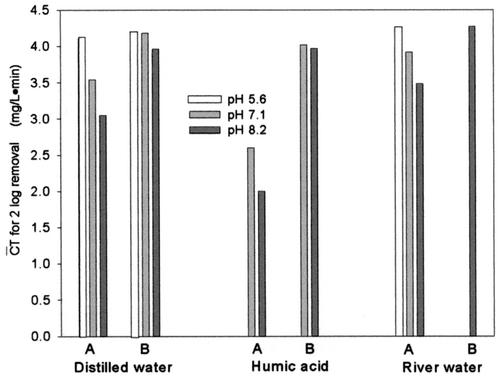
The 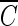 T values for achieving 2-log B. subtilis endospore inactivation, which were found in several different types of water, are compared in Fig. 5. Figure 5 shows that the
T values for achieving 2-log B. subtilis endospore inactivation, which were found in several different types of water, are compared in Fig. 5. Figure 5 shows that the 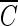 T values for achieving 2-log B. subtilis endospore inactivation, which were obtained in the presence of t-butanol, are all close to 4 mg/liter · min (3.96 to 4.27 mg/liter · min), within a 10% margin, regardless of not only pH but also the type of water. This result is greatly different from that of the previous study (5) in terms of the pH dependence of B. subtilis endospore inactivation in ozone disinfection. Facile et al. (5) reported a higher inactivation at lower pH since ozone decay was considered as a function of pH. However, even in the previous study (5),
T values for achieving 2-log B. subtilis endospore inactivation, which were obtained in the presence of t-butanol, are all close to 4 mg/liter · min (3.96 to 4.27 mg/liter · min), within a 10% margin, regardless of not only pH but also the type of water. This result is greatly different from that of the previous study (5) in terms of the pH dependence of B. subtilis endospore inactivation in ozone disinfection. Facile et al. (5) reported a higher inactivation at lower pH since ozone decay was considered as a function of pH. However, even in the previous study (5), 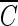 T values for achieving 2-log B. subtilis endospore inactivation, which ranged from 3.02 to 3.56 mg/liter · min at two pHs, were not greatly different from this study. On the other hand, the
T values for achieving 2-log B. subtilis endospore inactivation, which ranged from 3.02 to 3.56 mg/liter · min at two pHs, were not greatly different from this study. On the other hand, the 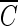 T values vary widely when hydroxyl radical formation is not suppressed. For example, for water containing humic acid, the
T values vary widely when hydroxyl radical formation is not suppressed. For example, for water containing humic acid, the 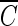 T value for achieving 2-log B. subtilis endospore inactivation in the absence of t-butanol was only about 50% of that observed in the presence of t-butanol. This means that as long as the microorganism is subjected to the same integrated ozone exposure without hydroxyl radicals, the same or a similar level of B. subtilis endospore inactivation is eventually achieved. This result has practical implications for optimum disinfection with ozone, taking into consideration hydroxyl radical formation in the presence of NOM.
T value for achieving 2-log B. subtilis endospore inactivation in the absence of t-butanol was only about 50% of that observed in the presence of t-butanol. This means that as long as the microorganism is subjected to the same integrated ozone exposure without hydroxyl radicals, the same or a similar level of B. subtilis endospore inactivation is eventually achieved. This result has practical implications for optimum disinfection with ozone, taking into consideration hydroxyl radical formation in the presence of NOM.
FIG. 5.
Comparison of 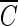 T values in several types of water for achieving 2-log B. subtilis spore inactivation (A, absence of t-butanol; B, presence of t-butanol).
T values in several types of water for achieving 2-log B. subtilis spore inactivation (A, absence of t-butanol; B, presence of t-butanol).
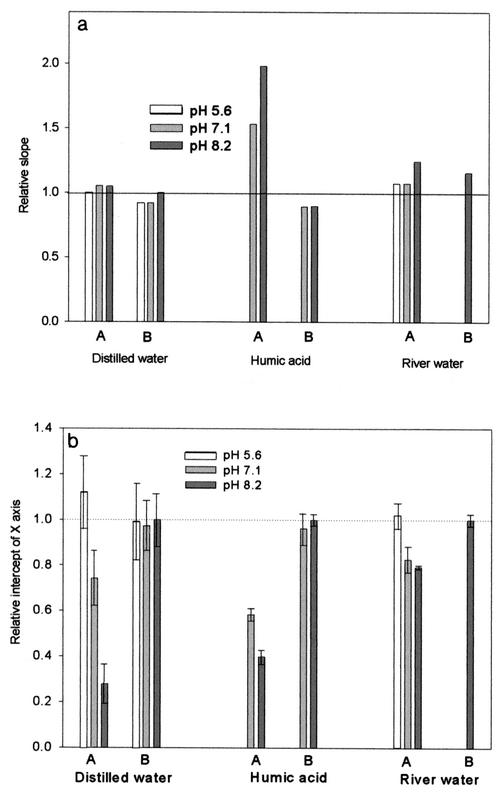
The inactivation curves of the delayed Chick-Watson model obtained from the disinfection experiments were further analyzed, because the 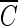 T values are affected by the inactivation kinetics curves. The curves are composed of the slope (kI) and x-axis intercept (
T values are affected by the inactivation kinetics curves. The curves are composed of the slope (kI) and x-axis intercept (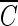 Tlag), representing the inactivation rate constant with ozone and the magnitude of the lag phase of the B. subtilis endospore inactivation with ozone, respectively. The relative ratios of slope and the relative ratios of the lag phase, in the x axis in Fig. 6, represent the slope and lag phase of the inactivation curves under each experimental condition, in relation to those for pH-controlled distilled water at pH 8.2. Figure 6a indicates that the presence of hydroxyl radicals has a limited influence on the change of the slope of the inactivation curves, except for those obtained for samples of water containing humic acid. On the other hand, as shown in Fig. 6b, the presence of hydroxyl radicals has a significant influence on the change of lag phase of the inactivation curves in all cases. This means that in the presence of the same amounts of hydroxyl radicals, the lag phase (x-axis intercept) of the inactivation curve is more changeable than the slope. In most cases, the level of hydroxyl radicals, which reduced the lag phase of the B. subtilis endospore, had a negligible effect on the change of slopes of the inactivation curves.
Tlag), representing the inactivation rate constant with ozone and the magnitude of the lag phase of the B. subtilis endospore inactivation with ozone, respectively. The relative ratios of slope and the relative ratios of the lag phase, in the x axis in Fig. 6, represent the slope and lag phase of the inactivation curves under each experimental condition, in relation to those for pH-controlled distilled water at pH 8.2. Figure 6a indicates that the presence of hydroxyl radicals has a limited influence on the change of the slope of the inactivation curves, except for those obtained for samples of water containing humic acid. On the other hand, as shown in Fig. 6b, the presence of hydroxyl radicals has a significant influence on the change of lag phase of the inactivation curves in all cases. This means that in the presence of the same amounts of hydroxyl radicals, the lag phase (x-axis intercept) of the inactivation curve is more changeable than the slope. In most cases, the level of hydroxyl radicals, which reduced the lag phase of the B. subtilis endospore, had a negligible effect on the change of slopes of the inactivation curves.
FIG. 6.
Comparison of the slope (a) and lag phase (b) of inactivation curves determined by the delayed Chick-Watson model. Relative slope is defined as (slope in the absence of t-butanol under each experimental condition)/(slope in the presence of t-butanol at pH 8.2). Relative intercept of the x axis is defined as (intercept in the absence of t-butanol under each experimental condition)/(intercept in the presence of t-butanol at pH 8.2). A, absence of t-butanol; B, presence of t-butanol.
The activity of hydroxyl radicals in reducing the lag phase of the inactivation curves may be explained by the ozone disinfection mechanism. It was reported that the attack, oxidation, and disruption of the cell wall and membrane by molecular ozone may cause disintegration of the cell and variations in ozone permeability, resulting in microbial inactivation. However, the inner component of the B. subtilis endospore, surrounded by the rigid structure of the bacterial endospore, might act as a barrier to the disinfectant, as suggested by the initial sluggish inactivation (lag phase) in Fig. 1 to 3 (5, 7, 13, 14, 15, 20, 24).
In light of this ozone disinfection scheme, the presence of hydroxyl radicals along with ozone may have a synergistic effect in attacking the cell wall and inactivating the B. subtilis endospore. This explanation is plausible, considering the diverse characteristics of hydroxyl radicals. First, the ability of a chemical disinfectant to oxidize the cell surface is closely related to its oxidation potential. Hydroxyl radicals, which have a higher oxidation potential (2.70 V) than does ozone (2.07 V), may be more effective in the destruction of the cell membrane or wall (21, 23). Second, hydroxyl radicals react nonselectively with the components of the outer structure of the cell, which is sometimes resistant to ozone, thus causing the microorganism to be inactivated.
Determination of 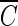 T values for hydroxyl radicals.
T values for hydroxyl radicals.
The determination of the 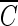 T values of hydroxyl radical was attempted, in order to improve our understanding of the correlation between the inactivation rate of the B. subtilis endospore and the quantity of hydroxyl radicals generated from ozone decomposition, in the experiments using pCBA as a hydroxyl radical probe compound in water containing NOM, in the presence (10 mM) or absence of t-butanol at pH 8.2.
T values of hydroxyl radical was attempted, in order to improve our understanding of the correlation between the inactivation rate of the B. subtilis endospore and the quantity of hydroxyl radicals generated from ozone decomposition, in the experiments using pCBA as a hydroxyl radical probe compound in water containing NOM, in the presence (10 mM) or absence of t-butanol at pH 8.2.
It is well known that NOM is involved in producing hydroxyl radicals through the ozone chain reaction. According to the reaction scheme suggested by Xiong and Legube (26), NOM can have four different types of reaction sites, one each for direct ozone reaction (HSd), hydroxyl radical initiation (HSi), promotion (HSp), and scavenging reactions (HSs). If the site pertaining to the radical promotion reaction is large compared with that for the radical-scavenging reaction (or [HSp] >> [HSs]), greater hydroxyl radical formation can be expected in the presence of NOM (8), depending on the type of organic matter present in the water. Therefore, organic matter such as humic acid may contribute to increased hydroxyl radical formation in the presence of ozone.
Quantification of the hydroxyl radical concentration, in both river water and water containing humic acid, was attempted from Fig. 4, using the Rct concept originally proposed by Elovitz et al. (4). Rct is defined in equation 6 as the ratio of ozone exposure to hydroxyl radical exposure:
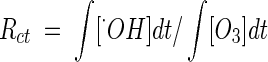 |
(6) |
The decrease of the pCBA level can be expressed as
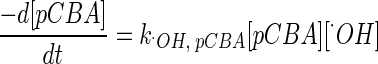 |
(7) |
Integrating equation 7 and substituting for the integral of [·OH] with equation:
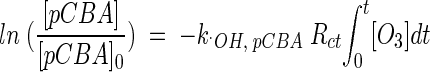 |
(8) |
From equation 8 and the results in Fig. 4, Rct can be found in water containing humic acid and river water. It was found that water containing humic acid has approximately 100 times more hydroxyl radicals present than does river water. Rct was 5.54 × 10−5 (R2 = 0.955) in humic substance containing water at pH 8.2, 4.25 × 10−7 (R2 = 0.964) in Han River water at pH 8.2 and 8.75 × 10−8 (R2 = 0.989) in Han River water at pH 7.1. The lower hydroxyl radical concentration is to be expected at the lower pH value of 7.1 than at pH 8.2, according to ozone decomposition chemistry. These results imply that the higher concentration of hydroxyl radicals in water containing humic acid led to a more rapid inactivation of the B. subtilis endospore.
With the assumption that there is no synergistic effect between molecular ozone and the hydroxyl radicals present in the inactivation of the B. subtilis endospore, determination of the 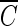 T values of hydroxyl radicals was attempted. Although this assumption may not be perfectly true, it enables us to establish at least approximate
T values of hydroxyl radicals was attempted. Although this assumption may not be perfectly true, it enables us to establish at least approximate 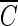 T values for hydroxyl radicals. The total inactivation of the microorganism can be expressed as the sum of the inactivation by ozone and by hydroxyl radicals, as in equation 9. Since ln (N/N0), corresponding to direct ozone inactivation, can be found only from the results of B. subtilis inactivation in the presence of t-butanol, the inactivation of B. subtilis by hydroxyl radicals can be determined by equation 10.
T values for hydroxyl radicals. The total inactivation of the microorganism can be expressed as the sum of the inactivation by ozone and by hydroxyl radicals, as in equation 9. Since ln (N/N0), corresponding to direct ozone inactivation, can be found only from the results of B. subtilis inactivation in the presence of t-butanol, the inactivation of B. subtilis by hydroxyl radicals can be determined by equation 10.
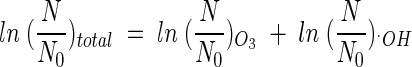 |
(9) |
where
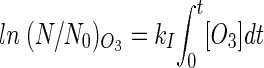 |
and
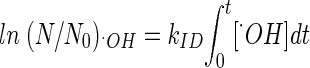 |
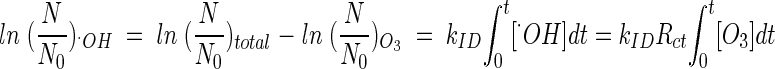 |
(10) |
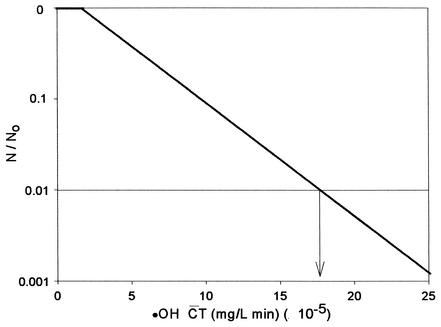
Figure 7 shows the  T curve of hydroxyl radicals constructed from the results of B. subtilis endospore inactivation experiments. As mentioned above, since the slopes of the inactivation curves do not change significantly unless the hydroxyl radical concentration is sufficiently high, as observed for water containing humic acid in Fig. 2, only the inactivation data for water containing humic acid were used to determine the
T curve of hydroxyl radicals constructed from the results of B. subtilis endospore inactivation experiments. As mentioned above, since the slopes of the inactivation curves do not change significantly unless the hydroxyl radical concentration is sufficiently high, as observed for water containing humic acid in Fig. 2, only the inactivation data for water containing humic acid were used to determine the  T values for hydroxyl radicals. From Fig. 7, the
T values for hydroxyl radicals. From Fig. 7, the 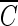 T value of hydroxyl radicals for 2-log removal was found to be approximately 1.7 × 10−4 mg/liter · min. It should be noted that the
T value of hydroxyl radicals for 2-log removal was found to be approximately 1.7 × 10−4 mg/liter · min. It should be noted that the 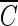 T value for hydroxyl radicals is based on the assumption that there is no synergistic effect between ozone and hydroxyl radicals. The
T value for hydroxyl radicals is based on the assumption that there is no synergistic effect between ozone and hydroxyl radicals. The 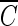 T values of several important disinfectants were determined at pH 8.2, in order to compare them with that of hydroxyl radicals for achieving 2-log removal of the B. subtilis endospore. The
T values of several important disinfectants were determined at pH 8.2, in order to compare them with that of hydroxyl radicals for achieving 2-log removal of the B. subtilis endospore. The 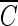 T value of free chlorine (450 mg/liter · min) was measured in this study. The
T value of free chlorine (450 mg/liter · min) was measured in this study. The 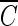 T value of hydroxyl radicals (1.7 × 10−4 mg/liter · min) is 2.4 × 104 times lower than that of ozone (4.1 mg/liter · min) and was 106 and 105 times lower than those of free chlorine (see above) and chlorine dioxide (50 mg/liter · min at pH 8.0 [17]), respectively.
T value of hydroxyl radicals (1.7 × 10−4 mg/liter · min) is 2.4 × 104 times lower than that of ozone (4.1 mg/liter · min) and was 106 and 105 times lower than those of free chlorine (see above) and chlorine dioxide (50 mg/liter · min at pH 8.0 [17]), respectively.
FIG. 7.
Theoretical inactivation curves of B. subtilis spores by hydroxyl radicals.
Acknowledgments
This research was partially supported by the Brain Korea 21 Program (of the Ministry of Education). This support is greatly appreciated.
REFERENCES
- 1.Bancroft, K., P. Chrostowski, R. L. Wright, and I. H. Suffet. 1984. Ozonation and oxidation competition values: relationship to disinfection and microorganisms regrowth. Water Res. 4:473-478. [Google Scholar]
- 2.Cho, M., H. Chung, and J. Yoon. 2002. Effect of pH and importance of ozone initiated radical reactions in inactivating Bacillus subtilis spore. Ozone Sci. Eng. 24:145-150. [Google Scholar]
- 3.Dahi, E. 1976. Physicochemical aspects of disinfection of water by means of ultrasound and ozone. Water Res. 10:677-684. [Google Scholar]
- 4.Elovitz, M. S., U. von Gunten, and H. P. Kaiser. 2000. Hydroxyl radical/ozone ratios during ozonation processes. II. The effect of temperature, pH, alkalinity, and DOM properties. Ozone Sci. Eng. 22:123-150. [Google Scholar]
- 5.Facile, N., B. Barbeau, M. Prevost, and B. Koudjonou. 2000. Evaluating bacterial aerobic spores as a surrogate for Giardia and Cryptosporidium inactivation by ozone. Water Res. 34:3238-3246. [Google Scholar]
- 6.Farooq, S., E. S. K. Chian, and R. S. Engelbrecht. 1977. Basic concepts in disinfection with ozone. J. Water Pollut. Control Fed. 49:1819-1831. [PubMed] [Google Scholar]
- 7.Finch, G. R., E. K. Black, L. Gyurek, and M. Belosevic. 1993. Ozone inactivation of Cryptosporidium parvum in demand-free phosphate buffer determined by in vitro excystation and animal infectivity. Appl. Environ. Microbiol. 59:4203-4210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guittonneau, S., D. Thibaudeau, and P. Meallier. 1996. Free radicals formation induced by the ozonation of humic substances in aqueous medium. Catalysis Today 29:323-327. [Google Scholar]
- 9.Gyurek, L. L., H. Li, M. Belosevic, and G. R. Finch. 1999. Ozone inactivation kinetics of Cryptosporidium in phosphate buffer. J. Environ. Eng. 125:913-914. [Google Scholar]
- 10.Haas, C. N., J. Joffe, U. Anmangandla, J. C. Hornberger, M. S. Heath, J. Jacangelo, and J. Glicker. 1995. Development and validation of radiational design methods of disinfection. AWWA Research Foundation, Denver, Colo.
- 11.Hoigne, J., and H. Bader. 1979. Ozonation of water: selectivity and rate of oxidation of solutes. Ozone Sci. Eng. 1:73-85. [Google Scholar]
- 12.Hunt, N. K., and B. J. Marinas. 1999. Kinetics of Escherichia coli inactivation with ozone: chemical and inactivation kinetics. Water Res. 33:2633-2641. [Google Scholar]
- 13.Khadre, M. A., and A. E. Yousef. 2001. Sporicidal action of ozone and hydrogen peroxide: a comparative study. Int. Food Sci. Technol. 71:131-138. [DOI] [PubMed] [Google Scholar]
- 14.Langlais, B., and D. Perrine. 1989. Study of the mechanisms of cyst killing action of ozone on free amoebae. Tech. Sci. Methodes 84:215-219. [Google Scholar]
- 15.Montgomery, J. M. 1985. Water treatment principles and design. John Wiley & Sons, New York, N.Y.
- 16.Nakayama, A., Y. Yano, S. Kobayashi, M. Ishikawa, and K. Sakai. 1996. Comparison of pressure resistances of spores of six Bacillus strains with their heat resistances. Appl. Environ. Microbiol. 62:3897-3900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Radziminski, C., L. Ballantyne, J. Hodson, R. Creason, R. C. Andrews, and C. Chauret. 2002. Disinfection of Bacillus subtilis spores with chlorine dioxide: a bench-scale and pilot-scale study. Water Res. 36:1629-1639. [DOI] [PubMed] [Google Scholar]
- 18.Rennecker, J. L., B. J. Marinas, J. H. Owens, and E. W. Rice. 1999. Inactivation of Cryptosporidium parvum oocysts with ozone. Water Res. 33:2481-2488. [Google Scholar]
- 19.Roy, D., R. S. Englebrecht, and E. S. Kchian. 1982. Comparative inactivation of six entero viruses by ozone. J. Am. Water Works Assoc. 74:660-664. [Google Scholar]
- 20.Setlow, B., and P. Setlow. 1993. Binding of small, acid-soluble spore proteins to DNA plays a significant role in the resistance of Bacillus subtilis spores to hydrogen peroxide. Appl. Environ. Microbiol. 59:3418-3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sonntag, V., 1986. Disinfection by free radicals and UV-radiation. Water Supply 4:11-18. [Google Scholar]
- 22.Staehelin, J., and J. Hoigne. 1985. Decomposition of ozone in water in the presence of organic solutes acting as promoters and inhibitors of radical chain reactions. Environ. Sci. Technol. 19:1206-1212. [DOI] [PubMed] [Google Scholar]
- 23.Watts, R. J., S. Kong, M. P. Orr, G. C. Miller and B. E. Henry. 1995. Photocatalytic inactivation of coliform bacteria and viruses in secondary wastewater effluent. Water Res. 29:95-100. [Google Scholar]
- 24.Wickramanayake, G. B., A. J. Rubin, and O. J. Sproul. 1984. Inactivation of Naegleria and Giardia cysts in water by ozonation. J. Water Pollut. Control Fed. 56:983-988. [Google Scholar]
- 25.Wolfe, R. L., M. H. Stewart, K. N. Scott, and M. J. MaGuire. 1989. Inactivation of Giardia muris and indicator organisms seeded in surface water supplies by PEROXONE and ozone. Environ. Sci. Technol. 23:744-745. [Google Scholar]
- 26.Xiong, F., and B. Legube. 1991. Enhancement of radical chain reaction of ozone in water in the presence of an aquatic fulvic acid. Ozone Sci. Eng. 13:349-363. [Google Scholar]