Abstract
A new laccase (EC 1.10.3.2) produced by Streptomyces cyaneus CECT 3335 in liquid media containing soya flour (20 g per liter) was purified to homogeneity. The physicochemical, catalytic, and spectral characteristics of this enzyme, as well as its suitability for biobleaching of eucalyptus kraft pulps, were assessed. The purified laccase had a molecular mass of 75 kDa and an isoelectric point of 5.6, and its optimal pH and temperature were 4.5 and 70°C, respectively. The activity was strongly enhanced in the presence of Cu2+, Mn2+, and Mg2+ and was completely inhibited by EDTA and sodium azide. The purified laccase exhibited high levels of activity against 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonate) (ABTS) and 2,6-dimethoxyphenol and no activity against tyrosine. The UV-visible spectrum of the purified laccase was the typical spectrum of the blue laccases, with an absorption peak at 600 nm and a shoulder around 330 to 340 nm. The ability of the purified laccase to oxidize a nonphenolic compound, such as veratryl alcohol, in the presence of ABTS opens up new possibilities for the use of bacterial laccases in the pulp and paper industry. We demonstrated that application of the laccase from S. cyaneus in the presence of ABTS to biobleaching of eucalyptus kraft pulps resulted in a significant decrease in the kappa number (2.3 U) and an important increase in the brightness (2.2%, as determined by the International Standard Organization test) of pulps, showing the suitability of laccases produced by streptomycetes for industrial purposes.
Because of their potential for biotechnological applications in areas such as biobleaching, increasing the strength of cellulose fibers, textile dye or stain bleaching, and bioremediation, attention is currently being paid to laccases (2, 23, 30, 43, 50). These enzymes are widely distributed in plants and fungi, but until now laccase activity has been reported in only a few bacteria, including Azospirillum lipoferum, Marinomonas mediterranea, Streptomyces griseus, and Bacillus subtilis (1, 15, 20, 22, 41, 45). A number of roles for laccases in bacterial systems have been suggested and include roles in melanin production and spore coat resistance and involvement in morphogenesis (15, 22). In Streptomyces cyaneus, a laccase-type phenol oxidase was found to be produced during growth under solid-substrate fermentation conditions, and it was suggested that this enzyme was involved in the solubilization and mineralization of lignin from wheat straw (5). Further studies demonstrated that this organism could be used to improve the qualities of pulp after 2 weeks of incubation under solid-substrate fermentation conditions (6). However, to date there have been no reports describing the involvement of bacterial laccases in the oxidation of nonphenolic compounds in the presence of mediators. The specific activities of these enzymes with lignin and related substrates have not been examined yet.
Laccases are considered some of the most promising enzymes for future industrial applications in the pulp and paper industry (4, 10, 31, 33, 38). In particular, laccases from fungi have been found to reduce the kappa number and enhance the bleaching of kraft pulp when they are used in the presence of chemical mediators, such as 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonate) (ABTS) (8, 11, 49). In this paper we describe the behavior of the phenol oxidase produced by S. cyaneus CECT 3335 in submerged cultures as a laccase-type behavior, and in this study we also confirmed the usefulness of the purified enzyme in the biobleaching of kraft pulps.
MATERIALS AND METHODS
Microorganism and growth conditions.
S. cyaneus CECT 3335 was maintained as a suspension of spores and hyphal fragments in 20% glycerol at −70°C and was routinely cultured on Bennet agar (29) or soya flour mannitol agar containing (per liter) 20 g of soya flour, 20 g of mannitol, and 20 g of agar. Distilled water suspensions of sporulating growth (107 CFU ml−1) were used as inocula. Standard spore suspensions (2 ml) were used to inoculate 500-ml flasks containing 100 ml of basal mineral medium (13) supplemented with soya flour (20 g per liter). Cultures were shaken at 200 rpm and incubated at 28°C for 10 days. Flasks were removed every 24 h to determine the enzyme activity. Culture supernatants were obtained by centrifugation of the contents of flasks at 10,000 × g at 4°C for 10 min. Cell-free culture supernatants were stored at −20°C until they were used.
Enzyme assays and protein determination.
Laccase activity was determined by determining the oxidation of 5 mM ABTS (Sigma-Aldrich) (48) in 0.1 M McIlvaine buffer (pH 4.4). The increase in absorbance at 436 nm was monitored with a Beckman DU-50 spectrophotometer connected to an Ultraterm thermostatic bath, which maintained the temperature of the reaction mixture at 60 ± 2°C. To calculate enzyme activity, we used an absorption coefficient of 29,300 M−1 cm−1 for oxidized ABTS.
The time course of growth was estimated by measuring intracellular protein concentrations. Cells were obtained daily by centrifugation at 5,000 × g for 10 min, and then they were washed with distilled water and resuspended in 10 ml of 0.1 M sodium phosphate buffer (pH 6.8). The cells were disrupted with a French pressure cell (SLM AMINCO), and the intracellular protein content was estimated by the Bradford method (9).
Protein contents in the supernatant were estimated as described above, and the specific activities were expressed as milliunits per minute per milligram of culture supernatant protein.
Purification and characterization of laccase.
Proteins were precipitated from the supernatant by addition of ammonium sulfate (up to 50% saturation) at 4°C and centrifugation at 10,000 × g for 30 min. The precipitate was resuspended in 0.1 M sodium phosphate buffer (pH 6.8) and extensively dialyzed at 4°C against the same buffer. The sample was precipitated again with ammonium sulfate (up to 20%) and centrifuged as described above.
Liquid chromatography was carried out with a GP-250 Plus fast-performance liquid chromatographic system (Pharmacia). Supernatant (2 ml) was loaded onto a hydrophobic column (Econo-Pac Methyl cartridge; Bio-Rad). The column was equilibrated with 0.1 M phosphate buffer (pH 6.8) supplemented with 1.1 M ammonium sulfate. The adsorbed proteins were eluted with the same buffer without ammonium sulfate by using a continuous gradient (1.1 to 0 M ammonium sulfate) at a flow rate of 1 ml min−1. Fractions with laccase activity were pooled, concentrated, and applied to an anion-exchange column (Econo Pac Q cartridge; Bio-Rad). In this step the column was equilibrated with 0.1 M phosphate buffer (pH 6.8), and the adsorbed proteins were eluted with the same buffer by using the following NaCl gradient: 35% NaCl (7 min) and 100% NaCl (5 min) at a flow rate of 1 ml min−1. Chromatograms were obtained by monitoring the absorbance at 280 nm. The ABTS-laccase activity and proteins were assayed in all fractions collected (1 ml).
Throughout the purification process, fractions were analyzed by native and denaturing polyacrylamide gel electrophoresis (PAGE).
Sodium dodecyl sulfate (SDS)-PAGE on 9% polyacrylamide gels was performed as described by Laemmli (27). Culture supernatants were obtained daily, and native PAGE (9% polyacrylamide) was used to separate the extracellular proteins. Gels were developed as zymograms for laccase activity by using ABTS as the substrate. After electrophoresis, the gels were overlaid onto a 1.5% (wt/vol) agarose gel sheet (thickness, 1 mm) containing 5 mM ABTS in 0.1 M McIlvaine buffer (pH 4.5). Both the polyacrylamide gel and the agarose gel were placed on a glass plate and incubated for 15 to 30 min at 45°C. During the purification process, SDS-PAGE analysis was also carried out. Protein bands were stained with Coomassie brilliant blue R-250 or silver stain (Bio-Rad) and compared with the bands of molecular weight markers (Bio-Rad). Isoelectric focusing was carried out by using a Bio-Rad mini isoelectric focusing cell system according to the manufacturer's instructions at pH 3 to 9; standard Bio-Rad markers were included. Protein bands were visualized by Coomassie brilliant blue R-250 staining.
Estimates of the optimal temperature and pH for activity and estimates of the thermal and pH stabilities of the purified laccase were obtained by using a temperature range of 30 to 100°C and a pH range of 3 to 8 with 10 mM McIlvaine buffer (pH 3 to 7) and 10 mM phosphate buffer (pH 7 to 8).
Michaelis-Menten kinetic parameters (Km and Vmax) for the purified laccase (481 μg ml−1) were determined at the optimal temperature (70°C) in 0.1 M McIlvaine buffer (pH 4.5) by using ABTS at concentrations of 0 to 50 mM as the substrate.
The effects of metal ions (CuSO4, MnSO4, MgSO4, ZnCl2, CaCl2, and FeSO4) at concentrations ranging from 0 to 50 mM were also determined. In addition, the effects of EDTA (0 to 50 mM), sodium azide (0 to 100 mM), and other potential inhibitors (phenylthiourea, cinnamic acid, and tropolone, each at a concentration of 10 mM; Sigma-Aldrich) were also examined. Additional assays with metal ions (CuSO4, MnSO4, and MgSO4) at concentrations of 0 to 50 mM were performed in order to determine their effects on the recovery of the activity inhibited by EDTA.
Spectrophotometric measurements of substrate oxidation by the purified enzyme were obtained at the optimal pH and optimal temperature by using 1-ml reaction mixtures containing the test substrates l-tyrosine (475 nm), l-3,4-dihydrophenylalanine (475 nm), tyramine (460 nm), dopamine (475 nm), protocatechuic acid (400 nm), ABTS (436 nm), 2,6-dimethoxyphenol (2,6-DMP) (468 nm), guaiacol (470 nm), and syringaldazine (525 nm) dissolved in 0.1 M McIlvaine buffer. Most of the substrates were used at a concentration of 5 mM; the only exception was syringaldazine, which was used at a concentration of 0.1 mM. For substrate specificity, enzymatic activity was expressed in absorbance units (AU), and 1 AU was defined as the amount of enzyme required to increase the absorbance at each characteristic wavelength indicated above by 1 U per min per ml. All chemicals were purchased from Sigma-Aldrich.
The UV-visible absorption spectrum of the purified laccase produced by S. cyaneus was determined at wavelengths between 700 and 200 nm at room temperature in 50 mM phosphate buffer (pH 6) by using a Beckman DU 50 spectrophotometer.
Oxidation of veratryl alcohol by the purified laccase with ABTS as the mediator.
Oxidation of veratryl alcohol (3,5-dimethoxybenzyl alcohol) by the purified enzyme in the presence of ABTS was carried out by using the following stock solutions: purified laccase (2.5 U ml−1), veratryl alcohol (5 mM), ABTS (5 mM), and McIlvaine buffer (0.1 M, pH 4.5). The standards used (each at a concentration of 1 mM) were veratryl alcohol, veratraldehyde (3,5-dimethoxybenzaldehyde), and veratric acid (3,5-dimethoxybenzoic acid). The controls contained laccase (10 μl), veratryl alcohol (50 μl), and buffer (40 μl). The samples contained laccase (10 μl), veratryl alcohol (50 μl), ABTS (30 μl), and buffer (10 μl). The samples were incubated at room temperature for 24, 36, and 48 h and 5 days in the dark. At the end of the incubation period, samples were centrifuged at 10,000 × g for 5 min. Each sample (10 μl) was analyzed by high-performance liquid chromatography (LC-9A; Shimadzu) by using a 25-cm column (Nucleosil 10-5 C18). The column was previously equilibrated with 15% (vol/vol) acetonitrile and a solution containing phosphoric acid (0.02 M) and NaOH (0.02 M) at pH 3 and was eluted at a rate of 1 ml min−1 with the following acetonitrile gradient: 15% acetonitrile (1 min), 35% acetonitrile (10 min), 70% acetonitrile (15 min), and 97% acetonitrile (16 min; maintained until 18 min). Eluted compounds were monitored at 310 nm by photodiode detection (Waters 996). Standard solutions containing different concentrations (0.1 to 10 mM) of veratryl alcohol, veratraldehyde, and veratric acid were chromatographed under the conditions described above, and calibration curves were obtained. Quantification of the compounds was carried out by integrating the area under each peak at 280 nm. All chemicals were obtained from Sigma-Aldrich.
Application of purified laccase to the biobleaching of eucalyptus kraft pulp.
Purified laccase produced by S. cyaneus was evaluated for biobleaching of eucalyptus kraft pulps by using ABTS as the mediator. The eucalyptus kraft pulp used in this assay was soaked with distilled water for 4 h at room temperature and then filtered and dried at 90°C to obtain a constant dry mass.
Enzymatic bleaching of the pulp with laccase and laccase-mediator systems was carried out in 500-ml Erlenmeyer flasks containing 7.5 g of pulp, 100 mU (16 μg) of purified laccase per g of pulp, and (in the laccase-mediator system assay) 5 mM ABTS. The volume of each assay mixture was adjusted to 100 ml with 50 mM McIlvaine buffer (pH 5) to obtain a final pulp content of 7.5% (wt/vol). The reaction mixtures were incubated at 45°C for 3 h with shaking and were periodically oxygenated. Control assay mixtures were prepared by using heat-inactivated enzyme.
Controls and pulps treated with enzyme and with the enzyme-mediator system were further extracted with 37.5 mM NaOH at 80°C for 3 h, and the kappa number (ISO-302-1981 E) was determined. After alkaline extraction, controls and treated pulps were further bleached by peroxide extraction. Peroxide bleaching of pulps was carried out in 250-ml Erlenmeyer flasks containing 2.5% (vol/vol) H2O2 (obtained from a 33% H2O2 [wt/vol] commercial solution), 0.05% (wt/vol) MgSO4, and 3.5% (wt/vol) Na2SiO3 in an appropriate volume of distilled water to obtain a final pulp content of 10% (wt/vol). The peroxide extraction was carried out at 60°C for 1 h with shaking. Finally, the kappa number and brightness (ISO 2496) were determined. All the assays were performed in triplicate.
RESULTS
Production of laccases in submerged culture.
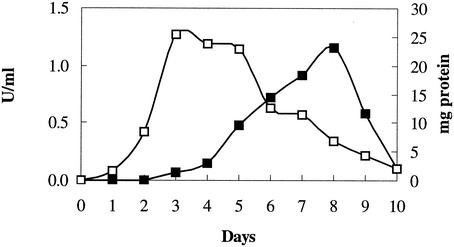
The time course for the production of extracellular laccase activity during the growth of S. cyaneus in submerged culture is shown in Fig. 1. The maximum laccase activity (1.2 U ml−1) was obtained after 8 days of growth. The maximum growth, as determined by using intracellular protein, was detected after 3 days. After 8 days the intracellular protein concentration had declined to 6.8 mg ml−1 (Fig. 1).
FIG. 1.
Growth of S. cyaneus (as determined by intracellular protein content) (□) and production of extracellular laccase activity (▪) over 10 days in basal mineral medium supplemented with soya flour (2 g per liter).
Purification of laccase.
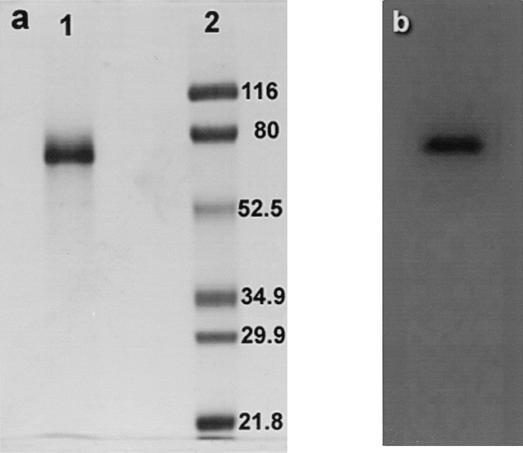
The laccase activity in the culture supernatant (100 ml) obtained after 8 days of growth (approximately 14 mg) was purified by hydrophobic interaction chromatography followed by anion-exchange chromatography as described in Materials and Methods. At the end of the purification process a purified laccase (Fig. 2) was obtained; this laccase had a specific activity of 6.3 U mg of protein−1, and the purification factor was 6.16-fold, which corresponded to a final yield of 57.8% (Table 1).
FIG. 2.
(a) SDS-PAGE of purified laccase produced by S. cyaneus stained with Coomassie brilliant blue (lane 1) and molecular mass markers (lane 2). (b) Zymogram of laccase stained with ABTS. Electrophoresis was performed after 25 μg of protein was loaded.
TABLE 1.
Purification of laccase produced by S. cyaneus
| Purification step | Total protein (mg) | Total activity (U) | Sp act (U mg of protein−1) | Purification (fold) | Yield (%) |
|---|---|---|---|---|---|
| Initial supernatant, (NH4)2SO4 | 87.67 | 88.33 | 1.02 | 1 | 100 |
| Precipitation (50%), (NH4)2SO4 | 62.76 | 83.71 | 1.33 | 1.3 | 93.7 |
| Precipitation (20%) | 45.8 | 70.84 | 1.55 | 1.52 | 79.3 |
| Econo Methyl | 21.44 | 52.53 | 2.44 | 2.39 | 58.8 |
| Econo Q | 8.19 | 51.64 | 6.3 | 6.16 | 57.8 |
Characterization of the purified laccase.
The purified laccase produced one band on an SDS-PAGE gel at a molecular mass of approximately 75 kDa (Fig. 2). The isoelectric point for this laccase was 5.6. The optimum pH for the enzyme was 4.5, and the optimum temperature for the purified laccase was 70°C. The enzyme retained up to 100% of its activity after 120 min of incubation at 40°C at pH values between 5 and 8 and lost 50% of its activity when it was incubated at pH 3 to 4. The laccase retained more than 75% of its activity after incubation for 120 min at 50°C and 60% of its activity after incubation at 60°C for 60 min at pH 4.5. The laccase activity was enhanced in the presence of CuSO4 at concentrations up to 10 mM (maximum activity at 10 mM), in the presence of MnSO4 at concentrations up to 40 mM (maximum activity at 20 mM), and in the presence of MgSO4 at concentrations up to 40 mM (maximum activity at 10 mM) and was reduced in the presence of FeSO4, ZnCl, and CaCl2 at the range of concentrations assessed (up to 50 mM).
Inhibition of laccase activity was observed in the presence of 10 mM tropolone (31.0% inhibition), phenylthiourea (95.0%), and cinnamic acid (α-phenylpropenoic acid) (75.7%). In the presence of EDTA and sodium azide, complete inhibition was detected at concentrations of 30 and 20 mM, respectively. In the case of EDTA inhibition, activity was recovered after addition of CuSO4 (100% recovery at a concentration of 40 mM), MnSO4 (150% recovery at a concentration of 35 mM), or MgSO4 (30% recovery at a concentration of 40 mM).
The kinetic constants of the purified laccase were determined under the optimal assay conditions. The Km was 0.38 mM, the Vmax for ABTS was 5.55 U mg of protein−1, and the efficiency of the enzyme against ABTS was 14.60 U mg of protein−1 mg of substrate−1.
In terms of substrate specificity, the purified laccase showed high activity against ABTS (0.763 AU 100 μg of protein−1) and 2,6-DMP (0.830 AU 100 μg of protein−1), lower activity against syringaldazine (0.03 AU 100 μg of protein−1) and protocatechuic acid (0.028 AU 100 μg of protein−1), and very low activity against tyramine (0.01 AU 100 μg of protein−1), l-3,4-dihydrophenylalanine (0.015 AU 100 μg of protein−1), dopamine (0.01 AU 100 μg of protein−1), and guaiacol (0.005 AU 100 μg of protein−1). No activity against tyrosine was detected.
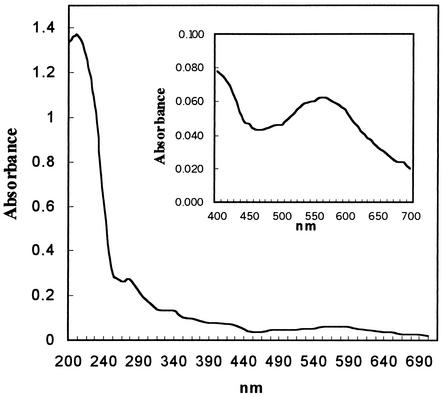
The UV-visible spectrum of the purified laccase had a peak of absorption at around 600 nm and a shoulder at around 330 to 340 nm (Fig. 3).
FIG. 3.
UV-visible spectrum of laccase produced by S. cyaneus. The spectrum was obtained by using 25 μg of purified laccase per ml.
Effect of the purified enzyme on the oxidation of veratryl alcohol when ABTS was used as the mediator.
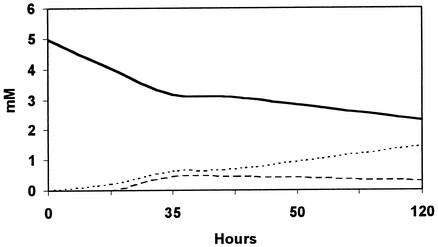
The results obtained after 5 days of incubation are shown in Fig. 4. A decrease in the relative area of veratryl alcohol was observed along with an increase in the concentration of veratraldehyde. In addition, a small increase in the veratric acid concentration was detected over the first 36 h. In all control experiments veratraldehyde and veratric acid were not detected.
FIG. 4.
Oxidation of veratryl alcohol by laccase produced by S. cyaneus in the presence of ABTS. Solid line, veratryl alcohol; dashed line, veratraldehyde; dotted line, veratric acid.
Application of purified laccase to biobleaching of eucalyptus kraft pulps.
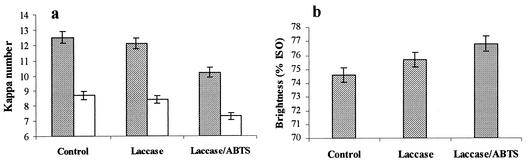
The effect of the purified laccase on biobleaching of eucalyptus kraft pulp was evaluated after the most common pulp properties, kappa number and brightness, were analyzed (Fig. 5). Following pulp treatment with laccase supplemented with ABTS and further alkaline extraction, a reduction in the kappa number of 2.3 U was observed (Fig. 5a). When laccase was used by itself (i.e., in the absence of the mediator ABTS), a reduction in the kappa number of only 0.4 U (compared with the control) was observed. In addition, laccase treatment followed by alkaline extraction yielded pulp with substantially increased brightness (1.1-U increase). Application of laccase plus ABTS as the mediator resulted in even greater increases in brightness (2.2 U compared with the control) after alkaline extraction (Fig. 5b). After H2O2 was applied to control pulp, a significant reduction in the kappa number (3.8 U) was observed. Following pulp treatment with both laccase and laccase plus ABTS in a bleaching sequence with H2O2, considerable reductions in the kappa number were observed (0.3 and 1.4 U, respectively). Increases in brightness were also observed after H2O2 was applied to the pulps treated with enzyme and with enzyme plus ABTS (1.1 and 2.2 U compared with the control) (Fig. 5b).
FIG. 5.
(a) Kappa number values of eucalyptus kraft pulp after enzymatic treatment with laccase produced by S. cyaneus followed by alkaline extraction (grey bars) and alkaline and peroxide extraction (open bars). (b) Brightness (expressed as a percentage, as determined by the International Standard Organization [ISO] test) of eucalyptus kraft pulp after enzymatic treatment with laccase produced by S. cyaneus followed by alkaline and peroxide extraction.
DISCUSSION
The present work was initiated by studying the production kinetics of laccase activity from S. cyaneus in submerged cultures by using soya medium. Although maximum growth was observed after 3 days of incubation, extracellular laccase activity was detected after 4 days of growth, and the maximum extracellular laccase activity was detected after 8 days of incubation, when the specific activity was 250 mU mg of protein−1, a value which is slightly greater than previously reported bacterial laccase activities (40, 41). This pattern shows that laccase activity can be detected once a strain has reached the stationary phase of growth and increases with cell autolysis. In general, the bacterial laccases that have been described are intracellular or cell bound. Furthermore, most of them are involved in basic cellular processes (e.g., the Azospirillum and Marinomonas enzymes and CotA from B. subtilis) (18, 19, 30). Recently, an extracytoplasmic phenol oxidase produced by the actinomycete S. griseus was described, and it has been suggested that this enzyme may be involved in the onset of morphogenesis (15). Therefore, to date, there have been no previous studies based on purified enzymes to determine the potential applications of bacterial laccases in the pulp industry, and in this paper we describe for the first time the use of a streptomycete laccase in biobleaching.
The purification procedure used for the laccase in this study resulted in sixfold purification and a yield of 58%. The molecular mass (75 kDa) and the isoelectric point (pI 5.6) reported here are different from those reported for the intracellular laccases of B. subtilis (molecular mass, 65 kDa; pI 7.7) (30) and S. griseus (molecular mass, 100 kDa) (15). However, the values which we obtained are similar to the previously described values for extracellular lignin-degrading laccases from the fungi Pleurotus ostreatus (35, 36) and Marasmius quercophilus (16).
The results which we obtained for the stability of the laccase at a high temperature and the optimum temperature can be considered industrially advantageous when they are compared with the results obtained for laccases produced by other organisms. The optimal temperature range for fungal laccases is 30 to 60°C (14, 32, 42). The enzyme activity of our purified laccase was maintained in the pH range determined for other fungal and bacterial laccases. In comparison, the optimum pH for the laccase activity from B. subtilis was reported to be pH 3 when ABTS was used as the substrate (30). However, no other comparative data were found for the stability of streptomycete laccases at different pH values and temperatures.
The effects of different metal ions on the laccase activity showed that copper, manganese, and magnesium activated the purified laccase, while ferric iron, calcium, and zinc reduced the activity. No equivalent data for bacterial laccase activity has been published. However, a copper requirement for bacterial laccase activity has been reported previously for S. griseus (15), B. subtilis, and A. lipoferum (20).
The effects of inhibitors showed that the purified laccase was sensitive to the inhibitors tropolone, phenylthiourea, and cinnamic acid. The observed responses to these inhibitors appear to be a unique feature of the purified laccase; this enzyme is similar to tyrosinases in terms of its sensitivity to tropolone and cinnamic acid (25, 47) and differs from fungal laccases (18). Nevertheless, the possibility that the behavior of phenol oxidases with different inhibitors could be affected by the conditions of the assay must be considered. The observation that EDTA completely inhibited laccase activity, which could then be recovered after addition of either copper, manganese, or magnesium, again suggests that bacterial laccases have a unique feature (12). Moreover, the enzyme was also inhibited by sodium azide, as described previously for other bacterial and fungal laccases (18, 20, 28).
Regarding substrate specificity, the laccase from S. cyaneus showed strong activity against two substrates which are considered highly specific for laccases (ABTS and 2,6-DMP) and showed much lower activity at the pH used against syringaldazine and guaiacol, which are also considered specific substrates for these enzymes (19). Nevertheless, difficulty oxidizing guaiacol has been also reported for some specific laccases (40, 44). The enzyme also showed low specificity against o-diphenolic substrates, such as protocatechuic acid, which is considered a substrate for catecholase (19). No activity against tyrosine was detectable; this is interesting because tyrosinases are the most common phenol oxidases produced by streptomycetes (26).
The UV-visible spectrum of the purified laccase was the typical spectrum of the blue laccases (with a peak at around 600 nm and a shoulder at around 330 nm). In fact, typical laccases isolated from fungi belong to the class containing the blue oxidases, which contain four copper atoms per molecule arranged in three different copper-binding sites (39, 46). Nevertheless, recent work on the laccase from B. subtilis determined that this oxidase has four copper-binding sites (22). All of these sites produce spectra with a maximum at 605 nm, which corresponds to a type 1 or blue copper atom. Type 2 copper exhibits weakly visible absorbance, and type 3 copper has two copper centers and is responsible for a shoulder at around 330 nm in the absorbance spectrum of native laccase (35, 37). A bacterial laccase, the CotA protein from B. subtilis, also showed the typical UV-visible spectrum of blue multicopper oxidases (30). The cloned gene corresponding to the multipotent phenol oxidase of M. mediterranea also had the motifs corresponding to the four copper-binding sites characteristic of most laccases (41).
The oxidation of veratryl alcohol by the purified laccase in the presence of ABTS described here is the first oxidation of a nonphenolic substrate by a purified bacterial laccase that has been reported. This activity opens up the potential for using this enzyme-mediator system in the pulp and paper industry as an alternative to fungus-based systems (7). A number of researchers have focused their efforts on description and characterization of laccases produced by lignin-degrading fungi. Although these enzymes have limited abilities to degrade lignin, in the presence of oxygen and an organic compound as an electron carrier (or mediator), they are able to delignify kraft pulps. This delignification of kraft pulps has been patented and named the Laccase Mediator System (LMS) (10). This LMS is highly selective and yields pulp with little carbohydrate loss or damage (49). The success achieved at the laboratory and pilot-plant scales (11, 34) makes this process a desirable system for industrial applications of bleaching of kraft pulps for the manufacture of paper (17).
In this work we demonstrated that laccase produced by S. cyaneus can delignify eucalyptus kraft pulp in the presence of ABTS as a mediator. The results which we obtained are comparable to those described by other workers who used different laccases produced by ligninolytic fungi and ABTS as the mediator. The level of delignification achieved with the LMS of S. cyaneus was an 18.4% reduction in the kappa number after alkaline extraction of the pulp. Taking into consideration the fact that the biobleaching experiment was carried out with 100 mU per g of pulp during a 2-h treatment, this result can be compared to the decrease in the kappa number of a softwood kraft pulp treated with 5 U of laccase produced by Trametes versicolor per g of pulp (3) and also with the decrease obtained by using 100 mU of the same laccase after 1 day of incubation (7). Moreover, the reduction in the kappa number obtained with the LMS of S. cyaneus after peroxide extraction was similar to the reduction obtained with a commercial laccase from Novo Nordisk (49). Moreover, the increase in brightness obtained after addition of hydrogen peroxide to LMS-treated pulps was significant. This result also confirms the potential application of the laccases from streptomycetes to biobleaching of kraft pulps in the presence of a synthetic mediator. Because the use of ABTS as a mediator for biobleaching of pulps is not considered cost-effective at an industrial scale, the search for natural mediators is a current research priority. The detection of low-molecular-weight aromatic compounds, such as p-hydroxybenzoic acid, after growth of streptomycetes on wheat straw (21) could open new possibilities for the use of natural mediators together with bacterial laccases for biobleaching. In fact, p-hydroxybenzoic acid has been considered a mediator as efficient as ABTS for the oxidation of polycyclic aromatic hydrocarbons (24).
In conclusion, we isolated, purified, and characterized a new extracellular bacterial laccase. Evidence for this was obtained from substrate specificity data and the kinetic, biochemical, and spectral characteristics combined with the ability of the enzyme to oxidize nonphenolic substrates in the presence of ABTS as a mediator. For the first time, the potential of laccases from streptomycetes for use in combination with mediators for biobleaching of kraft pulps has been demonstrated.
Acknowledgments
This work was supported by CICYT project BIO97-0808 and by Universidad de Alcalá project UAH2002/065. This work was aided by a fellowship to M.E.A. from the Spanish Ministry of Education.
REFERENCES
- 1.Alexandre, G., and R. Bally. 1999. Emergence of laccase-positive variant of Azospirillum lipoferum occurs via a two-step phenotypic switching process. FEMS Microbiol. Lett. 174:371-378. [DOI] [PubMed] [Google Scholar]
- 2.Alexandre, G., and I. B. Zhulin. 2000. Laccases are widespread in bacteria. Trends Biotechnol. 18:41-42. [DOI] [PubMed] [Google Scholar]
- 3.Archibald, F. S., R. Bourbonnais, L. Jurasek, M. G. Paice, and I. D. Reid. 1997. Kraft pulp bleaching and delignification by Trametes versicolor. J. Biotechnol. 53:215-236. [Google Scholar]
- 4.Bermek, H., K. C. Li, and K.-E. L. Ericksson. 1998. Laccase-less mutant of the white rot fungus Pycnoporus cinnabarinus cannot delignify kraft pulp. J. Biotechnol. 66:117-124. [Google Scholar]
- 5.Berrocal, M., J. Rodríguez, A. S. Ball, M. I. Pérez-Leblic, and M. E. Arias. 1997. Solubilisation and mineralisation of [14C]lignocellulose from wheat straw by Streptomyces cyaneus CECT 3335 during growth in solid-state fermentation. Appl. Microbiol. Biotechnol. 48:379-384. [Google Scholar]
- 6.Berrocal, M., A. S. Ball, S. Huerta, J. M. Barrasa, M. Hernández, M. I. Pérez-Leblic, and M. E. Arias. 2000. Biological upgrading of wheat straw through solid-state fermentation with Streptomyces cyaneus. Appl. Microbiol. Biotechnol. 54:764-771. [DOI] [PubMed] [Google Scholar]
- 7.Bourbonnais, R., and M. G. Paice. 1992. Demethylation and delignification of kraft pulp by Trametes versicolor laccase in the presence of 2,2′-azinobis(3-ethylbenzthiazoline-6-sulfonate). Appl. Microbiol. Biotechnol. 36:823-827. [Google Scholar]
- 8.Bourbonnais, R., and M. G. Paice. 1996. Enzymatic delignification of kraft pulp using laccase and a mediator. TAPPI (Tech. Assoc. Pulp Pap. Ind.) J. 79:199-204. [Google Scholar]
- 9.Bradford, M. M. 1976. A rapid sensitive method for quantitation of microgram quantities of proteins utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 10.Call, H.-P. December 1994. Process for modifying, breaking down or breaching lignin, materials containing lignin or like substances. PCT World patent WO 94/29510.
- 11.Call, H-P., and I. Mücke. 1997. History, overview and applications of mediated ligninolytic systems, especially laccase-mediator-systems (Lignozym-process). J. Biotechnol. 53:163-202. [Google Scholar]
- 12.Coll, P. M., J. M. Fernández-Abalos, J. R. Villanueva, R. Santamaría, and P. Pérez. 1993. Purification and characterization of a phenol oxidase (laccase) from the lignin-degrading basidiomycete PM1 (CECT 2971). Appl. Environ. Microbiol. 59:2607-2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crawford. D. L. 1978. Lignocellulose decomposition by selected Streptomyces strains. Appl. Environ. Microbiol. 35:1041-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eggert, C., U. Temp, and K. L. Ericksson. 1996. The ligninolytic system of the white rot fungus Pycnoporus cinnabarinus: purification and characterization of the laccase. Appl. Environ. Microbiol. 62:1151-1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Endo, K., K. Hosono, T. Beppu, and K. Ueda. 2002. A novel extracytoplasmatic phenol oxidase of Streptomyces: its possible involvement in the onset of morphogenesis. Microbiology 148:1767-1776. [DOI] [PubMed] [Google Scholar]
- 16.Farnet, A. M., S. Criquet, S. Tagger, G. Gil, and J. Le Petit. 2000. Purification, partial characterization, and reactivity with aromatic compounds of two laccases from Marasmius quercophilus strain 17. Can. J. Microbiol. 46:189-194. [DOI] [PubMed] [Google Scholar]
- 17.Farrel, R. L., L. Viikari, and D. Senior. 1996. Enzyme treatment of pulp, p. 363-377. In C. W. Dence and D. W. Reeve (ed.), Pulp bleaching: principles and practice. Tappi Press, Atlanta, Ga.
- 18.Faure, D., M. L. Bouillant, and R. Bally. 1995. Comparative study of substrates and inhibitors of Azospirillum lipoferum and Pyricularia oryzae laccases. Appl. Environ Microbiol. 61:1144-1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fernández, E., A. Sánchez-Amat, and F. Solano. 1999. Location and catalytic characteristics of a multipotent bacterial polyphenol oxidase. Pigm. Cell Res. 12:331-339. [DOI] [PubMed] [Google Scholar]
- 20.Givaudan, A., A. Effosse, D. Faure, P. Potier, M.-L. Bouillant, and R. Bally. 1993. Polyphenol oxidase in Azospirillum lipoferum isolated from rice rhizosphere: evidence for laccase activity in non-motile strains of Azospirillum lipoferum. FEMS Microbiol. Lett. 108:205-210. [Google Scholar]
- 21.Hernández-Coronado, M. J., M. Hernández, J. Rodríguez, and M. E. Arias. 1998. Gas chromatography/mass spectrometry as a suitable alternative technique to evaluate the ability of Streptomyces to degrade lignin from lignocellulosic residues. Rapid Commun. Mass Spectrom. 12:1744-1748. [Google Scholar]
- 22.Hullo, M.-F., I. Moszer, A. Danchin, and I. Martín-Verstraete. 2001. CotA of Bacillus subtilis is a copper-dependent laccase. J. Bacteriol. 183:5426-5430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johannes, C., A. Majcherczyk, and A. Hüttermann. 1996. Degradation of anthracene by laccase of Trametes versicolor in the presence of different mediator compounds. Appl. Microbiol. Biotechnol. 46:313-317. [DOI] [PubMed] [Google Scholar]
- 24.Johannes, C., and A. Majcherczyk. 2000. Natural mediators in the oxidation of polycyclic aromatic hydrocarbons by laccase mediator systems. Appl. Environ. Microbiol. 66:524-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kahn, V., and A. Andrawis. 1985. Inhibition of mushroom tyrosinase by tropolone. Phytochemistry 24:905-908. [Google Scholar]
- 26.Küster, E. 1976. Chromogeneity of actinomycetes, p. 43-54. In T. Arai (ed.), Actinomycetes: the boundary microorganisms. Toppan, Tokyo, Japan.
- 27.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 28.Leontievsky, A. A., N. N. Pozdnyakova, N. M. Myasoedova, and L. A. Golovleva. 1996. Comparative characterization of oxidase-1 from Panus tigrinus 8-18 and laccase from Coriolus versicolor VKM F-116. Biochemistry 61:1262-1268. [Google Scholar]
- 29.Locci, R., E. Baldacci, and B. Petrolini-Baldam. 1969. The genus Streptoverticillium. A taxonomic study. G. Microbiol. 17:1-60. [Google Scholar]
- 30.Martins, L. O., C. M. Soares, M. M. Pereira, M. Teixeira, T. Costa, G. H. Jones, and A. O. Henriques. 2002. Molecular and biochemical characterization of a highly stable bacterial laccase that occurs as a structural component of the Bacillus subtilis endospore coat. J. Biol. Chem. 277:18849-18859. [DOI] [PubMed] [Google Scholar]
- 31.Mayer, A. M., and R. C. Staples. 2002. Laccase: new functions for an old enzyme. Phytochemistry 60:551-565. [DOI] [PubMed] [Google Scholar]
- 32.Muñoz, C., F. Guillén, A. T. Martínez, and M. J. Martínez. 1997. Laccase isoenzymes of Pleurotus eryngii: characterization, catalytic properties, and participation in activation of molecular oxygen and Mn2+ oxidation. Appl. Environ. Microbiol. 63:2166-2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oriaran, T. P. H., P. Labosky, Jr., and P. R. Blankenhern. 1991. Kraft pulp and papermarking properties of Phanerochaete chrysosporium degraded-red oak. Wood Fiber Sci. 23:533-542. [Google Scholar]
- 34.Paice, M. G., R. Bourbonnais, I. D. Reid, F. S. Archibald, and L. Jurasek. 1995. Oxidative bleaching enzymes: a review. J. Pulp Pap. Sci. 21:280-284. [Google Scholar]
- 35.Palmieri, G., P. Giardina, C. Bianco, A. Scaloni, A. Capasso, and G. Sannia. 1997. A novel white laccase from Pleurotus ostreatus. J. Biol. Chem. 272:31301-31307. [DOI] [PubMed] [Google Scholar]
- 36.Palmieri, G., P. Giardina, C. Bianco, B. Fontanella, and G. Sannia. 2000. Copper induction of laccase isoenzymes in the ligninolytic fungus Pleurotus ostreatus. Appl. Environ. Microbiol. 66:920-924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Piontek, K., M. Antorini, and T. Choinowski. 2002. Crystal structure of a laccase from the fungus Trametes versicolor at 1.90-Å resolution containing a full complement of coppers. J. Biol. Chem. 227:37663-37669. [DOI] [PubMed] [Google Scholar]
- 38.Reid, I. D. 1998. Fate of residual lignin during delignification of kraft pulp by Trametes versicolor. Appl. Environ. Microbiol. 64:2117-2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reinhammar, B., and B. G. Malstrom. 1981. Blue copper-containing oxidases, p0.1-35. In L. Lontie (ed.), Copper proteins and copper enzymes. CRC Press Inc., Boca Raton, Fla.
- 40.Sánchez-Amat, A., and F. Solano. 1997. A pluripotent polyphenol oxidase from the melanogenic marine Alteromonas sp. shares catalytic capabilities of tyrosinases and laccases. Biochem. Biophys. Res. Commun. 240:787-792. [DOI] [PubMed] [Google Scholar]
- 41.Sánchez-Amat, A., P. Lucas-Elío, E. Fernández, J. C. García-Borrón, and F. Solano. 2001. Molecular cloning and functional characterization of a unique multipotent polyphenol oxidase from Marinomonas mediterranea. Biochim. Biophys. Acta 1547:104-116. [DOI] [PubMed] [Google Scholar]
- 42.Slomczynski, D., J. P. Nakas, and S. W. Tanenbaum. 1995. Production and characterization of laccase from Botrytis cinerea 61-34. Appl. Environ. Microbiol. 61:907-912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Soares, G. M. B., M. T. Pessoa de Amorim, and M. Costa-Ferreira. 2001. Use of laccase together with redox mediators to decolourize Remazol Brilliant Blue R. J. Biotechnol. 89:123-129. [DOI] [PubMed] [Google Scholar]
- 44.Solano, F., A. García, E. Pérez de Egea, and A. Sánchez-Amat. 1997. Isolation and characterization of strain MMB-1 (CECT 4803), a novel melanogenic marine bacterium. Appl. Environ. Microbiol. 63:3499-3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Solano, F., P. Lucas-Elío, D. López-Serrano, E. Fernández, and A. Sánchez-Amat. 2001. Dimethoxyphenol oxidase activity of different microbial blue multicopper proteins. FEMS Microbiol. Lett. 204:175-181. [DOI] [PubMed] [Google Scholar]
- 46.Solomon, E. I., U. M. Sundaram, and T. E. Machonkin. 1996. Multicopper oxidases and oxygenases. Chem. Rev. 96:J2563-J2605. [DOI] [PubMed] [Google Scholar]
- 47.Walker, J. R. L., and R. F. McCallion. 1980. The selective inhibition of ortho- and para-diphenol oxidases. Phytochemistry 19:373-377. [Google Scholar]
- 48.Werner, W., H. G. Rey, and H. Wielenger. 1970. Uber die Eigenschaften eines neven Chromogens für die Blutzuckerbestimmnug nach der GOD/pod-method. Anal. Chem. 252:224-228. [Google Scholar]
- 49.Wong, K. K. Y., K. D. Anderson, and R. P. Kibblewhite. 1999. Effect of the laccase-mediator system on the handsheet properties of two high kappa kraft pulps. Enzyme Microbiol. Technol. 25:125-131. [Google Scholar]
- 50.Wong, K. K. Y., J. D. Richardson, and S. D. Mannsfield. 2000. Enzymatic treatment of mechanical pulp fibers for improving papermaking properties. Biotechnol. Prog. 16:1025-1029. [DOI] [PubMed] [Google Scholar]