Abstract
Wastewater disinfection is used in many countries for reducing fecal coliform levels in effluents. Disinfection is therefore frequently used to improve recreational bathing waters which do not comply with microbiological standards. It is unknown whether human enteric viruses (which are responsible for waterborne disease) are simultaneously inactivated alongside fecal coliforms. This laboratory study focused on the chlorination of primary treated effluent with three doses (8, 16, and 30 mg/liter) of free chlorine as sodium hypochlorite. Seeding experiments showed that inactivation (>5 log10 units) of Escherichia coli and Enterococcus faecalis was rapid and complete but that there was poor inactivation (0.2 to 1.0 log10 unit) of F+-specific RNA (FRNA) bacteriophage (MS2) (a potential virus indicator) at all three doses. However, seeded poliovirus was significantly more susceptible (2.8 log10 units) to inactivation by chlorine than was the FRNA bacteriophage. To ensure that these results were not artifacts of the seeding process, comparisons were made between inactivation rates of laboratory-seeded organisms in sterilized sewage and inactivation rates of organisms occurring naturally in sewage. Multifactorial analysis of variance showed that there was no significant difference (P > 0.05) between the inactivation rates for seeded and naturally occurring FRNA bacteriophage. However, laboratory-grown poliovirus was inactivated much more rapidly than were naturally occurring, indigenous enteroviruses (P < 0.001). This may reflect differences in the way indigenous virus is presented to the disinfectant. Inactivation rates for indigenous enteroviruses were quite similar to those seen for FRNA bacteriophage at lower doses of chlorine. These results have significance for the effectiveness of chlorination as a sewage treatment process, particularly where virus contamination is of concern, and suggest that FRNA bacteriophage would be an appropriate indicator of such viral inactivation under field conditions.
Although it is under scrutiny, chlorine is still a widely used chemical disinfectant for the treatment of wastewater discharges. In the United States, many municipal discharges are treated in this way, usually after secondary treatment. In the United Kingdom, chlorination has been considered for the disinfection of primary or crude discharges to improve water quality in the vicinity of bathing waters to aid compliance with the European Community's Bathing Waters Directive (1). Prescribed water quality standards for recreational and other water uses, such as shellfish production, are typically based on contamination levels as indicated by fecal bacteria. However, bacterial indicators, such as the fecal coliforms, may not be representative of the behavior of human enteric viruses following wastewater disinfection (16, 22, 26). This may be significant, as enteric viruses are recognized to be important agents of disease following exposure to fecally polluted waters (4) or following consumption of bivalve shellfish grown in such waters (13, 20). The European Community's Bathing Waters Directive (1) includes an enterovirus parameter in recognition of the importance of viruses as disease-causing agents.
The study of the inactivation of gastroenteritis-causing viruses following wastewater disinfection is complicated by the low and variable levels of enteric virus frequently seen in effluents and by the absence of suitable viability assays for the Norwalk-like viruses, which are frequently associated with illness. Consequently, most studies have relied on the use of either laboratory-adapted strains of enterovirus, such as poliovirus, or indicator viruses, such as F+-specific RNA (FRNA) bacteriophage (8), seeded into reactor vessels in the laboratory. Previous work in this laboratory using such an approach demonstrated that the inactivation of bacterial indicators (Escherichia coli and Enterococcus faecalis) by chlorination was rapid in comparison to the inactivation of MS2 (FRNA bacteriophage type strain). Seeded poliovirus was significantly more susceptible to chlorine than MS2 but was more resistant than the bacterial indicators (26). Such studies have provided useful information on the likely virucidal efficacy of sewage disinfectants and, in particular, have highlighted the problem of differential inactivation of bacterial indicators compared with enteric viruses. In such circumstances, overreliance on fecal bacterium-based standards for water quality compliance would significantly overestimate the protection afforded against contamination with human enteric viruses.
Seeding experiments provide valuable information, in a controlled way, for the inactivation of organisms. There are, however, limitations to seeding experiments because seeded organisms may not be representative of those occurring naturally in sewage effluents. For instance, organisms naturally present in sewage may be encapsulated in cell debris, aggregated, or adhered to solid particles, which might afford protection from inactivation by a disinfectant. It is important, therefore, to compare microorganism inactivation rates in seeding experiments with those observed for naturally occurring (indigenous) organisms. In this study we compared the inactivation by chlorine disinfectant of seeded bacterial (E. coli and Enterococcus faecalis) and viral (MS2) indicators and poliovirus with that of their respective indigenous counterparts (E. coli, enterococci, FRNA bacteriophage, and enterovirus) in primary treated sewage effluent. Of particular interest was the relative inactivation of the viruses, since seeding experiments (26) suggest that FRNA bacteriophage is significantly more resistant to disinfection than enterovirus. This may limit the usefulness of the phage as a viral indicator.
MATERIALS AND METHODS
Wastewater treatment.
Samples of primary treated wastewater were obtained from a sewage treatment works at Dorchester, Dorset, United Kingdom. The mean levels of suspended solids, 5-day biochemical oxygen demand (BOD5), and ammoniacal nitrogen [NH3(N)] were 70, 68, and 12 mg/liter, respectively. On sampling, the effluent was mixed well and divided into two 3-liter samples. One sample was left untreated and stored at 4°C, while the other sample was sterilized by gamma irradiation from a cobalt 60 source (Isotron, Swindon, United Kingdom) at a dose of 25 kGy. Half of each 3-liter sample was stored at −20°C for later experiments using enterovirus. The remaining portions of each sample were stored at 4°C until E. coli, enterococci, and FRNA bacteriophage chlorine inactivation experiments were performed. These experiments were completed within 26 h of the collection of wastewater.
Effluent physicochemical properties.
BOD5, pH, suspended solids, NH3(N), and chemical oxygen demand in both irradiated and nonirradiated effluent samples were determined by Wessex Water plc (Bristol, United Kingdom) by using standard water industry methods (5). All determinations were performed on samples previously stored at −20°C.
Preparation of glassware and chlorine dose.
All glassware used had been acid washed and rinsed three times with chlorine demand-free water (Elgastat UHQ II; Elga Ltd., High Wycombe, United Kingdom) and dried. Chlorine stock solutions were prepared by adding quantities of sodium hypochlorite (14% [wt/vol]) (BDH, Poole, United Kingdom) to chlorine demand-free water. Free chlorine was detected colorimetrically at 520 nm by the Palintest N,N-diethyl-p-phenylenediamine method (5), using a PT 240 Chlorometer 1,000 (Palintest Ltd., Tyne and Wear, United Kingdom).
Bacterial indicators.
The E. coli strain (NCTC 10418) used in this study was obtained from the National Collection of Type Cultures, London, United Kingdom. Enterococcus faecalis (NCIMB 12756) was obtained from the National Collections of Industrial and Marine Bacteria Ltd., Aberdeen, United Kingdom. E. coli and Enterococcus faecalis were cultured at 37°C for 18 ± 2 h in nutrient broth (Oxoid, Basingstoke, United Kingdom). For seeding experiments, bacteria were sedimented (3,000 × g for 15 min), washed three times in phosphate-buffered saline, and filtered through a 5-μm-pore-size membrane (E. coli) or through a 1.2-μm-pore-size membrane (Enterococcus faecalis). This ensured that any clumps or aggregates of bacteria were removed. Before chlorination inactivation experiments commenced, gamma-irradiated wastewater was inoculated with bacterial indicators to give an approximate final concentration of 108 CFU/100 ml.
Cultured and indigenous E. coli organisms were detected by incubation for 24 ± 2 h at 44°C, using membrane filtration with membrane lauryl sulfate broth (Oxoid) as the selective medium. Measured volumes (usually 10 ml) of serial dilutions of wastewater were filtered through a presterilized 0.45-μm-pore-size cellulose nitrate membrane (Whatman, Kent, United Kingdom), using a membrane filtration apparatus supplied by Gelman Sciences, Ann Arbor, Mich. (All yellow colonies were considered to be presumptive E. coli.) This was verified with a proportion of colonies by subculturing to lactose peptone water (Oxoid) and to tryptone water (Oxoid) to confirm gas and indole production at 44°C after 24 ± 2 h, and the final counts were adjusted proportionally, according to the outcome of the confirmation method. Cultured Enterococcus faecalis and indigenous enterococci were detected by incubation for 48 ± 2 h at 44°C by using membrane filtration with Slanetz and Bartley agar (Oxoid) as the selective medium. All pink or red colonies of 0.5 mm were considered to be presumptive enterococci. This was verified with a proportion of colonies by subculturing on kanamycin esculin azide agar (Oxoid) and counting colonies surrounded by a black halo (ca. 1 cm in diameter) after 48 ± 2 h at 44°C, and the final results were adjusted accordingly.
FRNA bacteriophage.
The prototype FRNA bacteriophage strain MS2 (NCIMB 10108) was grown by inoculating 100 ml of tryptone-yeast extract-glucose broth (9) with E. coli (NCIMB 9481) and incubating for 18 ± 2 h at 37°C with shaking at 150 rpm. MS2 (1 ml) at a final concentration of 105 PFU/ml was added to this overnight culture and incubated for 4 to 5 h, and further growth was stopped by the addition of 10 ml of chloroform (CHCl3). Following overnight incubation at 4°C, the aqueous phase was decanted and centrifuged at 9,000 × g for 20 min, and the supernatant was dispensed in 1-ml volumes and stored at −20°C. Before use in seeding experiments, MS2 was sonicated (model VCX600 Vibra Cell; Sonica and Materials Inc.) for 30 s at 20 kHz (600 W) and filtered through a 0.2 μm-pore-size membrane (Sartorius, Gottingen, Germany) directly into the sterilized wastewater. This ensured that any clumps or aggregates of virus were removed. The final concentration of phage was approximately 105 PFU/100 ml.
Indigenous FRNA bacteriophage and MS2 were enumerated by using the genetically engineered Salmonella enterica serovar Typhimurium host WG 49 in a double agar overlay method as described by Havelaar and Hogeboom (9). Briefly, to 2.5 ml of molten 1% tryptone-yeast extract-glucose agar held at 43°C were added duplicate 1-ml volumes of undiluted or serially diluted wastewater and 1 ml of WG 49 host culture. This was mixed and poured onto a previously prepared 2% tryptone-yeast extract-glucose agar base in a 90-mm-diameter petri dish. Overlays were inverted and incubated for 18 ± 2 h at 37°C, and characteristic 1- to 2-mm-diameter plaques were counted by visual inspection.
Enterovirus.
Poliovirus type 1 (VR-192) was obtained from the American Type Culture Collection. Coxsackievirus B5 was isolated from river water by R. Morris. Both enterovirus strains were grown and titrated in BGM cells (ICN Pharmaceuticals Ltd., Basingstoke, United Kingdom). Virus for seeding experiments was prepared from infected monolayers by freeze-thaw lysis (three times) and clarified (3,000 × g for 5 min), and supernatants were stored at −70°C. Before use in seeding studies, the poliovirus suspensions were thawed, sonicated for 30 s at 20 kHz (600 W), and filtered through a 0.2 μm-pore-size membrane. This ensured that any large clumps or aggregates of virus were removed. The virus was filtered directly into the gamma-irradiated wastewater to give an approximate final concentration of 107 PFU/100 ml immediately before chlorine inactivation experiments were performed. Undiluted or serially diluted sewage samples were adsorbed onto triplicate 5-cm2 BGM cell monolayers for 1 h, overlaid with minimum essential medium (Sigma, Poole, United Kingdom) with 1% carboxymethylcellulose (Sigma), and incubated for up to 3 days in a 5% CO2 atmosphere at 37°C. Monolayers were fixed with 10% neutral buffered formalin, and plaques were visualized by staining with 0.05% (wt/vol) methyl crystal violet.
Indigenous enteroviruses were assayed by the adsorption of 16 ml of undiluted sewage in four 175-cm2 flasks (4 ml per flask) onto BGM cell monolayers for 2 h; every 20 min the flasks were shaken to redistribute the material. The cells were then overlaid with 56 ml of medium 199 (GIBCO BRL, Paisley, Scotland, United Kingdom) containing 50 IU of penicillin (Sigma) per ml, 50 μg of streptomycin (Sigma) per ml, 10% fetal bovine serum (Sigma), and 9 g of Noble agar (Difco, Detroit, Mich.) per liter and incubated in a 5% CO2 atmosphere for 6 to 7 days at 37°C. Plaques were visualized by addition of 30 ml of overlay medium containing (per liter) 222 mg of KCl (BDH, Poole, United Kingdom), 222 mg of KH2PO4 (BDH), 8.89 g of NaCl (Sigma), 1.56 g of Na2HPO4 · 2H2O (BDH), 10 g of Noble agar (Difco), and 3.3 g pf Neutral Red (Sigma).
Inactivation experiments.
Each wastewater type and the chlorine stock solutions were equilibrated to 15°C in a temperature-controlled room. Experiments were performed at the same temperature in 500-ml Pyrex beakers, each containing a magnetic stirrer rotating at 100 rpm. Wastewater (180 ml) was adjusted to pH 8 with 1 M HCl or 1 M NaOH and was mixed with 20 ml of disinfectant prepared at 10 times the required final residual. Prior to the addition of disinfectant in the seeding experiments, E. coli, Enterococcus faecalis, poliovirus, and MS2 were added to the same sample of wastewater at the same time. After the addition of disinfectant, samples of wastewater (25 ml) were taken at 5, 15, and 30 min, and the chlorine was immediately neutralized in 100 μl of 46-g/liter sodium thiosulfate. Total chlorine residuals were also measured after 5- and 30-min contact periods. Each experiment was conducted three times. A second set of three samples of wastewater was prepared with the addition of 20 ml of sterile distilled water and processed as before to act as a disinfection control. Results were plotted as mean log10 survivors (±1 standard deviation [SD]) or log10 (Ns/No), where Ns is the number of survivors after disinfection and No is the original number of organisms before disinfection, against contact time (minutes).
Statistical analyses.
The data from the inactivation studies were analyzed by using a multifactorial analysis of variance (ANOVA) with StatGraphics version 5.1. Log10-transformed data were used in the analysis. The main effects in the analysis of variance were the effects of seeded versus indigenous organisms, dose of chlorine, and contact time. Tukey's correction was performed when appropriate.
RESULTS
Sterilization of primary effluent.
Prior to chlorine inactivation experiments, samples of primary effluent were taken from Dorchester sewage treatment works and exposed to gamma irradiation at two different doses, 25 and 33 kGy. Presumptive E. coli, presumptive enterococci, FRNA bacteriophage, and enterovirus, with initial starting concentrations of 4 × 104 CFU/100 ml, 1 × 103 CFU/100 ml, 7 × 103 PFU/100 ml, and 3 × 102 PFU/100 ml, respectively, were inactivated beyond the limit of detection by both doses of gamma irradiation.
For comparative experiments, primary effluent samples were split, and half was sterilized by exposure to 25 kGy of gamma irradiation prior to seeding as described in Materials and Methods. The physicochemical properties of both irradiated and nonirradiated effluent were assayed (n = 4) to ensure that irradiation did not significantly affect sample matrix properties. The irradiated sewage had a mean BOD5 of 38 mg/liter (SD, 20 mg/liter), the pH was 7.7 (SD, 0.6), the suspended solids content was 125 mg/liter (SD, 21 mg/liter), and the NH3(N) content was 9.7 mg/liter (SD, 4 mg/liter). The nonirradiated effluent had a mean BOD5 of 39 mg/liter (SD, 18 mg/liter), a pH of 7.7 (SD, 0.4), a suspended solids content of 111 mg/liter (SD, 29 mg/liter), and an NH3(N) content of 8.7 mg/liter (SD, 3.3 mg/liter). The Mann-Whitney statistical tests showed that the physicochemical parameters of primary effluent were not altered significantly (P > 0.05 for all parameters) by sterilization with gamma irradiation.
Chlorine demand.
To establish the relationship between chlorine dose and residual for both seeded irradiated and nonirradiated effluent, total chlorine residuals and pH were monitored during all experiments at 5 and 30 min of contact time. A summary of the results is shown in Table 1. During all chlorine dose experiments, the pH rose after chlorine was added and continued to rise marginally throughout each experiment. Total chlorine residuals at 5 and 30 min fluctuated only very slightly during each experiment; some of this variation may have resulted from sewage components interfering with the N,N-diethyl-p-phenylenediamine test reagents.
TABLE 1.
pH and total chlorine residuals measured at 5 and 30 min for chlorine inactivation experiments performed on seeded effluent and untreated effluent (both fresh and frozen)a
| Chlorine dose, mg/liter (n) | Mean (SD) pH
|
Mean (SD) total chlorine residual (mg/liter) at:
|
||
|---|---|---|---|---|
| 5 min | 30 min | 5 min | 30 min | |
| 8 (4) | 8.1 (0.17) | 8.3 (0.08) | 6.8 (0.93) | 5.8 (1.25) |
| 18 (5) | 8.2 (0.19) | 8.5 (0.13) | 13.8 (1.52) | 12.6 (1.78) |
| 30 (4) | 8.3 (0.22) | 8.5 (0.10) | 25.9 (2.41) | 23.8 (1.94) |
A different batch of effluent was used for each dose experiment.
Disinfection of seeded sterilized effluent.
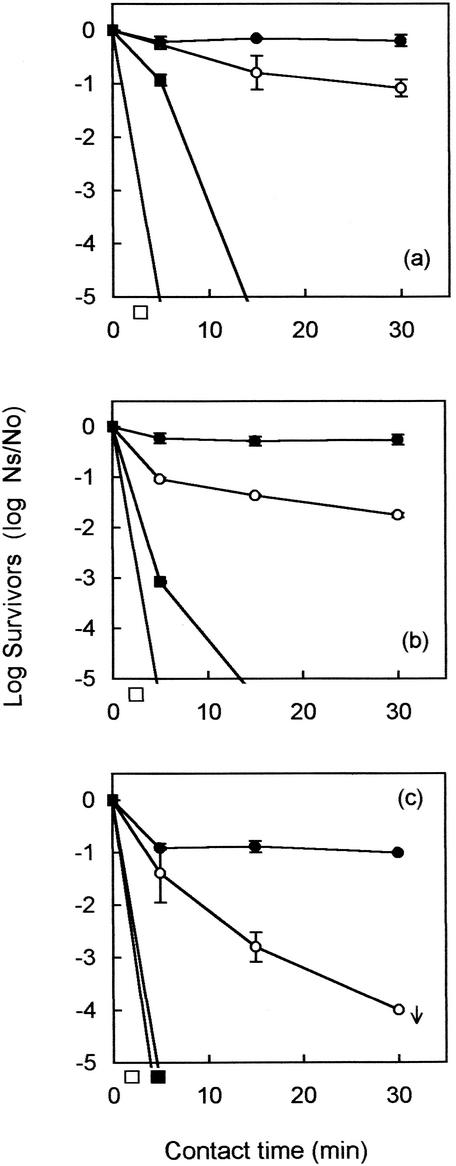
Initial studies on disinfection showed that monodispersed E. coli in sterilized primary effluent was rapidly inactivated within the first 5 min at all of the chlorine dosages used (Fig. 1). Monodispersed Enterococcus faecalis was also rapidly inactivated when 30 mg of chlorine per liter was used (Fig. 1c). At the lower dosages of 8 and 16 mg/liter, Enterococcus faecalis was marginally more resistant to chlorination than E. coli but still showed a 4-log10-unit inactivation within 15 min (Fig. 1a and b). In contrast, FRNA bacteriophage (MS2), a potential indicator for human enteric viruses, was very resistant at all three chlorine doses (Fig. 1). At the higher chlorine concentrations, there was an initial rapid inactivation of MS2 in the first 5 min followed by little or no inactivation thereafter. This probably corresponded to the initial presence of large quantities of free chlorine followed by its conversion to combined chlorine as the disinfectant reacted with the sample matrix.
FIG. 1.
Log10 survival (Ns/No) curves (±1 SD) for seeded MS2 (•), poliovirus (○), Enterococcus faecalis (▪), and E. coli (□) organisms in primary effluent dosed with 8 (a), 16 (b), and 30 (c) mg of free chlorine per liter. The arrow indicates that the result is at or near the limit of assay sensitivity.
The poliovirus seeding studies, using thawed sterilized effluent, showed the inactivation of monodispersed poliovirus with chlorine to be very dose dependent. When 30 mg of chlorine per liter was applied to seeded effluent, poliovirus was inactivated to below the detection limit within the first 15 min (Fig. 1c). However, the virucidal effectiveness of chlorination diminished as the dose was lowered. When 16 mg of chlorine per liter was used, poliovirus was inactivated by 1 log10 unit within the first 5 min. Thereafter, further inactivation was slow and incomplete, giving a final overall reduction of only 1.76 log10 units after 30 min (Fig. 1b). Similarly, the inactivation of poliovirus was poor when 8 mg of chlorine per liter was applied, with a contact time of 30 min required to achieve a 1-log10-unit reduction of virus (Fig. 1a).
Multifactorial ANOVA showed that both chlorine doses and time of exposure had significant effects on the survival of the organisms tested (P < 0.001).
Disinfection of indigenous organisms in effluent.
To determine whether seeding experiments were representative of the natural presentation of organisms within the effluent sample matrix, the experiments described above were repeated with untreated wastewater. The initial concentration of the test organisms in the effluent sample ranged between 1 × 106 and 5 × 106 CFU of E. coli per ml, between 1 × 105 and 8 × 105 CFU of enterococci per ml, between 1 × 103 and 1 × 104 PFU of FRNA bacteriophage per ml, and between 4 × 102 and 7 × 102 PFU of enterovirus per ml.
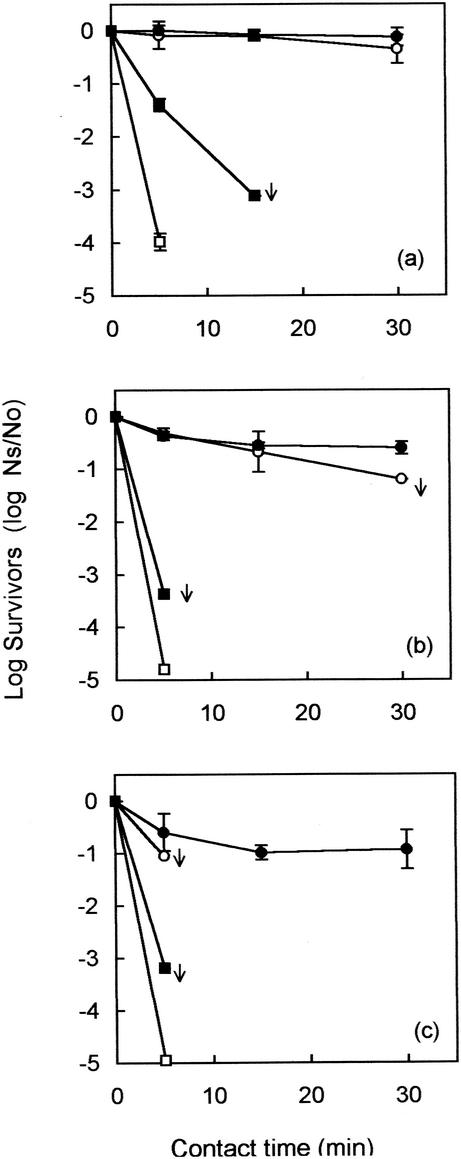
Figure 2 shows that indigenous E. coli was rapidly inactivated within the first 5 min at all chlorine dosages applied to the wastewater. These results were similar to those seen in seeding experiments (Fig. 1). Indigenous enterococci were slightly more resistant than E. coli when wastewater was dosed with 8 mg of chlorine per liter (Fig. 2a). However, when 16 and 30 mg of chlorine per liter were applied, enterococci were inactivated within the first 5 min (Fig. 2b and c).
FIG. 2.
Log10 survival (Ns/No) curves (±1 SD) for indigenous FRNA bacteriophage (•), enterovirus (○), presumptive enterococcus (▪), and presumptive E. coli (□) organisms in primary effluent dosed with 8 (a), 16 (b), and 30 (c) mg of free chlorine per liter. The arrows indicate results at or near the limit of assay sensitivity.
Indigenous FRNA bacteriophage showed sustained resistance to chlorination at all dosages used even after a 30-min contact time (Fig. 2). These levels of resistance were very similar to those seen in the seeding experiments (Fig. 1). In contrast, the sensitivity of enterovirus present naturally in wastewater was dependent on the dose of chlorine used. Chlorine inactivation experiments performed on thawed wastewater samples showed that indigenous enteroviruses were inactivated to below the detection limit within the first 5 min when a dose of 30 mg of chlorine per liter was applied (Fig. 2c). However, when 16 mg of chlorine per liter was used, a contact time of 30 min was needed to achieve a 1.2-log10-unit reduction (Fig. 2b). With 8 mg of chlorine per liter, only 0.35 log10 unit of enterovirus was inactivated after a 30-min contact period (Fig. 2a). In contrast to the behavior of enterococci, cultured monodispersed poliovirus appeared to be more sensitive to chlorination than indigenous enterovirus, particularly at the lower chlorine doses used.
Multifactorial ANOVA showed that both dose and contact time had a significant effect (P < 0.001) on survival in naturally contaminated samples. More interestingly, however, enteroviruses were significantly more resistant in naturally contaminated samples (P < 0.001) than seeded poliovirus, while the bacteriophage, natural or seeded, behaved similarly (P > 0.05). Comparisons of seeded and indigenous E. coli and enterococci were not performed because organisms often were inactivated within the first 5 min to, or near, the limit of assay sensitivity.
Comparison of poliovirus and coxsackievirus B5 inactivations.
A possible factor contributing to the apparent resistance of indigenous enteroviruses, compared with the monodispersed seeded poliovirus, could be that indigenous enteroviruses consist of a mixed population, with some strains potentially more resistant to chlorination. This hypothesis was tested by seeding sterilized primary effluent with coxsackievirus B5, an enterovirus which has been reported to be very resistant to chlorine (19). An environmental isolate which had been passaged twice in BGM cells before seeding was used in this study. The resistance of this virus was compared with the resistance of laboratory-cultured poliovirus and MS2 under the same experimental conditions.
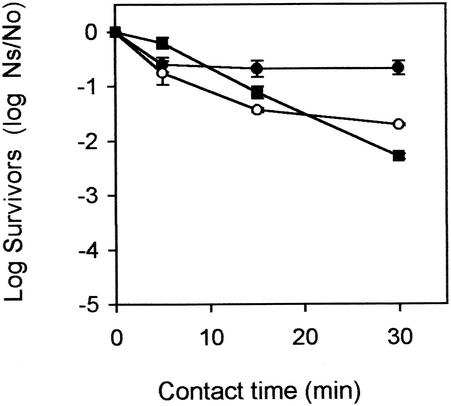
The poliovirus and coxsackievirus inactivation experiment (Fig. 3) showed that in the first 5 min coxsackievirus B5 was more resistant to chlorine than poliovirus. However, after 5 min coxsackievirus B5 continued to be inactivated, and a further reduction of 2.10 log10 units was seen. Poliovirus, in contrast, was not as easily inactivated, with a further drop of only 0.96 log10 unit. After 30 min, higher overall reductions were observed for coxsackievirus B5 than for poliovirus (Fig. 3). MS2 was included in this experiment to act as an internal control, to ensure consistency with the previous chlorination experiments. MS2 was inactivated by 0.66 log10 unit in this experiment, similar to the low levels of inactivation seen in the other seeding experiment (0.22 log10 unit) (Fig. 1b).
FIG. 3.
Log10 survival (Ns/No) curves (±1 SD) for seeded MS2 (•), coxsackievirus B5 (○), and poliovirus (▪) in primary effluent dosed with 16 mg of free chlorine per liter.
Multifactorial ANOVA showed that the survival of MS2 was significantly different from that of coxsackievirus and poliovirus (P < 0.05). In contrast, the survivals of coxsackievirus and poliovirus were similar (P > 0.05) over time at a dose of 16 mg of applied chlorine per ml.
DISCUSSION
Several studies have shown biphasic inactivation curves for microorganisms during chlorine disinfection experiments, with an initial rapid inactivation phase followed by more gradual inactivation (7, 10, 14). Biphasic curves could indicate the presence of a subpopulation of organisms more resistant to disinfection (3) or the presence of aggregated individuals more resistant to inactivation than individual organisms (25). However, perhaps more significant is the activity of chlorine species during disinfection of organic matrices. Free chlorine, a potent disinfectant, is only momentarily available before combination with ammoniacal nitrogen to form monochloramine, with reduced, but sustained, disinfecting power (10). The contributions of these two forms of chlorine may lead to a biphasic reaction curve.
In this study a variety of inactivation curves were seen. First-order kinetics were seen for laboratory-cultured and indigenous E. coli, suggesting a high sensitivity to free and combined chlorine. Enterococci were more resistant to disinfection than E. coli, particularly at the lower doses of chlorine, but were still completely inactivated within 15 min under all experimental conditions. Other workers have also observed enhanced resistance of enterococci to disinfection compared with E. coli (10, 18, 27). However, in contrast to the case for the bacterial indicators, inactivation curves for the viruses studied were essentially biphasic in nature. This was particularly noticeable for enteroviruses, where an initial rapid phase of inactivation was followed by a phase of slower inactivation, possibly due to the weaker effect of combined chlorine. Continued inactivation by combined chlorine with increasing contact time was not, however, a characteristic of inactivation curves of either laboratory-cultured or indigenous FRNA bacteriophage. Instead, inactivation occurred only in the first 5 min. These results suggest that phage is susceptible to free chlorine but resistant to chloramines. Shah and McCamish (21) demonstrated that the FRNA bacteriophage strain f2 was more resistant than poliovirus type 1 to combined chlorine. It appears, therefore, that inactivation of FRNA bacteriophage is dose, rather than residual, dependent. Similar conclusions were reached by Havelaar and Nieuwstad (10) and Sobsey et al. (24).
Many studies have compared the inactivation rates of seeded laboratory-cultured organisms in a range of effluent types. However, it is questionable how representative seeding studies are of the inactivation of organisms naturally occurring in sewage effluents. A few studies have compared the inactivation rates of indigenous organisms in primary effluent (2, 16), but we are not aware of any similar studies directly comparing the inactivation of seeded organisms with the inactivation of indigenous organisms under the same experimental conditions. Our findings demonstrate that, in general, there was little difference between the inactivation of either seeded or indigenous bacterial indicators and FRNA bacteriophage. However, there were significant differences with enteroviruses, where indigenous viruses proved to be more resistant to chlorination than seeded poliovirus. Comparison of poliovirus and coxsackievirus B5 inactivations suggested that these differences were unlikely to be associated with enterovirus strain type. These findings suggest that laboratory seeding studies may overestimate the level of human enteric virus inactivation in the field and that such seeding studies should therefore be interpreted with caution. This could be a significant finding given the importance of enteroviruses as a marker of human enteric viral inactivation during disinfection studies.
The enhanced resistance of indigenous enterovirus, compared to laboratory-cultured poliovirus, may be influenced by the way the virus is presented to the disinfectant. Other work has shown that enteric viruses in water are often embedded in or otherwise associated with suspended solids which may partially shield virus against disinfectant action (11, 12, 17, 23). This seems to be the most likely explanation for the enhanced resistance of indigenous enteroviruses to chlorination seen in this study. It is likely that indigenous bacterial indicators are also aggregated or attached to debris. However, the inactivation rates for indigenous bacteria were similar to those for the seeded bacteria; this is possibly because these bacteria were extremely sensitive to all of the doses of chlorine applied in this study, and hence aggregation offered no protection. MS2 and indigenous FRNA bacteriophage appeared to be similarly resistant to chlorination, indicating that seeding studies are representative of the behavior of phage in the environment. Possibly FRNA bacteriophage exist in sewage effluents predominantly as individual organisms, which are inherently resistant to chlorination. This would be consistent with their natural history.
Our results show that sewage-related microorganisms demonstrate different sensitivities to chlorination and that these patterns of sensitivity are dependent on the species of chlorine present, the dose of chlorine applied, and the way that the target organism is presented to the disinfectant. A wide variety of chlorine-based disinfection studies have been performed. However, conditions vary between studies, making comparison difficult, and often the goals of particular studies will differ as well (15). We present here a comprehensive set of data comparing, for the first time, inactivation of a suite of microorganisms under both laboratory and simulated field conditions. The results clearly confirm that enterococci are more resistant to chlorination than E. coli and that both were more rapidly inactivated than the viruses examined. Increased resistance of poliovirus compared to bacterial indicators has been previously reported (16, 22, 26) and is confirmed in this study, with the added observation that this may be even more apparent in field situations. FRNA bacteriophage was clearly the organism most resistant to disinfection by chlorination. The resistant nature of FRNA bacteriophage has also been recognized by other workers (6, 7,10, 27). It follows from the results described here that neither E. coli nor enterococci are adequate indicators of virus removal during sewage disinfection. In contrast, FRNA bacteriophage was more resistant to inactivation than poliovirus under both laboratory and field conditions. Unlike poliovirus, however, the phage showed similar behavior in both seeded and naturally contaminated samples. This suggests that FRNA bacteriophage would be a useful and conservative (fail-safe) model and indicator of virus inactivation during sewage chlorination.
Acknowledgments
This investigation was funded by the Ministry of Agriculture, Fisheries and Food, United Kingdom.
We thank the staff of Wessex Water for their cooperation and support during this work. We also thank Raymond Gani (Centre for Applied Microbiology and Research [CAMR]) and Steve Leach (CAMR) for performing the statistical analyses described in this study.
REFERENCES
- 1.Anonymous. 1976. Council Directive of 8th December 1975 concerning the quality of bathing water (76/160/EEC). Off. J. Eur. Communities L31:1-7.
- 2.Berg, G., D. R. Dahling, G. A. Brown, and D. Berman. 1978. Validity of fecal coliforms, total coliforms, and fecal streptococci as indicators of viruses in chlorinated primary sewage effluents. Appl. Environ. Microbiol. 36:880-884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bitton, G. 1994. Wastewater microbiology. Wiley-Liss, New York, N.Y.
- 4.Gray, J. J., J. Green, C. Gallimore, J. V. Lee, K. Neal, and D. W. G. Brown. 1997. Mixed genogroup SRSV infections among a party of canoeists exposed to contaminated recreational water. J. Med. Virol. 52:425-429. [PubMed] [Google Scholar]
- 5.Greenberg, A. E., L. S. Clesceri, and A. D. Eaton (ed.). 1992. Standard methods for the examination of water and wastewater, 18th ed. American Public Health Association, Washington, D.C.
- 6.Hajenian, H. G., and M. Butler. 1980. Inactivation of viruses in municipal effluent by chlorine. J. Hyg. Camb. 84:63-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harakeh, M., and M. Butler. 1984. Inactivation of human rotavirus, SA11 and other enteric viruses in effluent by disinfectants. J. Hyg. Camb. 93:157-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Havelaar, A. H. 1987. Bacteriophages as model organisms in water treatment. Microbiol. Sci. 4:362-364. [PubMed] [Google Scholar]
- 9.Havelaar, A. H., and W. M. Hogeboom. 1984. A method for the enumeration of male-specific bacteriophages in sewage. J. Appl. Bacteriol. 59:219-228. [DOI] [PubMed] [Google Scholar]
- 10.Havelaar, A. H., and T. J. Nieuwstad. 1985. Bacteriophages and fecal bacteria as indicators of chlorination efficiency of biologically treated wastewater. J. Water Pollut. Control Fed. 57:1084-1088. [Google Scholar]
- 11.Hejkal, T. E., F. M. Wellings, P. A. LaRock, and A. L. Lewis. 1979. Survival of poliovirus within organic solids during chlorination. Appl. Environ. Microbiol. 38:114-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hejkal, T. W., F. M. Wellings, A. L. Lewis, and P. A. LaRock. 1981. Distribution of viruses associated with particles in wastewater. Appl. Environ. Microbiol. 41:628-634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jaykus, L. A., M. T. Hemard, and M. D. Sobsey. 1994. Human enteric pathogenic viruses, p. 92-153. In C. R. Hackney and M. D. Pierson (ed.), Environmental indicators and shellfish safety. Chapman and Hall, London, United Kingdom.
- 14.Longley, K. E., B. E. Moore, and C. A. Sorber. 1980. Comparison of chlorine and chlorine dioxide as disinfectants. J. Water Pollut. Control Fed. 52:2098-2105. [PubMed] [Google Scholar]
- 15.Mariam, T. W., and D. O. Cliver. 2000. Small round coliphages as surrogates for human viruses in process assessment. Diary Food Environ. San. 20:684-689. [Google Scholar]
- 16.Morris, R. 1993. Reduction of microbial levels in sewage effluents using chlorine and peracetic acid disinfectants. Water Sci. Technol. 27:387-393. [Google Scholar]
- 17.Narkis, N., R. Armon, R. Offer, F. Orshansky, and E. Friedland. 1995. Effect of suspended solids on wastewater disinfection efficiency by chlorine dioxide. Water Res. 29:227-236. [Google Scholar]
- 18.Narkis, N., and Y. Kott. 1992. Comparison between chlorine dioxide and chlorine for use as a disinfectant of wastewater effluents. Water Sci. Technol. 26:1483-1492. [Google Scholar]
- 19.Payment, P., M. Tremblay, and M. Trudel. 1985. Relative resistance to chlorine of poliovirus and coxsackievirus isolates from environmental sources and drinking water. Appl. Environ. Microbiol. 49:981-983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rippey, S. R. 1994. Infectious diseases associated with molluscan shellfish consumption. Clin. Microbiol. Rev. 7:419-425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shah, P. C., and J. McCamish. 1972. Relative chlorine resistance of poliovirus I and coliphages f2 and T2 in water. Appl. Microbiol. 24:658-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sobsey, M. D. 1989. Inactivation of health-related microorganisms in water by disinfection processes. Water. Sci. Technol. 21:179-196. [Google Scholar]
- 23.Sobsey, M. D., T. Fuji, and R. M. Hall. 1991. Inactivation of cell-associated and dispersed hepatitis A virus in water. J. Am. Water Works Assoc. 11:64-67.
- 24.Sobsey, M. D., T. Fuji, and P. A. Shields. 1988. Inactivation of hepatitis A virus and model viruses in water by free chlorine and monochloramine. Water Sci. Technol. 20:385-391. [Google Scholar]
- 25.Stagg, C. H. 1982. Evaluating chemical disinfectants for virucidal activity, p. 331-345. In C. P. Gerba and S. M. Goyal (ed.), Methods in environmental virology. Marcel Dekker, New York, N.Y.
- 26.Tree, J. A., M. R. Adams, and D. N. Lees. 1997. Virus inactivation during disinfection of wastewater by chlorination and UV irradiation and the efficacy of F+ bacteriophage as a viral indicator. Water Sci. Technol. 35:227-232. [Google Scholar]
- 27.Tyrrell, S. A., S. R. Rippey, and W. D. Watkins. 1995. Inactivation of bacterial and viral indicators in secondary sewage effluents, using chlorine and ozone. Water Res. 29:2483-2490. [Google Scholar]