Abstract
Seawater used in mariculture has been suspected of being a potential source of infection. In this study, the lethal effects of low-amperage electric treatment on microorganisms were examined in natural seawater and in seawater inoculated with Vibrio parahaemolyticus. In both cases, bacteria including V. parahaemolyticus in seawater were completely eliminated in 100 ms by a 0.5-A, 12-V direct current. Electron microscopic investigation of the electrically treated bacteria revealed substantial structural damage at the cellular level. In conclusion, our results indicate that low-amperage electric treatment is effective for rapid inactivation of microorganisms in seawater.
The prevalence and distribution of bacteria such as Vibrio species in aquatic environments are of great public health concern because Vibrio spp. are pathogenic for both humans and animals (18). Recent outbreaks of Vibrio parahaemolyticus in the United States have been associated with eating behavior such as the consumption of raw or undercooked seafood, including fish and shellfish (1, 4, 9). Hence, seawater used for the cultivation and washing of fish and shellfish is another potential source of infection. However, conventional techniques of water sterilization are typically unsuitable for seawater. Antibiotics, for example, have come to be commonly used to prevent diseases due to seafood in fishery and culture, but chemical disinfectants may be toxic, deteriorate with an abnormal smell, and cause increased resistance to antibiotics (8). Heat sterilization is not suitable for large-scale treatment and culturing of fish (12). Ozone and UV light are efficient and produce harmless derivatives but are more costly (3, 5, 19). Recently, the use of pulsed electric fields, which are nonthermal and nonchemical, has been researched for sterilization of water (13, 16, 21, 22). However, the industrial plant required for such treatment represents a major investment for manufacturers because of the limited application of high-voltage electric fields. We also have studied an electric sterilization method for seawater that is based on low-amperage direct-current transfer between bacterial cells and electrodes rather than on the generation of toxic substrates such as H2O2 (10). In contrast to a pulsed electric field, the method described here uses a low-intensity electric current and is therefore potentially much simpler and cheaper to implement. In the present study, the sterilizing effect of low-amperage electric treatment for various times was studied with natural seawater and seawater inoculated with V. parahaemolyticus. Because V. parahaemolyticus is known to cause gastrointestinal illness in humans, it seemed to be suitable as our experimental model.
The natural seawater used in this study was collected in the seashore of In-cheon in Korea. V. parahaemolyticus (ATCC 17802; Shirasu food-poisoning isolate) was grown in nutrient broth (Difco Laboratories, Detroit, Mich.) containing 3% (wt/vol) NaCl at 37°C. At the end of the exponential growth phase, the cells were resuspended in filtered seawater to a density of 104/ml before transfer to an electrolysis vessel containing two platinum electrodes. The electrolysis vessel used in this study, an acrylic resin cylinder with two internally attached platinum electrodes immersed in seawater, was made as a small-batch treatment prototype. The two electrodes, 10 mm wide and 80 mm long, were 10 mm apart from each other and connected to a DC power supply (model 525C; Metronix Corp., Tokyo, Japan). Electric treatments were carried out in triplicate with 5 ml of seawater, and the results shown are expressed as the mean ± the standard deviation. The voltage for these treatments was set to 12 V, and the current, in the range of 1.55 mA to 2 A, was applied for 1.55 to 2,000 ms. Following electric treatment, the treated seawater was spread on nutrient agar (Difco) plates containing 3% NaCl and the plates were incubated at 37°C for 48 h. CFU counting determined the number of viable microorganisms in the treated seawater. The detection limit of this procedure was 10 CFU/ml.
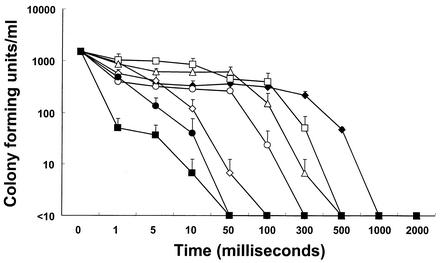
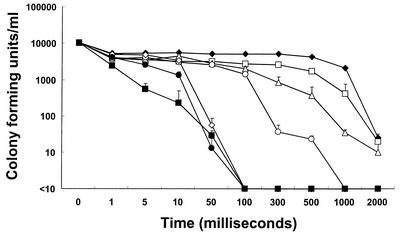
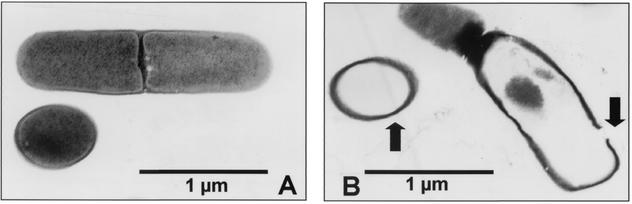
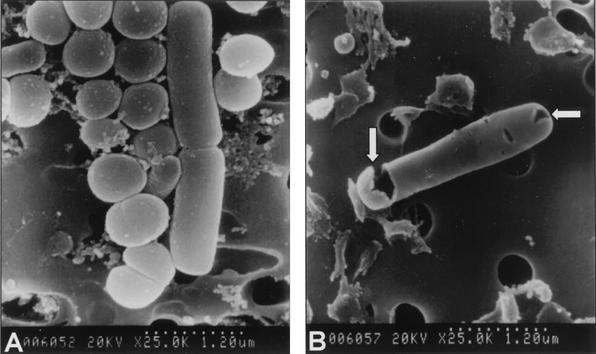
As shown in Fig. 1, the population of microorganisms in natural seawater decreased proportionally to the current duration and intensity. Under these conditions, the seawater microflora was entirely inactivated within 1 s by all of the current settings used. At a current of 1∼2 A, it took at least 50 ms for microorganisms to be fully inactivated. In the case of seawater inoculated with V. parahaemolyticus, the sterilizing power was diminished under the same conditions (Fig. 2). Even after 2 s of treatment at current settings of 1.55, 10, and 50 mA, V. parahaemolyticus in seawater was not completely inactivated. However, at 0.5∼2 A, all V. parahaemolyticus bacteria were inactivated within 100 ms. In this study, it was found that electric current intensity and duration significantly affected the inactivation of microorganisms in seawater. It seems that the differences in duration and intensity between the two experimental conditions were due to the dissimilarity in the population and kind of microorganisms in untreated seawater. No temperature change could be detected through the whole range of experimental settings used, even at 2 A and 2 s (data not shown). The impact of low-amperage electric current on the viability of microorganisms in seawater was confirmed by transmission electron microscopy (TEM) and scanning electron microscopy. Electron microscopic investigation of electrically treated microorganisms revealed substantial structural damage at the cellular level and irreversible cell membrane rupture at a number of locations with the apparent leakage of intracellular contents (Fig. 3 and 4). Although these results have significant implications, the underlying mechanisms of microbial inactivation remain to be fully elucidated.
FIG. 1.
Effects of current intensity and duration on the viability of microorganisms in natural seawater. The detection limit was 10 CFU/ml. Symbols: ♦, 1.55 mA; □, 10 mA; ▵, 50 mA; ○, 100 mA; ⋄, 500 mA; •, 1 A; ▪, 2 A. The inocula contained 1.0 × 103 cells/ml. The results shown are mean data from three experiments, and error bars indicate standard deviations.
FIG. 2.
Effect of current intensity and duration on the viability of microorganisms in seawater inoculated with V. parahaemolyticus. The detection limit is 10 CFU/ml. Symbols: ♦, 1.55 mA; □, 10 mA; ▵, 50 mA; ○, 100 mA; ⋄, 500 mA; •, 1 A; ▪, 2 A. The inocula contained 1.0 × 104 cells/ml. The results shown are mean data from three experiments, and error bars indicate standard deviations.
FIG. 3.
TEM of untreated (A) and electrically treated (B) cells in seawater (magnification, ×57,000). The TEM results revealed clear changes in the structural integrity of the cells. Cell membrane rupture and loss of intracellular contents are indicated by the arrows. These images are results of seawater treatment with 12 V and 1 A for 1 s.
FIG. 4.
Scanning electron microscopy of untreated (A) and treated (B) cells in seawater (magnification, ×25,000). Arrows indicate some pores on the membranes. These images are results of seawater treatment with 12 V and 1 A for 1 s.
There is general agreement that very little is known about what really occurs in the cell and its membranes at the molecular level. The mechanism of electric current activity may include disruption of bacterial membrane integrity or electrolysis of molecules on the cell surface (10). Among several theoretical models, one model considers the membrane a viscoelastic fluid so that the membrane may rupture because of electric stress (6). When a voltage is applied, it increases the energy of the membrane such that an increase in membrane pore size takes place up to a transition to hydrophilic pores, where free diffusion may occur (20). Another hypothesis is called dielectric breakdown (23). Because of the attraction of opposite charges induced on the inner and outer surfaces of the cell membrane, compression pressure occurs, resulting in a decrease in membrane thickness. When thinning of the membrane, which is considered a homogeneous solid, is too great, an irreversible rupture take place. The most widely accepted model is that of severe electroporation (i.e., the formation of pores in cell membranes by the action of high-voltage electric fields), where local instabilities in the membranes of treated microorganisms are formed by electromechanical compression and electrical field-induced tension due to the applied voltage (24). If critical electrical field strength is exceeded, the membrane is permeabilized by pore formation. This permeabilization can be reversible or irreversible, depending on the electrical field strength, treatment time, cell size, membrane surface charge, cytoplasm, and suspending liquid medium (11). It is generally thought that the critical membrane potential induced by electric fields causes microbial inactivation and is about 1 V. At this level, it is thought that the permeability of the membrane increases such that cell death occurs (17).
The highly lethal effects observed in this study are applicable only to specific conditions such as seawater because standard seawater contains electrolytic materials at a level of more than 35,000 ppm (2). It has been suggested that the greater the concentration of dissolved salts in the extracellular medium is, the greater is the yield of electropermeated cells (14, 15). Hence, this study has demonstrated much greater efficacy than that of Guillou and El Murr under similar current and voltage conditions (7). Because Guillou and El Murr used not bacteria but the yeast Saccharomyces cerevisiae and not seawater but phosphate buffer, the type of cellular membrane and the amount of electrolytic materials probably caused the difference in sterilizing power. Consequently, this method is distinct from other methods because it produces lethal effects on bacteria in seawater with a low-amperage electric current within a few seconds. Therefore, this low-amperage electric treatment, which prevents the infection of seafood and resolves the resistance problem arising from the use of antibiotics, can be utilized in practical industrial applications as well.
REFERENCES
- 1.Bean, N. H., E. K. Maloney, M. E. Potter, P. Korazemo, B. Ray, J. P. Taylor, S. Seigler, and J. Snowden. 1998. Crayfish: a newly recognized vehicle for Vibrio infections. Epidemiol. Infect. 121:269-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bearman, G. 1989. Ocean chemistry and deep-sea sediments. Pergamon Press, Sydney, Australia.
- 3.Blatchley, E. R., III, N. Dumoutier, T. N. Halaby, Y. Levi, and J. M. Laine. 2001. Bacterial responses to ultraviolet irradiation. Water Sci. Technol. 43:179-186. [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. 1998. Outbreak of Vibrio parahaemolyticus infections associated with eating raw oysters—Pacific Northwest, 1997. Morb. Mortal. Wkly. Rep. 47:457-462. [PubMed] [Google Scholar]
- 5.Christopher, M., and L. Richard. 1995. Oxidation behavior of aqueous contaminants in the presence of hydrogen peroxide and filter media. J. Hazard. Mater. 41:105-116. [Google Scholar]
- 6.Dimitrov, D. S., and R. K. Jain. 1984. Membrane stability. Biochim. Biophys. Acta 779:437-468. [DOI] [PubMed] [Google Scholar]
- 7.Guillou. S., and N. El Murr. 2002. Inactivation of Saccharomyces cerevisiae in solution by low-amperage electric treatment. J. Appl. Microbiol. 92:860-865. [DOI] [PubMed] [Google Scholar]
- 8.Hatha, A. A., and P. Lakshmanaperumalsamy. 1995. Antibiotic resistance of Salmonella strains isolated from fish and crustaceans. Lett. Appl. Microbiol. 21:47-49. [DOI] [PubMed] [Google Scholar]
- 9.Kaysner, C. A., C. Abeyta, R. F. Stott, M. H. Krane, and M. M. Wekel. 1990. Enumeration of Vibrio species, including V. cholerae, from samples of an oyster growing area, Grays Harbor, Washington. J. Food. Prot. 53:300-301. [DOI] [PubMed] [Google Scholar]
- 10.Liu, W. K., M. R. W. Brown, and T. S. J. Elliott. 1997. Mechanisms of the bactericidal activity of low amperage electric current. J. Antimicrob. Chemother. 39:687-695. [DOI] [PubMed] [Google Scholar]
- 11.Lojewska, Z., D. L. Farkas, B. Ehrenberg, and L. M. Loew. 1989. Analysis of the effect of medium and membrane conductance on the amplitude and kinetics of membrane potentials induced by externally applied electric fields. Biophys. J. 56:121-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mann, A., M. Knifer, and H. Leuenberger. 2001. Thermal sterilization of heat-sensitive products using high-temperature short-time sterilization. J. Pharm. Sci. 90:275-287. [DOI] [PubMed] [Google Scholar]
- 13.Manolache, S., E. B. Somers, A. C. Wong, V. Shamamian, and F. Denes. 2001. Dense medium plasma environments: a new approach for the disinfection of water. Environ. Sci. Technol. 35:3780-3785. [DOI] [PubMed] [Google Scholar]
- 14.Muraji, M., W. Tatebe, and H. Berg. 1998. The influence of extracellular alkali and alkaline-earth ions on electropermeation of Saccharomyces cerevisiae. Bioelectrochem. Bioenerg. 46:293-295. [Google Scholar]
- 15.Rols, M. P., and J. Teissié. 1990. Implications of membrane interface structural forces in electropermeabilization and electrofusion. Bioelectrochem. Bioenerg. 24:101-111. [Google Scholar]
- 16.Rowan, N. J., S. J. Macgregor, J. G. Anderson, D. Cameron, and O. Farish. 2001. Inactivation of Mycobacterium paratuberculosis by pulsed electric fields. Appl. Environ. Microbiol. 67:2833-2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rowan, N. J., Scott J. Macgregor, J. G. Anderson, D. Cameron, and O. Farish. 2000. Pulsed electric field inactivation of diarrhoeagenic Bacillus cereus through irreversible electroporation. Lett. Appl. Microbiol. 31:110-114. [DOI] [PubMed] [Google Scholar]
- 18.Sakazaki, R., and T. Shimada. 1982. Vibrio species as causative agents of food-borne infection. Dev. Food Microbiol. 2:123-151. [Google Scholar]
- 19.Smith, R. J., S. C Kehoe, K. G. Mcguigan, and M. R. Barer. 2000. Effect of simulated solar disinfection of water on infectivity of Salmonella typhimurium. Lett. Appl. Microbiol. 31:284-288. [DOI] [PubMed] [Google Scholar]
- 20.Sugar, I. P., and E. Neumann. 1984. Stochastic model for electric field-induced membrane pores—electroporation. Biophys. Chem. 19:211-225. [DOI] [PubMed] [Google Scholar]
- 21.Wouters, P. C., N. Dutreux, J. P. P. Smelt, and H. L. M. Lelieveld. 1999. Effects of pulsed electric fields on inactivation kinetics of Listeria innocua. Appl. Microbiol. Biotechnol. 67:3092-3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wouters, P. C., A. P. Bos, and J. Ueckert. 2001. Membrane permeabilization in relation to inactivation kinetics of Lactobacillus species due to pulsed electric fields. Appl. Microbiol. Biotechnol. 65:5364-5371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zimmerman, U., G. Pilwat, and F. Riemann. 1974. Dielectric breakdown of cell membranes. Biophys. J. 14:881-899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zimmermann, U. 1986. Electrical breakdown, electropermeabilization and electrofusion. Rev. Physiol. Biochem. Pharmacol. 105:175-256. [PubMed] [Google Scholar]