Abstract
The tumor suppressor Smad4/Dpc4 is a transcription activator that binds specific DNA sequences and whose nuclear localization is induced after exposure to type β transforming growth factor-like cytokines. We explored an inducible system in which Smad4 protein is activated by translocation to the nucleus when cell lines that stably express wild-type or mutant Smad4 proteins fused to a murine estrogen receptor domain are treated with 4-hydroxytamoxifen. This induced Smad4-mediated transcriptional activation and a decrease in growth rate, attributable to a cell cycle arrest at the G1 phase and an induction of apoptosis. A tumor-derived mutation (Arg-100 → Thr) affecting a residue critical for DNA-binding demonstrated an “oncogenic” phenotype, having decreases in both the G1 fraction and apoptosis and, consequently, an augmentation of population growth. This model should be useful in the exploration and control of components that lie further downstream in the Smad4 tumor-suppressor pathway.
Keywords: pancreatic cancer, type β transforming growth factor, signaling pathway, DNA-binding protein, nuclear translocation
SMAD4/DPC4/MADH4 belongs to a family of human SMAD genes (reviewed in ref. 1). The structural homologies are clustered at the N-terminal Mad homology 1 (MH1) domain and the C-terminal MH2 protein domain. A significant structural feature that distinguishes Smad4 from other Smads is the lack in Smad4 of the SSXS motif at the tail of the MH2 domain terminal that can be phosphorylated by the cognate receptor kinases (2–6). Based on biochemical studies, Smad4 is thought to act as a common Smad protein that couples the pathway-restricted Smads, those activated by TGF-β-like ligands, with downstream transcriptional events (2, 7). Phosphorylation of the pathway-restricted Smads is accompanied by their heterooligomerization with Smad4, which accompanies their transport into the nucleus (4, 7–11). This nuclear translocation of Smad4 is correlated with its activation of gene transcription. In the nucleus, Smad4 binds specific short sequences of DNA [Smad-binding elements (SBE)] and functions as a transcription activator (9, 12–14).
Although the biochemical properties of Smad proteins have been intensely studied, the tumor-suppressor pathway of the SMAD4 gene remains unclear. The SMAD4 gene is inactivated frequently in pancreatic (15, 16), biliary (15, 17), and colorectal (15, 18, 19) tumors and less frequently in other tumor types (16). The TGF-β pathways are in part mediated by Smad4 (7, 20), but some human colorectal and pancreatic cancers suffer inactivation of both the SMAD4 and TGF-β receptor genes (ref. 21; L. Meyeroff, W. Grandy, S. Thiagalingam, B. Vogelstein, and S. Markowitz, personal communication). These findings specifically raise interest in the evaluation of suppressive roles for Smad4 that may be independent of TGF-β signaling.
Tumor-derived SMAD4 mutations consistently abrogate Smad4-inducible gene activation by at least three mechanisms: missense mutations in the MH1 domain abolish binding to the SBE, missense mutations in the MH2 domain prevent Smad4 nuclear translocation, and truncation mutations in the MH2 domain ablate the transactivation ability (22). The universally null function of mutant Smad4 in SBE-mediated transcription activation strongly indicates that Smad4-inducible gene activation is the underlying mechanism for its tumor-suppressor functions, although direct genetic targets for these Smad4 functions remain unknown. The phenotypic changes induced by Smad4 are also largely undefined. Although some of these questions have been addressed by murine knock-out studies, the SMAD4-null mouse is early embryonic lethal (23, 24). The replacement of the SMAD4 gene into SMAD4-null cells represents another attractive approach. Most studies of this type have used transient protein overexpression that precludes a systematic examination of the phenotypic changes mediated by Smad4. In the few studies of stable integration of the wild-type SMAD4 gene, constitutive effects appear to be generated, perhaps initiated by an autocrine or paracrine loop (25, 26). As a result, only few clones survive, and they maintain a much slower growth rate compared with control cells having integrated a mutant SMAD4 gene (J.L.D. and S.E.K., unpublished data).
It appeared that some of these technical limitations might be overcome with a system that sequesters Smad4 protein in the cytoplasm. Therefore, we applied an established modified mouse estrogen receptor (MER) model (27) that has been successfully used in the functional studies of nuclear factors such as c-Myc (28), c-Raf-1 (29), and p53 (30) proteins. We previously used this system to control ligand-initiated signaling as detected by reporter gene activation and have proposed the direct control of Smad4 nuclear localization as a potential therapeutic strategy (22). Here we present our evaluation of some general biological effects with this model, our finding that Smad4 regulates both cell cycle progression and apoptosis, and related properties of a Smad4 mutant.
MATERIALS AND METHODS
Cell Culture and Generation of Stably Transfected Clones.
Constructs used in this study have been reported (22). Wild-type and mutant SMAD4-coding sequences fused 3′ to MER ligand-binding domain (27) were constructed in pcDNA3.1 (Smad4-MER or 100T-Smad4-MER, respectively; pcDNA3.1 from Invitrogen). The SMAD4-null breast cancer cell line MDA-MB-468 (ATCC) was transfected with the above plasmids and FuGENE6 reagent (Boehringer Mannheim) and selected with G418 (350 μg/ml, active concentrations, from Life Technologies, Gaithersburg, MD). G418-resistent clones were screened for protein expression by immunoblot analysis (see below).
Reporter Assays.
Stable cell lines were plated on six-well cluster dishes and transfected with 0.5 μg of 6SBE-Luc (22), which contains Smad4 consensus palindromic binding sites, 6MBE-Luc, which contains the mutant form of SBE, or a TGF-β-responsive reporter, 3TP-lux (31). In all experiments, 0.5 μg of β-galactosidase expression vector pCMVβ (CLONTECH) was cotransfected for normalization of transfection efficiency. Transfected cells were then treated with 100 nM 4-hydroxytamoxifen (4-OHT, Sigma), 1 ng/ml TGF-β1 (R&D Systems), or vehicle. Reporter assays were performed as described (22). Briefly, 20 h after ligand treatment, cells were harvested for β-galactosidase and luciferase reporter assays, according to the manufacturer’s instructions (Promega). Luciferase activities were normalized with β-galactosidase. β-Galactosidase activities remained stable among all transfection experiments.
Cell Counting and Cell Cycle Analysis.
Cells stably expressing MER fusion proteins were plated in six-well cluster dishes at a density of 2 × 105 cells per plate. After the cells had incubated overnight, media were replaced with 10% fetal bovine serum in the presence of 100 nM 4-OHT or vehicle. After 1, 2, and 4 days of incubation, cells were trypsinized, centrifuged, and resuspended in PBS. Cell numbers were determined by hemocytometer.
Flow cytometry was used to determine the cell cycle distributions. After the 4-OHT treatment, cells were trypsinized and washed with ice-cold PBS. Cell suspensions were then fixed dropwise in ice-cold 70% ethanol. Fixed cells were subsequently stained with 10 μg/ml propidium iodide containing 100 μg/ml RNase A and analyzed by flow cytometry for DNA content. Ten thousand forward scatter gated events were collected for each sample.
TUNEL (Terminal Deoxynucleotidyltransferase-Mediated dUTP Nick End Labeling) Assays.
Stable clones were plated on 35-mm culture dishes at a density of 5 × 105 cells per dish. After an overnight incubation, cells were treated with 100 nM 4-OHT or vehicle. In some experiments, membrane-permeant caspase inhibitor I [Z-VAD-FMK, Z-Val-Ala-Asp(OMe)-Ch2F] or caspase-3 inhibitor II [Z-DEVD-FMK, Z-Asp(OMe)-Glu(OMe)-Val-Asp(OMe)-Ch2F] (both from Calbiochem-Novabiochem) were added to the media 1 h before the 4-OHT treatment. After 48 h of 4-OHT treatment, the floating cells were first collected, and the adhering cells were then harvested by trypsinization. The Apo-BrdU kit (PharMingen) was used for the TUNEL assays, following the manufacturer’s instructions. Briefly, cells were fixed with 1% paraformaldehyde and further permeablized with 70% ethanol. Fragmented DNA ends, such as present in apoptotic cells, were labeled with dUTP-BrdUrd in a reaction containing terminal deoxynucleotide transferase and detected with fluorescein isothiocyanate-labeled monoclonal antibody against BrdUrd. Cells were then stained with propidium iodide solution that contained RNase A. Green fluorescein isothiocyanate emissions were acquired on a log scale, and red propidium iodide florescence was acquired on a linear scale. Ten thousand cells were analyzed with a Coulter Pics V flow cytometer (Coulter), and data were analyzed with multiparameter data acquisition and display system-86, version 2.0, software (Coulter). A window (identical for treated and untreated pairs) was selected to separate the “labeled” (inside, apoptotic) and “nonlabeled” (outside) cells. A subset (0.5–2%) of labeled cells was detectable in the absence of 4-OHT. Baseline variability among experiments, reflecting labeling efficiencies, initial populations, and choice of apoptotic window, was obviated by analysis of 4-OHT-induced changes as normalized to the untreated group (fold changes). A trapezoidal window was used to reflect the higher apoptotic labeling in G2/M phase cells having a higher DNA content.
Gene Expression Analysis.
Clones stably expressing Smad4–MER fusion proteins were screened by immunoblot. Total cellular protein was harvested in Laemmli buffer (without β-mercaptoethanol), and protein concentrations were determined with bicinchoninic acid reagents (Pierce). Total protein (20 μg) were separated by SDS/PAGE (10% gel) and detected by polyclonal antiserum against the estrogen receptor ligand-binding domain (working dilution 1:200, Santa Cruz Biotechnology).
Immunodetection of hyper- or hypophosphorylated Rb protein was performed with the monoclonal antibody recognizing both forms of human Rb (clone G3–245, from PharMingen). Cells were trypsinized and disrupted in lysis buffer [50 mM Tris⋅HCl, pH 7.5/250 mM NaCl/1% Nonidet P-40/1 mM EDTA/1 mM phenylmethanesulfonyl fluoride/1× protease inhibitor mixture (Boehringer Mannheim)]. After 10 min of incubation, the lysates were centrifuged and the supernatants collected. Protein extracts (25 μg total) were separated by SDS/PAGE (7% gel) and detected by the Rb antibody (2 μg/ml). Blots were incubated with a horseradish peroxidase-conjugated secondary antibody (Pierce). Detection was afforded by SuperSignal substrates (Pierce).
RESULTS
Stable Expression of Functional Smad4–MER.
With a transient transfection model, previously we controlled the nuclear localization of wild-type Smad4 by use of a fusion of Smad4 to a modular domain (a modified MER ligand-binding domain) and detected the consequent Smad4 transactivation function (22). We generated breast cancer MDA-MB-468 lines that stably expressed wild-type (Smad4–MER) or mutant (100T–Smad4–MER) Smad4. As a control, MER-expressing clones were also prepared. Six, five, and six drug-resistant clones were selected from the Smad4–MER, MER, and 100T–Smad4–MER cultures, respectively. Clones were verified by immunoblot analysis with a polyclonal antibody that recognized the ligand-binding domain of MER. The expression of intact protein from four wild-type Smad4–MER, four MER, and three mutant Smad4–MER clones was verified (Fig. 1A). No full-length endogenous estrogen receptor could be detected in the clones by immunoblotting, which is in agreement with a previous report that MDA-MB-468 cells are estrogen receptor negative (32). No difference in clonal outgrowth was observed in the absence of 4-OHT, with regard to time of clone appearance or the number of clones attained. This is in stark contrast to wild-type SMAD4 stably transfected cells (ref. 26; J.L.D. and S.E.K., unpublished data) in which a high selective pressure against wild-type Smad4 was seen when its nuclear localization was unregulated.
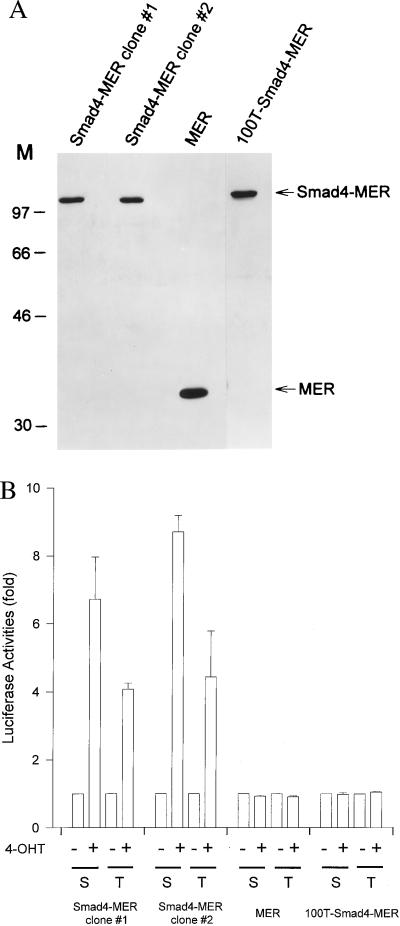
Figure 1.
Stable expression of functional Smad4-MER in transfected clones of the breast cancer line MDA-MB-468. (A) Immunoblots confirm the expression of full-length Smad4-MER, MER, and 100T-Smad4-MER. (B) Fusion proteins exhibit the expected activity with SBE-containing transcriptional reporters. Stable clones were transfected with 6SBE-Luc (S) or 3TP-lux (T) before being treated for 20 h with (+) or without (−) 100 nM 4-OHT. pCMVβ was cotransfected to normalize for transfection efficiencies. Luciferase and β-galactosidase activities were determined. The luciferase activities in the absence of 4-OHT are arbitrarily set at 1. The means of three independent experiments are shown. Bars represent SE.
We then examined whether the expressed Smad4 fusion proteins were functional. Two Smad4–MER, one MER, and one 100T–Smad4–MER clones were studied. As expected, in the presence of 4-OHT, the wild-type Smad4–MER mediated gene activation with either an Smad4/TGF-β reporter, 6SBE-Luc, or a TGF-β-responsive reporter, 3TP-lux (ref. 31; Fig. 1B). In contrast, neither the MER nor the 100T–Smad4–MER was active (Fig. 1B). In the absence of 4-OHT, reporter activities of 6SBE-Luc and 6MBE-Luc (containing mutant sites that fail to bind Smad4) were similar and very weak, indicating that Smad4 background activation is low when its localization is controlled by the tagged fusion protein (data not shown). This is consistent with the comparable clonal outgrowth during the generation of these clones (in the absence of 4-OHT described above). We also found that TGF-β failed to further increase the activities of Smad4/TGF-β-responsive (6SBE-Luc) or TGF-β-responsive (3TP-lux) reporters once Smad4 is transported to the nucleus (data not shown), consistent with our previous transient transfection studies (22). The two sets of experiments suggest that, in the mediation of signals from TGF-β and perhaps other ligands, Smad4 nuclear translocation may serve as a major rate-limiting step. These above results verify that Smad4, when stably expressed as a MER fusion protein, is functional. The effects of Smad4–MER were disrupted by a single tumorigenic amino acid change and thus were likely to be of physiological relevance. This model permitted the exploration of the phenotypic changes that occur on the translocation of Smad4 protein to the nucleus.
Growth of Smad4–MER Cells Inhibited by 4-OHT.
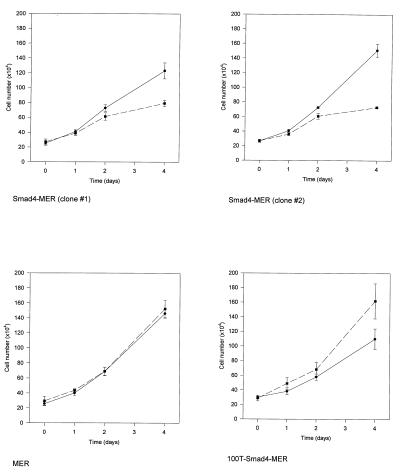
The growth responses of two clones expressing wild-type Smad4–MER were examined. In both, 4-OHT treatment resulted in a significant decrease in cell number. We compared cell growth under 1, 2, and 4 days of 4-OHT treatment and untreated conditions. Moderate and reproducible growth suppression was present at 1 and 2 days of 4-OHT treatment. At 4 days of 4-OHT exposure, the growth of Smad4–MER clones was markedly (≈40–50%) repressed compared with the untreated group (Fig. 2). This growth suppression is not attributable to the effect of 4-OHT on MER itself, because no growth inhibition was observed in the clone expressing MER (Fig. 2), substantiating the role of Smad4 in this growth arrest. Similar experiments were carried out with a 100T–Smad4–MER clone. Instead of growth suppression as in wild-type Smad4 clones, the 100T–Smad4–MER clone displayed growth stimulation after treatment with 100 nM 4-OHT. Gene transcription attributable to nuclear translocation specifically of wild-type Smad4 protein thus appears responsible for the phenotypic changes observed in this study.
Figure 2.
Growth suppression after Smad4 nuclear translocation. Clones that stably expressed Smad4-MER, MER, or 100T–Smad4–MER were treated with ( ■ and dashed line) or without (● and solid line) 100 nM 4-OHT for 0, 1, 2, or 4 days before harvesting for cell counting. Data were analyzed from three independent experiments and are presented as means ± SE.
G1 Accumulation on Smad4 Nuclear Translocation.
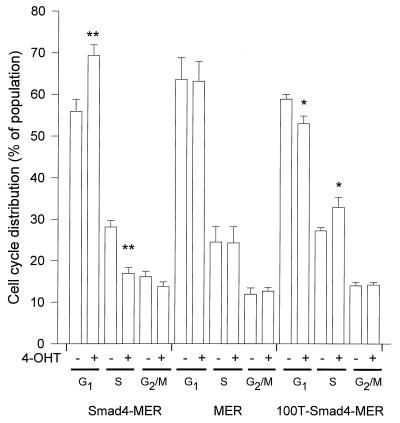
To identify the mechanism underlying the growth inhibition by nuclear Smad4, we first examined the alterations of cell cycle distribution after Smad4 activation. Stable clones were treated for 1, 2, or 4 days with 4-OHT, and the asynchronous cell populations were harvested for analyses of cell cycle distributions. There was a significant increase of G1 (or G1/G0) populations after 4-OHT treatment in the Smad4–MER clone 2 examined (Fig. 3). This change was accompanied by a decrease of the S phase cell population in Smad4–MER cells. The G1 accumulation by 4-OHT was relatively transient. The G1 accumulation declined at day 2 and was minimal by day 4, even with continued 4-OHT treatment (data not shown). Similar results were obtained in the Smad4–MER clone 1. No G1 accumulation was observed in MER cells, indicating that this effect was not because of the MER domain alone. In contrast, in the 100T mutant line, and consistent with the above-mentioned growth promotion on its nuclear translocation, there was a decrease of the G1 population together with an increase of S phase cells (Fig. 3). Rb protein and its phosphorylation status were examined in the stable clones by immunoblotting. We confirmed that the Rb protein was absent, in agreement with previous reports that the RB1 gene is deleted in MDA-MB-468 cells (33, 34).
Figure 3.
G1 cell cycle arrest on nuclear translocation of Smad4. Clones that stably expressed Smad4-MER, MER, or 100T–Smad4–MER were treated for 1 day with (+) or without (−) 100 nM 4-OHT. Data from three to four independent experiments are shown (mean ± SE; ∗, P < 0.05; ∗∗, P < 0.01; comparison to 4-OHT-untreated groups, with paired t test).
Apoptosis Induced by Smad4 Nuclear Translocation.
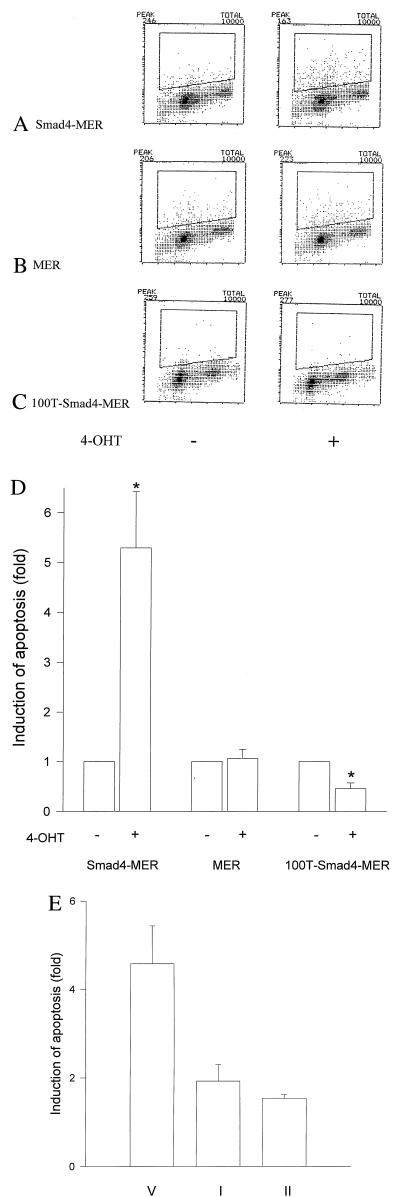
The moderate and transient G1 cell cycle arrest did not fully account for the profound decrease of cell number and delayed growth suppression after 4 days of 4-OHT treatment, as shown in the time course experiments (Figs. 2 and 3). Thus, we examined an alternate mechanism, the regulation of apoptosis. The nuclear translocation of Smad4 resulted in an induction of cell apoptosis (≈5-fold by TUNEL assay, P < 0.05, Fig. 4 A and D). Apoptotic induction was evident in all cell cycle phases and the sub-G1 fraction. This effect persisted after 4 days of 4-OHT exposure (data not shown). The apoptotic response was specific for wild-type Smad4, because this response was not observed in the MER control clone (Fig. 4 B and D). The TUNEL results were confirmed to reflect apoptotic cells, because the number of labeled cells were markedly reduced by coincubation with either caspase inhibitor I or caspase-3 inhibitor II (Fig. 4E). In contrast to this induction of apoptosis, there was a significant decrease (≈50%, P < 0.05) in apoptotic cell number of the 100T–Smad4–MER clone in response to 4-OHT (Fig. 4 C and D). This evasion of apoptosis is also consistent with the observed growth stimulation by 100T–Smad4 when translocated to nucleus.
Figure 4.
Apoptosis induced on nuclear translocation of Smad4. MDA-MB-468 cells that stably expressed Smad4–MER (A), MER (B), and 100T–Smad4–MER (C) were treated with (Right) or without (Left) 100 nM 4-OHT for 2 days. Both attached and unattached cells were harvested and processed for TUNEL assays. A representative set from three independent experiments is shown for each clone. (D) Data analyses of the TUNEL assays of cells in A–C. The induction was determined with percentages of apoptotic cells in the presence (+) of 4-OHT divided by those in the absence (−). Paired t test was used. ∗, P < 0.05 vs. in the absence of 4-OHT (mean ± SE). (E) Caspase inhibitors interfere with the Smad4-induced apoptosis in Smad4–MER cells. Cells were pretreated with 50 μM caspase inhibitor I (I), 50 μM caspase-3 inhibitor II (II), or vehicle (V) for 1 h before 100 nM 4-OHT was added. An average of two independent experiments is presented.
DISCUSSION
Nuclear translocation presents an inducible control of Smad4 function. In the absence of such manipulation, endogenous TGF-β-like ligand(s) and other perhaps more indirect signals are able to activate some Smad4-related functions, hampering the distinction of Smad4-dependent effects. In the current model, however, Smad4 proteins are sequestered in the cytoplasm. Thus, it is possible to achieve a low background for many chosen comparisons, as evidenced by comparable numbers of clonal outgrowth, growth rate, and SBE/MBE reporter activation among the null, wild-type, and mutant Smad4 cell clones grown in standard media (without 4-OHT). We have identified a G1 arrest and apoptosis induction attributable to the nuclear translocation of wild-type Smad4, phenotypes that are in agreement with its tumor-suppressive role.
The G1 arrest was relatively moderate but reproducible. This is consistent with earlier observations showing, in response to TGF-β treatment of MDA-MD-468 cells, a limited decrement (≈20%) in DNA synthesis that depended on the stable expression of wild-type Smad4 (ref. 26; unpublished data). Rb phosphorylation is critical for the cell entry from G1 to S phase (35). The Smad4-mediated cell cycle arrest in MDA-MB-468 is Rb independent because this cell line lacks the RB1 gene (33, 34). In these cells, one might consider that the absence of Rb protein could explain this modest degree of cell cycle regulation. But some Rb-positive cell lines also exhibit limited responses on activation of the Smad4/TGF-β pathway. For example, a colorectal cancer line, HCT116, which expresses wild-type SMAD4 and RB1 genes, exhibited a moderate growth suppression (≈30%) in response to TGF-β compared with parallel lines whose SMAD4 gene had been knocked out by somatic recombination (20). Although the cell cycle arrest mediated by Smad4 has received the most published attention, it is but one potential mechanism of tumor suppression.
The current study indicates that Smad4-inducible apoptosis has the greater consequence in growth control. Indeed, the period of the greatest growth suppression temporally was better correlated with the apoptotic responses than with the cell cycle arrest. The Smad4-induced apoptosis is apparently independent of p53 and Rb proteins, because the p53 (36, 37) and RB1 (33, 34) genes in the MDA-MB-468 line are inactivated. The current data are in agreement with the observations from genetic analyses of pancreatic cancer that showed SMAD4 inactivation commonly coexisted with p53 and p16 gene alterations (38). The combination of apoptotic and cell cycle components observed here reflects the multiple phenotypic responses seen for ligands such as TGF-β and bone morphogenic proteins, whose effector pathways can be shown to be mediated by Smad4 (20, 39–43).
The 100T–Smad4 and wild-type Smad4 protein display opposing phenotypic responses. The interaction of mutant Smad4 with Smad4-binding partners may explain this observation. Smad4 participates in Smad-mediated gene transactivation (in whole or in part) by interaction with other signal transduction proteins (44, 45). Recent studies indicate that certain transcription factors/cofactors such as p300/CBP (CREB-binding protein) and c-Jun/c-Fos cooperate with Smads through binding of the Smad MH2 domain (46–48). Thus, intact interactions of 100T–Smad4 with other binding partners including transcription factors, coupled with the inability to bind the SBE sequence (22), could allow 100T–Smad4 to titrate (squelch) other signal transduction proteins or general transcription factors. In tumors, missense mutations in the MH1 domain are rather infrequent. The other two major categories of mutation include missense mutations in the MH2 domain, which interfere with nuclear translocation, and truncation mutations in the MH2 domain, which cause loss of the transactivation function (22). The growth advantages offered by the 100T mutant in this study suggest that tumors containing certain mutant Smad4 may harbor specific properties that differ from SMAD4-null cells. The extent to which other SMAD4 mutations might have “oncogenic” phenotypes needs to be further explored.
The present studies provide additional evidence to confirm the expectation that Smad4 accomplishes its tumor-suppressive functions as a nuclear protein. Our earlier observations indicated that missense mutations in the MH2 domain extinguished Smad4 transcription activity by an impairment of Smad4 transport into the nucleus (22), probably by destroying the function of the Smad-interaction surface (49). Pathway-restricted Smads become phosphorylated on bone morphogenic protein (Smads 1 and 5; refs. 8 and 9) or TGF-β (Smads 1, 2, and 3; refs. 2, 7, and 50) signaling and heterooligomerize with Smad4. This heterooligomerization accompanies Smad protein nuclear translocation. In view of these data, we can consider two mechanisms by which the nuclear translocation of Smad4 (accomplished here by the action of 4-OHT mediated by the MER domain) might effect phenotypic changes. 4-OHT administration may facilitate the movement of preformed cytoplasmic heterocomplexes to allow their action at the DNA recognition sites. In this view, use of the MER domain simply eliminates nuclear localization as a rate-limiting step in signal transduction by Smad heterocomplexes. Alternatively, Smad4 may function at the transcription level independently of other Smads, controlled largely by its level of nuclear accumulation. These functions operate subsequent to and independent of heterologous interactions that bring Smad4 to the nucleus. These are not mutually exclusive views.
Smad4 thus may serve its tumor-suppressive role through participation in the signaling pathways of multiple ligands. If so, it would be difficult to imagine the therapeutic manipulation of multiple signaling pathways in cancer except by the targeting of the more distal and common aspects of these pathways. It would be useful to evaluate the means to overcome the rate-limiting components of such a common system. Practically, it is possible to envision chemical or peptide agents that would facilitate the direct nuclear translocation of individual proteins, such as Smad4. It is with this view that we have evaluated the phenotypic effects produced by the direct nuclear localization of Smad4. In addition, changes in gene transcription directly attributable to Smad4 nuclear translocation are believed to be responsible for the cell cycle regulation and apoptotic effects identified here. Thus, this model may be valuable for dissecting the downstream genes that mediate these phenotypic changes, to better explore the functional consequences of Smad4 action.
Acknowledgments
We thank Trevor Littleword for the modified MER ligand-binding domain. This work was supported by National Institutes of Health Grant CA68228.
ABBREVIATIONS
- 4-OHT
4-hydroxytamoxifen
- TGF-β
type β transforming growth factor
- SBE
Smad-binding element
- MH1 and MH2
Mad-homology domains 1 and 2
- TUNEL
terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling
References
- 1.Heldin C H, Miyazono K, ten Dijke P. Nature (London) 1997;390:465–471. doi: 10.1038/37284. [DOI] [PubMed] [Google Scholar]
- 2.Zhang Y, Feng X, We R, Derynck R. Nature (London) 1996;383:168–172. doi: 10.1038/383168a0. [DOI] [PubMed] [Google Scholar]
- 3.Kretzschmar M, Liu F, Hata A, Doody J, Massague J. Genes Dev. 1997;11:984–995. doi: 10.1101/gad.11.8.984. [DOI] [PubMed] [Google Scholar]
- 4.Macias-Silva M, Abdollah S, Hoodless P A, Pirone R, Attisano L, Wrana J L. Cell. 1996;87:1215–1224. doi: 10.1016/s0092-8674(00)81817-6. [DOI] [PubMed] [Google Scholar]
- 5.Yingling J, Das P, Savage C, Zhang M, Padgett R, Wang X. Proc Natl Acad Sci USA. 1996;93:8940–8944. doi: 10.1073/pnas.93.17.8940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu X, Sun Y, Constantinescu S N, Karam E, Weinberg R A, Lodish H F. Proc Natl Acad Sci USA. 1997;94:10669–10674. doi: 10.1073/pnas.94.20.10669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lagna G, Hata A, Hemmati-Brivanlou A, Massague J. Nature (London) 1996;383:832–836. doi: 10.1038/383832a0. [DOI] [PubMed] [Google Scholar]
- 8.Hoodless P A, Haerry T, Abdollah S, Stapleton M, O’Connor M B, Attisano L, Wrana J L. Cell. 1996;85:489–500. doi: 10.1016/s0092-8674(00)81250-7. [DOI] [PubMed] [Google Scholar]
- 9.Liu F, Hata A, Baker J C, Doody J, Carcamo J, Harland R M, Massague J. Nature (London) 1996;381:620–623. doi: 10.1038/381620a0. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Y, Musci T, Derynck R. Curr Biol. 1997;7:270–276. doi: 10.1016/s0960-9822(06)00123-0. [DOI] [PubMed] [Google Scholar]
- 11.Liu F, Pouponnot C, Massague J. Genes Dev. 1997;11:3157–3167. doi: 10.1101/gad.11.23.3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zawel L, Dai J L, Buckhaults P, Zhou S, Kinzler K W, Vogelstein B, Kern S E. Mol Cell. 1998;1:611–617. doi: 10.1016/s1097-2765(00)80061-1. [DOI] [PubMed] [Google Scholar]
- 13.Dennler S, Itoh S, Vivien D, ten Dijke P, Huet S, Gauthier J. EMBO J. 1998;17:3091–3100. doi: 10.1093/emboj/17.11.3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yingling J M, Datto M B, Wong C, Frederick J P, Liberati N T, Wang X F. Mol Cell Biol. 1997;17:7019–7028. doi: 10.1128/mcb.17.12.7019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hahn S A, Schutte M, Hoque A T M S, Moskaluk C A, da Costa L T, Rozenblum E, Weinstein C L, Fischer A, Yeo C J, Hruban R H, Kern S E. Science. 1996;271:350–353. doi: 10.1126/science.271.5247.350. [DOI] [PubMed] [Google Scholar]
- 16.Schutte M, Hruban R H, Hedrick L, Cho K, Nadasdy G M, Weinstein C L, Bova G S, Isaacs W B, Cairns P, Nawroz H, et al. Cancer Res. 1996;56:2527–2530. [PubMed] [Google Scholar]
- 17.Hahn S A, Bartsch D, Schroers A, Galehdari H, Becker M, Ramaswamy A, Schwarte-Waldhoff I, Maschek H, Schmiegel W. Cancer Res. 1998;58:1124–1126. [PubMed] [Google Scholar]
- 18.Thiagalingam S, Lengauer C, Leach F S, Schutte M, Hahn S A, Overhauser J, Willson J K V, Markowitz S D, Hamilton S R, Kern S E, et al. Nat Genet. 1996;13:343–346. doi: 10.1038/ng0796-343. [DOI] [PubMed] [Google Scholar]
- 19.Hoque A T M S, Hahn S A, Schutte M, Kern S E. Gut. 1996;40:120–122. doi: 10.1136/gut.40.1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou S, Buckhaults P, Zawel L, Bunz F, Riggins G, Dai J L, Kern S E, Kinzler K W, Vogelstein B. Proc Natl Acad Sci USA. 1998;95:2412–2416. doi: 10.1073/pnas.95.5.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goggins M, Shekher M, Turnaniglu K, Yeo C J, Hruban R H, Kern S E. Cancer Res. 1998;58:9329–9332. [PubMed] [Google Scholar]
- 22.Dai J L, Turnacioglu K K, Schutte M, Sugar A Y, Kern S E. Cancer Res. 1998;58:4592–4597. [PubMed] [Google Scholar]
- 23.Sirard C, de la Pompa J L, Elia A, Itie A, Mirtsos C, Cheung A, Hahn S, Wakeham A, Schwartz L, Kern S E, et al. Genes Dev. 1998;12:107–119. doi: 10.1101/gad.12.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zazuaki T, Oshima M, Miyoshi M, Matsui M, Seldin M F, Taketo M M. Cell. 1998;92:645–656. doi: 10.1016/s0092-8674(00)81132-0. [DOI] [PubMed] [Google Scholar]
- 25.de Caestecker M P, Hemmati P, Larisch-Bloch S, Ajmera R, Roberts A B, Lechleider R J. J Biol Chem. 1997;272:13690–13696. doi: 10.1074/jbc.272.21.13690. [DOI] [PubMed] [Google Scholar]
- 26.de Winter J P, Roelen B A, ten Dijke P, van der Burg B, van den Eijnden-van Raaij A J. Oncogene. 1997;14:1891–1899. doi: 10.1038/sj.onc.1201017. [DOI] [PubMed] [Google Scholar]
- 27.Littlewood T D, Hancock D C, Danielian P S, Parker M G, Evan G I. Nucleic Acids Res. 1995;23:1686–1690. doi: 10.1093/nar/23.10.1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alarcon R M, Rupnow B A, Graeber T G, Knox S J, Giaccia A J. Cancer Res. 1996;56:4315–4319. [PubMed] [Google Scholar]
- 29.Kerkhoff E, Rapp U R. Mol Cell Biol. 1997;17:2576–2586. doi: 10.1128/mcb.17.5.2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vater C A, Bartle L M, Dionne C A, Littlewood T D, Goldmacher V S. Oncogene. 1996;13:739–748. [PubMed] [Google Scholar]
- 31.Carcamo J, Weis F M, Ventura F, Wieser R, Wrana J L, Attisano L, Massague J. Mol Cell Biol. 1994;14:3810–3821. doi: 10.1128/mcb.14.6.3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thompson E W, Paik S, Brunner N, Sommers C L, Zugmaier G, Clarke R, Shima T B, Torri J, Donahue S, Lippman M. J Cell Physiol. 1992;150:534–544. doi: 10.1002/jcp.1041500314. [DOI] [PubMed] [Google Scholar]
- 33.Lee E Y-H, To H, Shew J, Bookstein R, Scully P, Lee W. Science. 1988;241:218–221. doi: 10.1126/science.3388033. [DOI] [PubMed] [Google Scholar]
- 34.T’Ang A, Varley J M, Chakraborty S, Murphree A L, Fung Y T. Science. 1988;242:263–266. doi: 10.1126/science.3175651. [DOI] [PubMed] [Google Scholar]
- 35.Weinberg R A. Cell. 1995;81:323–330. doi: 10.1016/0092-8674(95)90385-2. [DOI] [PubMed] [Google Scholar]
- 36.Nigro J M, Baker S J, Preisinger A C, Jessup J M, Hostetter R, Cleary K, Bigner S H, Davidson N, Baylin S, Devilee P, et al. Nature (London) 1989;342:705–708. doi: 10.1038/342705a0. [DOI] [PubMed] [Google Scholar]
- 37.Bartek J, Iggo R, Gannon J, Lane D P. Oncogene. 1990;5:893–899. [PubMed] [Google Scholar]
- 38.Rozenblum E, Schutte M, Goggins M, Hahn S A, Lu J, Panzer S, Zahurak M, Goodman S N, Hruban R H, Yeo C J, Kern S E. Cancer Res. 1997;57:1731–1734. [PubMed] [Google Scholar]
- 39.Zou H, Niswander L. Science. 1996;272:738–741. doi: 10.1126/science.272.5262.738. [DOI] [PubMed] [Google Scholar]
- 40.Hoosein N, Brattain D, McKnight M, Levine A, Brattain M. Cancer Res. 1987;47:2950–2954. [PubMed] [Google Scholar]
- 41.Yanagihara K, Tsumuraya M. Cancer Res. 1992;52:4042–4045. [PubMed] [Google Scholar]
- 42.Silberstein G B, Daniel C W. Science. 1987;273:291–293. doi: 10.1126/science.3474783. [DOI] [PubMed] [Google Scholar]
- 43.Martikainen P, Kyprianou N, Issacs J T. Endocrinology. 1990;127:2963–2968. doi: 10.1210/endo-127-6-2963. [DOI] [PubMed] [Google Scholar]
- 44.Chen X, Weisber E, Fridmacher V, Watanabe M, Naco G, Whitman M. Nature (London) 1997;389:85–89. doi: 10.1038/38008. [DOI] [PubMed] [Google Scholar]
- 45.Zhou S, Zawel L, Lengauer C, Kinzler K W, Vogelstein B. Mol Cell. 1998;2:121–128. doi: 10.1016/s1097-2765(00)80120-3. [DOI] [PubMed] [Google Scholar]
- 46.Janknecht R, Wells N J, Hunter T. Genes Dev. 1998;12:2114–2119. doi: 10.1101/gad.12.14.2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Feng X H, Zhang Y, Wu R Y, Derynck R. Genes Dev. 1998;12:2153–2163. doi: 10.1101/gad.12.14.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang Y, Feng X H, Derynck R. Nature (London) 1998;394:909–913. doi: 10.1038/29814. [DOI] [PubMed] [Google Scholar]
- 49.Shi Y, Hata A, Lo R S, Massague J, Pavletich N P. Nature (London) 1997;388:87–93. doi: 10.1038/40431. [DOI] [PubMed] [Google Scholar]
- 50.Liu X, Yue J, Frey R S, Zhu Q, Mulder K M. Cancer Res. 1998;58:4752–4757. [PubMed] [Google Scholar]