Abstract
Oligonucleotide microarrays were used to profile directly extracted rRNA from environmental microbial populations without PCR amplification. In our initial inspection of two distinct estuarine study sites, the hybridization patterns were reproducible and varied between estuarine sediments of differing salinities. The determination of a thermal dissociation curve (i.e., melting profile) for each probe-target duplex provided information on hybridization specificity, which is essential for confirming adequate discrimination between target and nontarget sequences.
The vast and previously unrecognized diversity of the microbial world discovered during the last decade reflects the rapid progress made in the development of molecular techniques for direct characterization of environmental systems. Among techniques now commonly employed, recovery of 16S rRNA sequences by using standard methods of PCR amplification, cloning, and sequencing has provided the most encompassing perspectives of microbial diversity (8). However, such descriptions of diversity have been accompanied by modest progress in characterizing the spatial and temporal patterns of abundance and activity of microorganisms. Thus, future advances will likely be technology driven and dependent upon new methods for both extensive and intensive monitoring of population abundance, distribution, and activity (22). In this regard, we anticipate that high-throughput DNA microarrays, which provide for parallel hybridizations to hundreds or thousands of genes per single experiment, will play a major role in the study of environmental systems (9, 11, 20, 27).
The major advantages of the microarray format are a low-sample-size requirement and the possibility for characterizing environmental nucleic acid pools without the biases associated with PCR and other amplification techniques. The level of sensitivity is particularly relevant to the development of this technology, since many molecular detection methods currently rely upon amplification to detect target sequences (12, 16). Amplification, however, often introduces biases that make quantification of natural populations difficult (5, 21, 24, 26). The ability to detect target rRNA sequences without amplification (i.e., direct profiling of rRNA sequences) would greatly improve our capacity to determine the relative number of sequences representing natural microbial populations. In our present work, we demonstrate the direct profiling of environmental microbial populations by hybridization of native rRNA recovered from two different sediment samples to a microarray designed to identify major microbial groups (PhyloChip).
The level of probe and hybridization specificity is also important to the development of DNA microarray technology, since probes targeting certain groups differ by as little as a single base pair. For example, probe BET42a (L-Sc-bProt-1027-a-A-17), which targets the β subclass of Proteobacteria, and probe GAM42a (L-Sc-gProt-1027-a-A-17), which targets the γ subclass of Proteobacteria, differ by a single T or A nucleotide (Table 1). The position and type (i.e., nucleotide) of a single-base-pair mismatch in a probe-target duplex (in addition to hybridization and wash conditions) have significant effects on hybridization specificity (25). Hence, it is necessary to recognize when a high level of discrimination is not feasible and to acknowledge instances when the identification of a particular target sequence was not achieved. This includes recognizing detection limits and/or the extent to which nucleic acid cross-hybridization compromises our ability to adequately distinguish target from nontarget sequences.
TABLE 1.
Oligonucleotide probes and targeted microbial groups
| Probe name | Full namea | Sequence (5′ to 3′) | % G+C | Major target group | Reference |
|---|---|---|---|---|---|
| UNIV907 | S-*-Univ-0907-a-A-22 | CCC CGT CAA TTC CTT TGA GTT T | 45 | All life | 3 |
| UNIV1390 | S-*-Univ-1390-a-A-18 | GAC GGG CGG TGT GTA CAA | 61 | All life | 28 |
| EUB336 | S-D-Bact-0336-a-A-18 | TGC CTC CCG TAG GAG TCT | 61 | Domain Bacteria | 4 |
| EUB338 | S-D-Bact-0338-a-A-18 | GCT GCC TCC CGT AGG AGT | 67 | Domain Bacteria | 2 |
| EUB927 | S-D-Bact-0927-a-A-17 | ACC GCT TGT GCG GGC CC | 76 | Domain Bacteria | 7 |
| PLA46 | S-P-Planc-0046-a-A-18 | GAC TTG CAT GCC TAA TCC | 50 | Planctomycetes | 18 |
| EUB338II | S-*-BactP-0338-a-A-18 | GCA GCC ACC CGT AGG TGT | 67 | Planctomycetes | 6 |
| EUB338III | S-*-BactV-0338-a-A-18 | GCT GCC ACC CGT AGG TGT | 67 | Verrucomicrobia | 6 |
| ALF968 | S-Sc-aProt-0968-a-A-18 | GGT AAG GTT CTG CGC GTT | 56 | α-Proteobacteria | 17 |
| BET42ab | L-Sc-bProt-1027-a-A-17 | GCC TTC CCA CTT CGT TT | 53 | β-Proteobacteria | 1515 |
| GAM42ab | L-Sc-gProt-1027-a-A-17 | GCC TTC CCA CAT CGT TT | 53 | γ-Proteobacteria | 15 |
| ACID20 | S-Div-Acido-0020-a-A-19 | CAG CGT TGA TTC TGA GCC A | 53 | Acidobacteria | This study |
| ACID294 | S-*-Acido-0294-a-A-17 | CCG TGC GCC CTC TCA GG | 76 | Groups of Acidobacteriac | This study |
| ACID351 | S-*-Acido-0351-a-A-20 | GAA CAA TTC CCC ACT GCT GC | 55 | Groups of Acidobacteriad | This study |
| ACID1399 | S-Div-Acido-1399-a-A-20 | TTT CGT GAT GTG ACG GGC GG | 60 | Acidobacteria | This study |
| GPOS1192 | S-*-Grps-1192-a-A-22 | TAA GGG GCA TGA TGA TTT GAC G | 45 | Gram-positive bacteria | This study |
| GPOS1199 | S-*-Grps-1192-a-A-15 | TAA GGG GCA TGA TGA | 47 | Gram-positive bacteria | This study |
| HGC69ab | L-Div-Hgc-1901-a-A-18 | TAT AGT TAC CAC CGC CGT | 50 | Actinobacteria | 19 |
| LGC353a | S-Div-Lgc-0353-a-A-16 | AAG ATT CCC TAC TGC T | 44 | Low G+C gram-positive bacteria | This study |
| LGC353b | S-Div-Lgc-0353-b-A-18 | GGA AGA TTC CCT ACT GCT | 50 | Low G+C gram-positive bacteria | This study |
| BACIL1136 | S-G-Bacil-1136-a-A-22 | AGT CAC CTT AGA GTG CCC AAC T | 50 | Bacillus | This study |
| NEG E7 | GAT GAT GAT GAT GAT GA | 35 | Negative control | S. Bavykin, unpublished data |
Full names have been standardized by Alm et al. (1).
23S rRNA probes.
Environmental clone MC22, MC103, and Acidobacterium capsulatum groups.
Environmental clone MB1228 and RB40 groups.
In conventional hybridization assays, the level of specificity is typically determined by adjusting the experimental conditions (e.g., temperature, ionic strength, and concentration of formamide) of association (i.e., hybridization) and the subsequent dissociation (i.e., washing or melting) of the probe-target duplex (28). In DNA microarray assays, however, this approach is difficult to apply since one set of conditions does not provide optimal target discrimination for all probes. Ideally, hybridization would be interrogated by using the association or dissociation condition that provides appropriate specificity for each probe. To this end, we have previously demonstrated that a thermal dissociation analysis of all probe hybrids, conducted in parallel on a single microarray, could be used to achieve higher discrimination between matched and mismatched probe-target duplexes and to provide a measure of the extent of cross-hybridization (13, 25). In the present study, we incorporated a thermal dissociation step in the characterization of native environmental rRNAs, demonstrating a format suitable both for direct detection and for confirmation that appropriate target sequence specificity has been realized.
Rationale for probe design.
Twenty-one oligonucleotide probes were used in this study (Table 1). Each probe targeted a given group or subgroup, was not complementary to any nontarget rRNA sequence in the ARB software (http://www.mikro.biologie.tu-muenchen.de) or GenBank database, and had a low number of self-complementarities (<4 nucleotides) as determined by online analysis of the Probe_Match program in the Ribosomal Database Project II (14). The positions of single-base-pair mismatches in nontarget sequences were designed to be not less than 2 bases from the 5′ or 3′ termini (25). To facilitate the correct interpretation of hybridization patterns on the microarray and to ensure probe fidelity, replicate and redundant probes were incorporated. While replicate probes served as internal hybridization controls, redundant and hierarchically nested probes served to confirm identification (13). Identification of a specific target molecule can be confirmed from the hybridization patterns of redundant probes that hybridize to multiple unique sites in rRNA. The hierarchical nesting of the probes provides phylogenetic information at several levels of taxonomic specificity (e.g., from domain to species) as well as information on hybridization specificity, since hybridization to multiple probes provides additional coverage of a single molecule. Lastly, since the secondary structures of probes and target sequences affect signal intensity, some redundant probes were designed to hybridize to different sites of the same rRNA molecule while maintaining taxonomic specificity (e.g., GPOS1192 and GPOS1199).
Sampling and RNA extraction.
Sediment cores (6.5-cm diameter) were collected during low tide from a high-salinity (20 ppt) site and a low-salinity (0 ppt) site in Plum Island Sound, Mass., in April 2001. Sediment samples at the high-salinity site consisted almost exclusively of sand, while those collected at the low-salinity site consisted primarily of silt and clay (10). Cores were kept in the dark and transported at the in situ water temperature to the laboratory where the top 0.5 cm was collected, homogenized, and stored at −80°C until processing.
Total RNA was extracted from approximately 0.5 g of sediment by bead beating (two times for 30 s each), phenol-chloroform extraction, and isopropanol precipitation as previously described (23). Replicate extracts from the high-salinity site (n = 4) and the low-salinity site (n = 3) were analyzed.
Fragmentation and labeling of rRNAs.
The RNA was fragmented and labeled simultaneously by using a silica-based column as previously described, with slight modifications (4). The silica matrix material was suspended twice with 500 μl of the binding buffer containing 50 μg of total RNA and centrifuged at 14,000 × g for 30 s. The column was washed twice with 70% ethanol, followed by a second wash using 100% ethanol, and air dried. The silica column containing bound RNA was sealed and incubated at 95°C for 4 min. Freshly prepared labeling cocktail (150 μl) containing 5 mM 1,10-phenanthroline, 0.5 mM CuSO4, 1 mM lissamine-rhodamine B ethylenediamine (Molecular Probes, Inc., Eugene, Oreg.), and 20 mM sodium phosphate (pH 7.0) was heated at 95°C for 30 s. Hydrogen peroxide (2 mM) and freshly prepared 20 mM NaCNBH3 were added to the cocktail, and the mixture was added to the column. After incubation of the mixture for 10 min at 95°C, the reaction was stopped by adding 9 μl of 500 mM EDTA (pH 8.0). Fragmented nucleic acids were immobilized on the column by adding 15 μl of 5 M ammonium acetate and 450 μl of 100% (vol/vol) ethanol followed by a 5-min incubation at room temperature. Excess fluorescent label was removed by washing the column twice with 500 μl of 100% (vol/vol) ethanol. The column was then dried with forced air, and the labeled product was eluted with 45 to 60 μl of diethyl pyrocarbonate-treated water.
Microarray fabrication and hybridization.
The DNA microarray was fabricated as previously described (25), with each gel pad containing a total of 3 pmol of probe. Hybridization buffer (40% formamide, 0.9 M NaCl, 20 mM Tris-HCl [pH 8.0]) containing approximately 2 μg of labeled RNA in 40 μl of buffer was passed through a 0.22-μm-pore-size filter to remove any particles that may damage the gel pads. The hybridization solution was added to a hybridization chamber (Grace Biolabs, Bend, Oreg.), and the hybridization chamber was affixed to a microarray. The microarray was then allowed to hybridize overnight at room temperature (20°C in the dark). After hybridization, the chamber and hybridization solution were removed and the microarray was washed twice for 10 s with 100 μl of washing buffer (20 mM Tris-HCl [pH 8.0], 5 mM EDTA, 4 mM NaCl). Analyses of images and thermal dissociation curves (i.e., melting profiles) were carried out as previously described (25).
Direct profiling of environmental rRNAs.
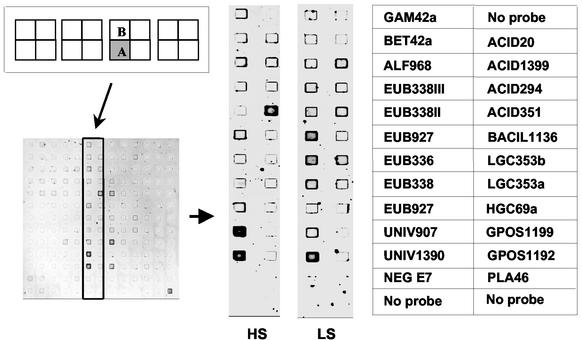
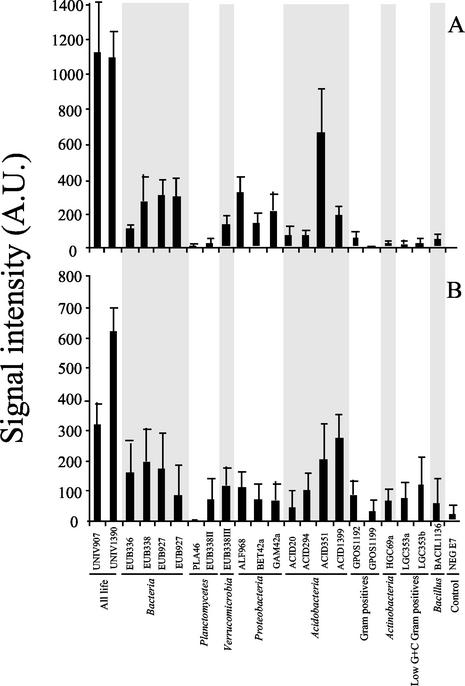
We achieved direct profiling of sediment microbial populations by hybridizing rRNA extracted from estuarine sediment samples to the microarray (Fig. 1). The profiles of sediment samples were reproducible and distinctly different between the sites (Fig. 2). The signal intensities of replicated bacterial domain probe EUB927 were not significantly different from one another (Student's t test), indicating that identical probes located at different positions on the microarray yielded identical results (Fig. 1 and 2).
FIG. 1.
DNA microarray images hybridized with rRNAs extracted from sediment samples collected at a high-salinity (HS) site and a low-salinity (LS) site, and position of the probes on the microarray. The microarrays were hybridized and washed at 20°C. Each probe was spotted in duplicate on the microarray. DNA microarray images of quadrant A are shown since quadrant B yielded virtually identical results. Probe names are listed in Table 1.
FIG. 2.
Signal intensity values of probe-target duplexes representing rRNA extracted from estuarine sediment samples. (A) High-salinity site (n = 8). (B) Low-salinity site (n = 6). Probe names are listed in Table 1. Each probe was spotted in duplicate on the microarray. Error bars indicate the standard deviations from the means of quadruplicate (high-salinity site) or triplicate (low-salinity site) experiments. Experimental error represents the variability obtained using two different microarrays and rRNA extracted, fragmented, and labeled from one low-salinity-site or two high-salinity-site sediment samples. A.U., arbitrary units.
On the other hand, the signal intensities of some redundant probes varied significantly for samples collected within the same field site. For example, in the low-salinity samples (Fig. 2B), probe UNIV907 had significantly lower (P < 0.02 for both quadrants A and B [Student's t test]; n = 3) signal intensities than probe UNIV1390. However, this within-site variation was not observed for the same probes hybridized to the high-salinity samples (Fig. 2A). Within-site variation was also observed for other redundant probes hybridized to rRNA extracted from high-salinity samples (Fig. 2A). For example, results obtained for probe EUB336 (Fig. 2A) yielded significantly lower (P < 0.03 for quadrant A and P < 0.01 for quadrant B [Student's t test]; n = 4) signal intensities than probes EUB927 and EUB338. Yet, there were no statistical differences among signal intensities for the same probes when using rRNA extracted from low-salinity samples (Fig. 2B). Site-specific differences in signal intensities originating from redundant probes might be due to subtle differences in fragmentation and/or fluorescent labeling of the target sequences (4). However, we anticipate that more-significant differences originate from minor sequence variation within the relatively highly conserved regions of the rRNAs targeted by these probes. Clearly, more-extensive experiments (beyond the scope of this study) are needed to reveal the source of sample-specific variability within redundant probe sets. Nonetheless, the results demonstrated that oligonucleotide microarrays can be used to (i) detect rRNA extracted from 0.5 g of sediment and (ii) provide information on the composition of microbial communities at different levels of taxonomic specificity.
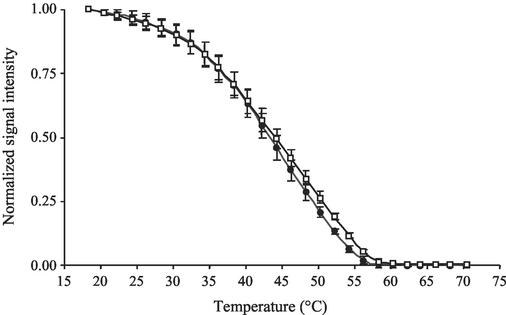
Melting profiles were used to evaluate the specificity of hybridization of environmentally derived rRNA to specific probes. We accomplished this by comparing the melting profiles of probe-target duplexes using rRNA extracted from pure cultures with those obtained from environmental samples. Figure 3 shows the melting profiles of universal probe UNIV1390 to (i) a perfect-match target sequence (Sulfobacillus acidophilus ATCC700253) and (ii) rRNA extracted from sediment collected at the high-salinity site. The melting profiles were virtually identical, demonstrating that environmentally derived rRNA included perfect-match target sequences to this probe. Similar results were also obtained for probes EUB338 and EUB927 (data not shown).
FIG. 3.
Normalized melting profiles of universal probe Univ1390 to a perfect-match target, Sulfobacillus acidophilus (white squares), and a high-salinity environmental sample (black circles). Error bars indicate the standard deviations from the means of triplicate experiments.
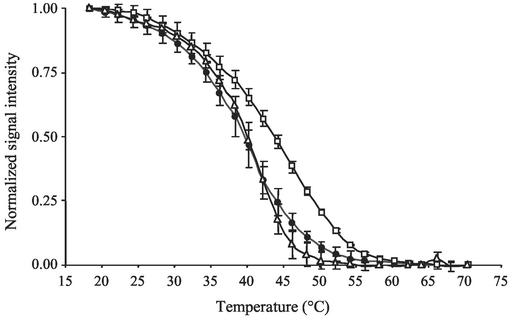
In the case of probe ALF968 (S-Sc-aProt-0968-a-A-18), targeting the α subclass of Proteobacteria (Fig. 4), there were considerable differences in melting profiles of rRNA extracted from sediment samples and those of perfect-match rRNA extracted from a pure culture of Sulfobacillus acidophilus. Apparently, the melting profile of the environmentally derived rRNA was more similar to that of rRNA extracted from a pure culture, Brevibacillus agri ATCC51663, which had a single-base-pair mismatch to probe ALF968. These results indicate that a significant fraction of the environmentally derived rRNA had at least one single-base-pair mismatch to probe ALF968.
FIG. 4.
Normalized melting profiles of probe ALF968 targeting α-Proteobacteria to perfect-match target rRNA of a pure culture Sulfobacillus acidophilus (white squares), to single-mismatch target Brevibacillus agri (white triangles), and to a high-salinity environmental sample (black circles). Error bars indicate the standard deviations from the means of triplicate experiments.
The advantage of comparing melting profiles of probe-target duplexes rather than comparing signal intensities at a single wash temperature of 20°C is that melting profiles provide more information on hybridization specificity. Moreover, since the relative abundances of target and closely related nontarget molecules in the environment are not known, it is difficult to determine the extent of cross-hybridization events from signal intensity data alone. However, the incorporation of melting-profile measurements in the oligonucleotide microarray analysis serves to identify circumstances when cross-hybridization clouds our ability to accurately discriminate target from nontarget sequences.
In summary, oligonucleotide microarrays were used to profile directly extracted rRNA from environmental microbial populations without PCR amplification. Recently, Small et al. (20) described a microarray system capable of detecting 0.5 μg of total rRNA by using a chaperone-detector probe strategy. This detection limit was in the level of PCR detection limits applied to environmental systems (109 to 1010 copies of 16S rRNA) and similar to the sensitivity we report here. The results achieved by Small et al. (20) were obtained by mixing purified reference nucleic acids with environmental extracts. Our study now demonstrates the feasibility of directly characterizing native rRNA. In our initial inspection of two distinct estuarine study sites, hybridization patterns were reproducible and varied between estuarine sediments of differing salinities. The incorporation of a melting-profile analysis for each probe duplex provided information on hybridization specificity, which is essential for confirming adequate discrimination between target and nontarget sequences.
Acknowledgments
We thank B. Chernov, A. Gemmell, J. Golova, A. Kukhtin, and S. Surzhikov for their efforts in manufacturing the oligonucleotide microarrays and for synthesis of the oligonucleotide probes. We are grateful to Y. Koizumi and S. Siripong for valuable discussions. We are also grateful to J. C. Smoot for reading the manuscript.
This work was support by grants from DARPA and NASA to D.A.S. and National Science Foundation grants DEB-0088879 to P.A.N. and MCB-9977897 to D.A.S. and from DARPA to J.J.K.
REFERENCES
- 1.Alm, E. W., D. B. Oerther, N. Larsen, D. A. Stahl, and L. Raskin. 1996. The oligonucleotide probe database. Appl. Environ. Microbiol. 62:3557-3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amann, R. I., B. J. Binder, R. J. Olson, S. W. Chisholm, R. Devereux, and D. A. Stahl. 1990. Combination of 16S rRNA-targeted oligonucleotide probes with flow cytometry for analyzing mixed microbial populations. Appl. Environ. Microbiol. 56:1919-1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amann, R. I., J. Stromley, R. Devereux, R. Key, and D. A. Stahl. 1992. Molecular and microscopic identification of sulfate-reducing bacteria in multispecies biofilms. Appl. Environ. Microbiol. 58:614-623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bavykin, S. G., J. P. Akowski, V. M. Zakhariev, V. E. Barsky, A. N. Perov, and A. D. Mirzabekov. 2001. Portable system for microbial sample preparation and oligonucleotide microarray analysis. Appl. Environ. Microbiol. 67:922-928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cottrell, M. T., and D. L. Kirchman. 2000. Community composition of marine bacterioplankton determined by 16S rRNA gene clone libraries and fluorescence in situ hybridization. Appl. Environ. Microbiol. 66:5116-5122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Daims, H., A. Bruhl, R. Amann, K.-H. Schleifer, and M. Wagner. 1999. The domain-specific probe EUB338 is insufficient for the detection of all Bacteria: development and evaluation of a more comprehensive probe set. Syst. Appl. Microbiol. 22:434-444. [DOI] [PubMed] [Google Scholar]
- 7.Giovannoni, S. J., E. F. DeLong, G. J. Olsen, and N. R. Pace. 1988. Phylogenetic group-specific oligodeoxynucleotide probes for identification of single microbial cells. J. Bacteriol. 170:720-726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giovannoni, S. J., T. B. Britschgi, C. L. Moyer, and K. G. Field. 1990. Genetic diversity in Sargasso Sea bacterioplankton. Nature (London) 345:60-63. [DOI] [PubMed] [Google Scholar]
- 9.Guschin, D. Y., B. K. Mobarry, D. Proudnikov, D. A. Stahl, B. E. Rittmann, and A. D. Mirzabekov. 1997. Oligonucleotide microchips as genosensors for determinative and environmental studies in microbiology. Appl. Environ. Microbiol. 63:2397-2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hopkinson, C. S., A. E. Giblin, J. Tucker, and R. H. Garritt. 1999. Benthic metabolism and nutrient cycling along an estuarine salinity gradient. Estuaries 22:863-881.
- 11.Koizumi, Y., J. J. Kelly, T. Nakagawa., H. Urakawa, S. El-Fantroussi, S. Al-Muzaini, M. Fukui, Y. Urushigawa, and D. A. Stahl. 2002. Parallel characterization of anaerobic toluene- and ethylbenzene-degrading microbial consortia by PCR-denaturing gradient gel electrophoresis, RNA-DNA membrane hybridization, and DNA microarray technology. Appl. Environ. Microbiol. 68:3215-3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu, W.-T., T. L. Marsh, H. Cheng, and L. J. Forney. 1997. Characterization of microbial diversity by determining terminal restriction fragment length polymorphisms of genes encoding 16S rRNA. Appl. Environ. Microbiol. 63:4516-4522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu, W.-T., A. D. Mirzabekov, and D. A. Stahl. 2001. Optimization of an oligonucleotide microchip for microbial identification studies: a non-equilibrium dissociation approach. Environ. Microbiol. 3:619-629. [DOI] [PubMed] [Google Scholar]
- 14.Maidak, B. L., J. R. Cole, T. G. Lilburn, C. T. Parker, Jr., P. R. Saxman, R. J. Farris, G. M. Garrity, G. J. Olsen, T. M. Schmidt, and J. M. Tiedje. 2001. The RDP-II (Ribosomal Database Project). Nucleic Acids Res. 29:173-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manz, W., R. Amann, W. Ludwig, M. Wagner, and K.-H. Schleifer. 1992. Phylogenetic oligodeoxynucleotide probes for the major subclasses of Proteobacteria: problems and solutions. Syst. Appl. Microbiol. 15:593-600. [Google Scholar]
- 16.Muyzer, G., E. C. de Wall, and A. G. Uitterlinden. 1993. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl. Environ. Microbiol. 59:695-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neef, A. 1997. Anwendung der in situ Einzelzell-Identifizierung von Bakterien zur Populationsanalyse in komplexen mikrobiellen Biozönosen. Ph.D. thesis. Technische Universität München, Munich, Germany.
- 18.Neef, A., R. Amann, H. Schlesner, and K.-H. Schleifer. 1998. Monitoring a widespread bacterial group: in situ detection of planctomycetes with 16S rRNA-targeted probes. Microbiology 144:3257-3266. [DOI] [PubMed] [Google Scholar]
- 19.Roller, C., M. Wagner, R. Amann, W. Ludwig, and K.-H. Schleifer. 1994. In situ probing of gram-positive bacteria with high DNA G+C content using rRNA-targeted oligonucleotides. Microbiology 140:2849-2858. [DOI] [PubMed] [Google Scholar]
- 20.Small, J., D. R. Call, F. J. Brockman, T. M. Straub, and D. P. Chandler. 2001. Direct detection of 16S rRNA in soil extracts by using oligonucleotide microarrays. Appl. Environ. Microbiol. 67:4708-4716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Speksnijder, A. G., G. A. Kowalchuk, S. De Jong, E. Kline, J. R. Stephen, and H. J. Laanbroek. 2001. Microvariation artifacts introduced by PCR and cloning of closely related 16S rRNA gene sequences. Appl. Environ. Microbiol. 67:469-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stahl, D. A. 2000. Small instruments for the study of small life. Environ. Microbiol. 2:5. [Google Scholar]
- 23.Stahl, D. A., B. Flesher, H. R. Mansfield, and L. Montgomery. 1988. Use of phylogenetically based hybridization probes for studies of ruminal microbial ecology. Appl. Environ. Microbiol. 54:1079-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suzuki, M. T., and S. J. Giovannoni. 1996. Bias caused by template annealing in the amplification of mixtures of 16S rRNA genes by PCR. Appl. Environ. Microbiol. 62:625-630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Urakawa, H., P. A. Noble, S. El Fantroussi, J. J. Kelly, and D. A. Stahl. 2002. Single-base-pair discrimination of terminal mismatches by using oligonucleotide microarrays and neural network analyses. Appl. Environ. Microbiol. 68:235-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang, G. C., and Y. Wang. 1996. The frequency of chimeric molecules as a consequence of PCR co-amplification of 16S rRNA genes from different bacterial species. Microbiology 142:1107-1114. [DOI] [PubMed] [Google Scholar]
- 27.Wu, L., D. K. Thompson, G. Li, R. A. Hurt, J. M. Tiedje, and J. Zhou. 2001. Development and evaluation of functional gene arrays for detection of selected genes in the environment. Appl. Environ. Microbiol. 67:5780-5790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zheng, D., E. W. Alm, D. A. Stahl, and L. Raskin. 1996. Characterization of universal small-subunit rRNA hybridization probes for quantitative molecular microbial ecology studies. Appl. Environ. Microbiol. 62:4504-4513. [DOI] [PMC free article] [PubMed] [Google Scholar]