Abstract
Hsp90, a molecular chaperone required for the functioning of glucocorticosteroid receptor (GR), ensures, by direct interaction, the conformational competence of the steroid-binding pocket. In addition to having this positive function, Hsp90 maintains steroid receptors in an inactive form in the absence of hormone. However, neither the participation of Hsp90 once the pathway has been activated by the ligand nor the importance of increased Hsp90 levels in determining the amplitude of the response has ever been assessed directly. Here, by increasing the Hsp90/GR ratio in the nuclear compartment, we found an attenuation of the response to glucocorticosteroids that was not due to a nonspecific or toxic effect of the Hsp90 modified by nuclear targeting. Since this negative effect was more pronounced at high levels of hormone, when receptor and Hsp90 are maximally dissociated, the possibility of an interaction between Hsp90 and GR, already activated to a DNA-binding form, was directly investigated. Indeed GR, after in vivo activation by ligand, was still able to reassociate with Hsp90, suggesting that this interaction plays a role in vivo, possibly in receptor recycling. Moreover, the GR binding to its DNA response element was inhibited by an excess of Hsp90, pointing to a function of Hsp90 in the nuclear compartment. It is thus proposed that an increased Hsp90/GR ratio influences the responsiveness to ligand at a step that is after receptor activation. This increased ratio may be of pathophysiological relevance in the different circumstances that lead to an elevated level of nuclear Hsp90.
The glucocorticosteroid receptor (GR) is a ligand-inducible transcription factor belonging to the nuclear receptor superfamily. Hormone binding to the ligand-binding domain (LBD) elicits a series of molecular events including dissociation from the Hsp90 chaperone and changes of conformation of the receptor itself, allowing the activation or repression of target genes (1–4). Interaction of the GR LBD with the Hsp90 moiety of a complex multichaperone machinery is required for optimal hormone binding and thus for all ligand-dependent activities of the receptor. The same interaction ensures the repression of the receptor in the absence of hormone (4–8). Collectively these data point to a dual positive and negative function of Hsp90.
The positive function has been demonstrated by genetic approaches, showing that in the presence of very low levels of Hsp90, the signal transduction by steroids is dramatically impaired (9). Remarkably, the antibiotic geldanamycin, which binds to the ATP pocket and blocks the ATPase activity of Hsp90 (10–12), abolishes the hormone binding and the transcriptional activation mediated by steroid receptors (13, 14). Moreover, the positive function of Hsp90, consisting in the maintenance of the conformational competence of steroid receptors, seems necessary not only during but also after receptor synthesis (15).
The inhibitory function of Hsp90 has been suggested by in vitro experiments showing that its association with steroid receptors, when stabilized by molybdate, prevents the interaction of the receptor with DNA consensus sequences (5, 7, 16–18) and by the finding that, in vivo, the LBD of steroid receptors confers on many fused proteins a repressed status, which is relieved by ligand binding and Hsp90 dissociation (19, 20).
Both conformational maturation and repression of steroid receptors in the absence of ligand seem thus intimately connected with the Hsp90-multicomponent chaperone machinery operating in the cytoplasm, as suggested by experiments using cellular extracts or reticulocyte lysate (4). Whether the same machinery or part of it also operates in the nucleus, where some Hsp90 and associated proteins have been found colocalized with or complexed to nuclear steroid receptors, remains to be firmly established (21–25).
Here, we investigated whether high nuclear levels of Hsp90 may modulate GR function and influence hormonal responses once the receptor has been activated by the hormone. When, by nuclear targeting, Hsp90 was overexpressed in the nucleus, where physiologically liganded GR regulates transcription in the presence of a relatively low level of Hsp90, the response to glucocorticosteroids was negatively modulated. This effect was not a toxic consequence of nuclear targeted Hsp90 and was specific for a GR-dependent promoter. Moreover, purified Hsp90 physically interacted in vitro with GR previously activated in vivo and inhibited the binding of the same receptor to its cognate DNA consensus sequence. Thus, the Hsp90–GR interaction, although dynamically shifted toward dissociation by the specific steroid, may take place in the nuclear compartment not only before but also after receptor activation to a DNA-binding form, thus modulating the responsiveness to hormone.
MATERIALS AND METHODS
Plasmids.
pSVK390WT and pSVK390NLS (WT, wild-type; NLS, nuclear localization signal) have been described previously (23). pSVK390Mut was obtained by a point mutation (G289A) generating the stop codon TAA instead of the Lys-99 codon. Plasmids pSVK390 (WT, NLS, or Mut)-MMTVLuc were constructed by insertion of the PvuII–AseI-filled fragment from pFC31Luc (26) into the XhoI-filled site of plasmids pSVK390 (WT, NLS, or Mut). Plasmids pSVK390 (WT, NLS, or Mut)-SVLuc, were constructed by inserting the fragment PvuII–SmaI from pSVD5′Luc (26) in pSVK390 (WT, NLS, or Mut) cut by XhoI and filled in. Plasmid GST⋅Hsp90 was obtained by insertion of a XbaI–XhoI fragment encoding the Hsp90 cDNA into the pGEX-2T vector (Pharmacia) cut by BamHI after treatment of both fragments with mung bean nuclease. Plasmid pCH110 (Pharmacia), expressing the β-galactosidase (β-gal) gene was used as an internal control for transfection.
Yeast Strains, Transformants, and β-gal Reporter Assay.
The two Saccharomyces cerevisiae strains (from the laboratory of S. Lindquist, University of Chicago) used in this paper were both derivatives of W303. The haploid ΔECU82a strain, deleted of both hsp82 and hsc82 (can1-100 his3-11,15 leu2-3,12 trp1-1 ura3-1 Δhsc82∷LEU2+ Δhsp82∷LEU2+) is rescued from lethality by a URA3-marked plasmid expressing wild-type Hsp90 (Hsp82 plasmid pKAT6) (15, 24, 27). Chicken Hsp90WT, -NLS, and -Mut were introduced into the pTGPD TRP1-marked, low copy expression vector and used to transform ΔECU82a. Transformants were plated onto SD (15, 27) minus tryptophan, and then replica plated onto SD minus tryptophan plus 5-fluoroorotic acid, at 30°C, to select for colonies that had lost the pKAT6 plasmid and have been rescued from lethality by chicken Hsp90WT or mutants.
The diploid iLEP1a strain (can1-100 ade2-1 his3-11 leu2-3,12 trp1-1 ura3-1 HSP82∷LEU2 HSC82∷LEU2), in which both Hsp82 and Hsc82 genes have been disrupted, expresses low levels of Hsp82 from its natural promoter deleted up to position −110, after integration of pRS303-F3 into the HIS3 locus. It is temperature sensitive. The plasmid p2A/GRGZ (from the Lindquist laboratory; ref. 15) is an ADE3-marked, high copy plasmid, which contains the mouse GR coding region under the control of the GPD constitutive promoter as well as a lacZ reporter gene under the control of glucocorticosteroid response elements (GREs). The transformants were selected on SD minus tryptophan and adenine. The cells expressing the GR and Hsp90WT (or Hsp90NLS) were grown to a concentration of about 2 × 106 cells per ml in SD minus tryptophan and adenine. Deoxycorticosterone (1 μM or 10 μM) was added for 1 h to activate GR. Yeast lysates were prepared by shaking cells with glass beads for 4 min at 4°C in lysis buffer (15). The β-gal activity was measured with the Galactolight Kit (Tropix, Bedford, MA) and normalized to the protein concentration as determined by the Bio-Rad protein assay.
Cell Culture and Transfection and β-gal and Luciferase Activities.
The 34i cell line is derived from a C3H mouse mammary epithelial carcinoma (26), the T47D cell line is derived from a human mammary carcinoma (American Type Culture Collection), and PR50 cells are a CV1 cell line stably transfected with the gene encoding rabbit progesterone receptor (J. F. Savouret, personal communication). All these cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal calf serum. Twenty-four hours before transfection, cells were plated in 6 ml of medium at 3.5 × 105 cells per 6-cm-diameter dish. Medium was changed 4 h before transfection. Each dish, containing 3 ml of medium, was transfected with 2.5 μg of tested plasmid and 2.5 μg of control plasmid (pCH110) and carrier DNA (pSP72 from Promega) by using the N-[1-(2,3-dioleoyloxy)propyl]-N,N,N-trimethylammonium methylsulfate (DOTAP) procedure (Boehringer). Briefly, DNA samples (100 μl in 10 mM Hepes buffer, pH 7.4) and 100 μl of DOTAP reagent in the same buffer were mixed and added drop by drop to the cells. Sixteen hours after transfection, the precipitate was removed by rinsing with phosphate-buffered saline (PBS), and fresh medium (6 ml) with or without hormone was added. After a further 24-h incubation, cells were harvested and lysed to determine β-gal and luciferase activities as previously described (28). The reporter activity is expressed in relative light units.
Indirect Immunofluorescence and Western Blot Analyses.
Double indirect immunofluorescence studies of endogenous GR and chicken Hsp90WT or Hsp90NLS expressed after transfection in 34i cell line were carried out as previously described with the anti-GR BUGR 2 antibody (NovoCastra; Newcastle, U.K.) diluted 1:500 and the BF4 antibody specific for chicken Hsp90 (23). Western blotting analyses using BF4 or anti-human GR antibodies have been described (23, 29).
Hsp90 Purification.
Chicken Hsp90α was obtained by one-step immunoaffinity purification with BF4 monoclonal antibody (30). This procedure, using as starting material a homogenate of 14-day-old chicken embryos, gave a homogenous Hsp90 preparation (purity >95%) on the basis of SDS/PAGE and Coomassie blue or silver staining followed by gel scanning densitometry.
Sf9 Cell Culture, Recombinant Human GR (hGR)-Expressing Baculovirus Infection, and Preparation of GR-Containing Extracts.
Recombinant baculovirus, expression in Sf9 cells of hGR cDNA and its derivative I550* lacking LBD (7), as well as treatment with triamcinolone acetonide (TA), preparation of high-salt nuclear extracts, and measurement of steroid-binding capacity of extracts were as described (29). High-salt GR extracts after 1 h of cell treatment with TA were at 3.2 mg/ml protein and 200 pmol/mg of protein of steroid-binding capacity.
Electrophoretic Mobility-Shift Assay (EMSA).
GR extracts (1 μl) were incubated with ≈0.4 ng of 32P-end-labeled synthetic GRE perfect palindrome sequence as described (29), and the binding reaction was allowed to proceed for 45 min at 25°C in the presence of BSA (5 μg). For competition experiments, various concentrations of purified Hsp90 were added to the mixture before the addition of the end-labeled nucleotide. For dissociation experiments, Hsp90 was mixed with a preformed complex of GR and labeled oligonucleotide and incubated for an additional 45 min. Free DNA and DNA–protein complexes were resolved on a nondenaturing polyacrylamide gel as described (29). The protein–DNA complexes were quantified by densitometric scanning of autoradiograms utilizing BioImage software on a BioImage Station (Millipore).
Purification and Immobilization of Glutathione S-Transferase (GST)-Hsp90 and Interaction with GR Extracts.
The fusion protein GST-Hsp90 was expressed in Escherichia coli strain BL21 after induction by isopropyl β-d-thiogalactoside (IPTG). The bacterial lysate was adsorbed onto glutathione-Sepharose 4B gel (Pharmacia). On 200 μl of gel, 600 μg of fusion protein was retained and incubated with 1 ml of diluted GR extract (corresponding to 65 pmol of GR) at different salt concentrations. At low salt concentration, ≈50% of the GR added was retained by the gel. Quantification of the GR–GST-Hsp90 interaction was performed by densitometric scanning of enhanced chemiluminescence (ECL; Amersham) signals obtained after SDS/PAGE and Western blotting of the proteins eluted by boiling in SDS Laemmli buffer, using a polyclonal anti-GR antibody (29).
RESULTS
Nuclear Targeted Hsp90 (Hsp90NLS) Sustains Viability and GR Function in Yeast.
In a nuclear cotranslocation assay using double immunofluorescence detection, Hsp90NLS interacted in vivo with cytoplasmic steroid receptors and, conversely, wild-type cytoplasmic Hsp90 interacted with nuclear progesterone or estrogen receptors in the absence of specific hormonal ligands (23, 24, 28). In this report, we tested whether the overexpression of Hsp90WT or Hsp90NLS, in the 34i cell line, which possesses a functional GR (26), can modulate the transcriptional activity of a glucocorticosteroid-responsive reporter gene. When the Hsp90NLS construct is used, an effective increase in the Hsp90/GR ratio is expected in the nuclear compartment, where Hsp90 is normally found at a low level (23).
Since nuclear targeted Hsp90 may be toxic or may display nonspecific effects in steroid signaling pathways, Hsp90WT and Hsp90NLS were subcloned in a yeast expression plasmid and then tested for complementation properties for viability and for steroid hormone-dependent transcriptional activation in appropriate yeast strains that allow avoidance of competition with endogenous Hsp90.
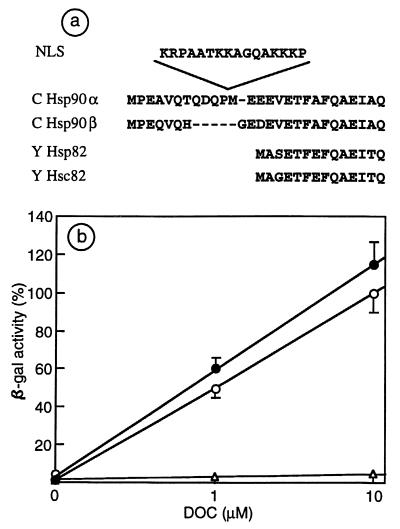
The yeast strain ΔEcu82a was used to replace, by a plasmid shuffling technique (27), a unique yeast Hsp90 (in the pKAT6 ura3 plasmid) with Hsp90WT or Hsp90NLS expressed from the cen-ars plasmid pTGPD. Both constructs were able to confer viability and normal growth at temperatures between 24° and 30°C, though cells died at 37°C (not shown). Indeed, both Hsp90WT and Hsp90NLS can complement for viability, except at elevated temperatures. Since the NLS sequence was inserted between Pro-11 and Met-12, after the N-terminal extension specific to vertebrate Hsp90α (see Fig. 1a) and before a sequence that is highly conserved from lower to higher eukaryotes, the viability and normal growth conferred by Hsp90 NLS were not unexpected.
Figure 1.
Hsp90NLS sustains GR function in yeast. (a) Insertion of the NLS of Xenopus laevis nucleoplasmin in the N-terminal region of chicken Hsp90α and comparison with chicken Hsp90β and yeast Hsp82 and Hsc82 N-terminal sequence. (b) Response to deoxycorticosterone (DOC) of the diploid iLEP1a strain, transformed with p2A/GRGZ, and pTGPD empty (▵) or bearing Hsp90WT (○) or Hsp90NLS (●).
Then the strain iLEP1a, almost devoid of Hsp90, was transformed by Hsp90WT or Hsp90NLS in the cen-ars plasmid and GRGZ (GR plus GRE β-gal reporter) in plasmid 2 μ. The expression of Hsp90WT or Hsp90NLS drastically and equally improved the hormonal response of the GRE β-gal reporter as compared with the strain transformed with empty vectors (Fig. 1b). Therefore, Hsp90WT and Hsp90NLS both possess the ability to confer viability and to support the function of the GR in yeast cells.
Hsp90NLS Specifically Inhibits in a Dose-Dependent Manner the Hormone-Dependent Transcriptional Activity of the Mouse Mammary Tumor Virus (MMTV) Promoter.
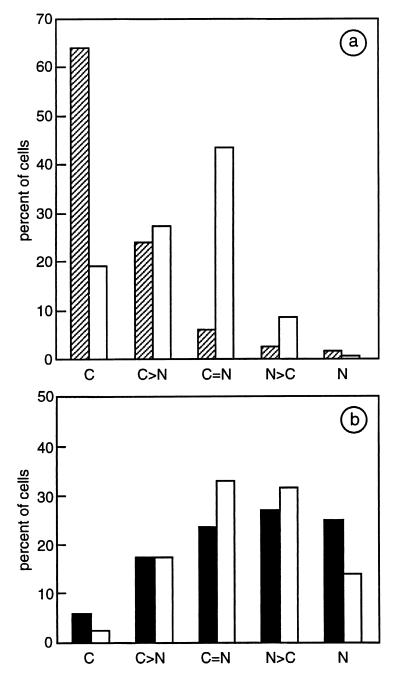
In the 34i cell line the expression level (≈5–10-fold increase in transfected cells) and subcellular localization of Hsp90WT or Hsp90NLS were comparable to those found in Cos7 cells (23). Fig. 2a shows the subcellular distribution of transfected Hsp90WT as essentially cytoplasmic, whereas Hsp90NLS was nuclear in about 60% of transfected cells (Fig. 2b). Moreover, in the absence of ligand, the expression of Hsp90NLS resulted in ≈50% shift of the cytoplasmic GR to the nuclear compartment (compare the subcellular distribution of GR in Fig. 2 a and b, while noting that the subcellular distributions of GR in the presence or absence of transfected Hsp90 WT were the same).
Figure 2.
Subcellular distribution of endogenous GR and chicken Hsp90WT or Hsp90NLS. One hundred transfected 34i cells were analyzed by double immunofluorescence and confocal microscopy for GR (white bars) and Hsp90WT (hatched bars in a) or Hsp90NLS (black bars in b). Subcellular localizations (C = cytoplasmic, N = nuclear) were categorized as follows: C, C>N, C=N, N>C, and N.
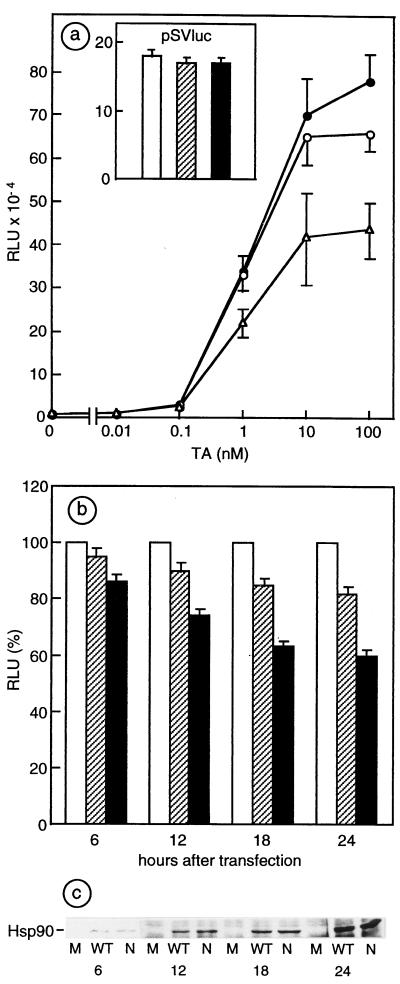
In the same cell line, the MMTV-luciferase reporter activity was stimulated by TA concentrations from 10−10 to 10−7 M. Only at the highest hormone concentrations (10−8 to M-10−7 M) was a constant and reproducible inhibition (15–20%) of the MMTV-driven luciferase activity found when the reporter was cotransfected with Hsp90NLS, compared with values obtained after cotransfection with Hsp90WT or Hsp90Mut (not shown). Since the percentage of cotransfected cells may have influenced the extent of the inhibition observed, plasmids bearing both the reporter gene and one of the three different Hsp90 cDNAs (WT, NLS, and Mut) have been constructed and transfected in the same cell line. Results of such experiments, illustrated in Fig. 3a, show that Hsp90NLS inhibited the response to 10−7 M TA by 40% compared with control plasmid (Hsp90Mut), whereas Hsp90WT produced only a limited inhibition. Thus, Hsp90NLS and, to a minor extent, Hsp90WT behave differently from Hsp90 mutants selected for decreased GR function, which all display impaired responses at all hormone concentrations (31). The specificity of inhibition of glucocorticosteroid responsiveness of the MMTV promoter by Hsp90NLS was tested by using a constitutive promoter, the MMTV luciferase reporter being replaced by the simian virus 40 early promoter driving the expression of the luciferase gene. Although Hsp90WT and Hsp90NLS were accumulated at the same level (data not shown) compared with the constructs bearing MMTV promoter, no effect of Hsp90NLS was detected on the constitutive hormone-independent reporter pSVluc (see Inset of Fig. 2a), thus demonstrating the specific involvement of Hsp90NLS in the attenuation of the glucocorticosteroid-dependent response.
Figure 3.
Effects of overexpressed Hsp90WT and Hsp90NLS on glucocorticosteroid-inducible MMTV promoter activity. (a) MMTV promoter activity in 34i cells transfected with plasmids bearing the reporter plus Hsp90Mut (●), Hsp90WT (○), or Hsp90NLS (▵) in the presence of increasing concentrations of TA. (Inset) Simian virus 40 (SV40) promoter activity in the presence of Hsp90Mut (white bars), Hsp90WT (hatched bars), or Hsp90NLS (black bars). The mean values (±SEM) of luciferase activity, expressed in relative light units (RLU), are from four experiments with each point in triplicate. (b) Inhibition of MMTV promoter activity by increasing levels of Hsp90WT and NLS. 34i cells transfected with the Hsp90 constructs described for a were stimulated or not with 10−7 M TA for 6 h at the indicated times after transfection. For each point (in triplicate) of the time course experiment, the values of the reporter activity of Hsp90 Mut (white bars) construct stimulated by TA are represented as 100%; Hsp90WT is represented by hatched bars and Hsp90NLS, by black bars. (c) SDS/PAGE and Western blot analysis. Equal amounts of cell lysates from experiments described for b were analyzed by SDS/PAGE and Western blotting to check the level of expression of chicken Hsp90WT and Hsp90NLS (WT and N, respectively).
To further confirm that the inhibition of the glucocorticosteroid-dependent MMTV promoter was directly related to the expression level of Hsp90NLS, cells transfected by the three constructs were treated with TA (6 h) at progressively increasing time intervals after the beginning of transfection, thus allowing the 34i cells to express increasing amounts of Hsp90WT or Hsp90NLS. As expected, Fig. 3b shows that an increased inhibition of MMTV-promoter-controlled luciferase activity stimulated by TA occurred when the expression level of Hsp90 (Fig. 3c) was increased. Again, the extent of inhibition was greater with Hsp90NLS than with Hsp90WT. The same constructs were tested in progesterone receptor-expressing cell lines (PR50 and T47D). A lack of negative modulation of the hormonal response by both Hsp90WT and Hsp90NLS was consistent with the inability of these cell lines to accumulate exogenous Hsp90 efficiently (not shown).
Hsp90 Modulates the Binding of GR to GRE.
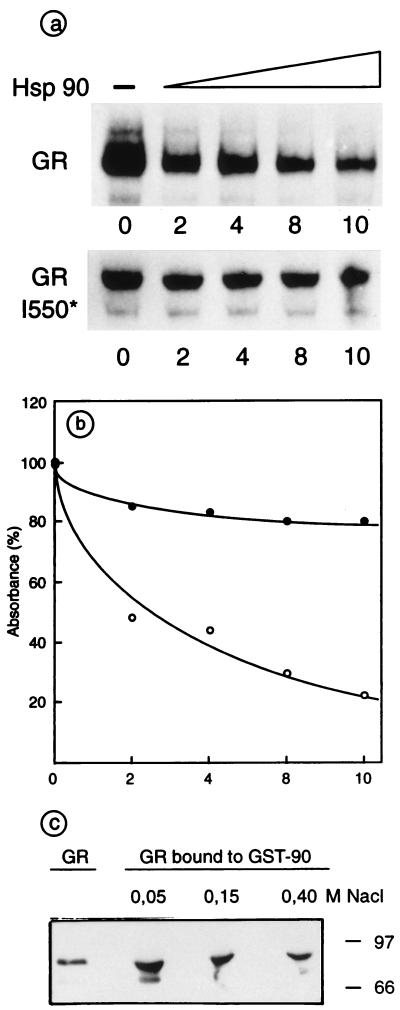
Because it has been reported that increasing concentrations of Hsp90 are able to inhibit the interaction of estrogen receptor (ER) and estrogen-responsive element (ERE) and to actively dissociate preformed high-affinity ER–ERE complexes (16), we directly tested whether purified Hsp90 may interfere with GR binding to GRE in an EMSA. As shown in Fig. 4 a and b, Hsp90 was able to dissociate preformed GR–GRE complexes, suggesting that Hsp90 competes with GRE possibly by binding to the wild-type receptor. This inhibitory effect was also found when GR and Hsp90 were simultaneously incubated with GRE (not shown) and it was inversely dependent on the concentration of Hsp90.
Figure 4.
Hsp90 modulates the binding of GR to GRE and interacts with activated GR. (a) Interaction of GR or GR I550* with GRE was followed by EMSA in the presence of increasing amount of Hsp90; the molar ratio of Hsp90 to GR was between 0 and 10. (b) Densitometric scanning of the GR–GRE complexes. Retarded bands obtained with GR (○) or GR I550* (●) in the absence of Hsp90 were each considered as 100% absorbance. (c) GR activated in vivo by TA in Sf9 cells, infected with baculovirus GR-expression vector, was salt extracted and incubated at the indicated NaCl concentration with GST-Hsp90 fusion protein fixed to glutathione-Sepharose beads. One-tenth of the added extract and 1/3 of the eluted material were analyzed by SDS/PAGE and Western blotting with anti-GR antibodies. The percentage of the GR that interacted with GST-Hsp90 was calculated by quantitative densitometry.
Moreover, as already described (18), we found that Hsp90 did not influence the binding of other transcription factors such as AP1 and NF1 to their consensus sequence (not shown), nor it did affect the GRE-binding properties of a GR mutant lacking the LBD (see Fig. 4 a and b), in agreement with the finding that this mutant does not interact with Hsp90 (7). These results strongly indicate that there is, at least, a transient interaction between Hsp90 and the LBD of GR during incubation and that Hsp90 is not a nonspecific inhibitor of the interaction between transcription factors and corresponding DNA consensus elements.
Because the inhibition of the GR–GRE interaction by Hsp90 was observed while using high salt nuclear extracts diluted into EMSA buffer and obtained after in vivo activation of GR by TA to a DNA-binding form, we tested whether the same GR preparations may still interact with Hsp90. Fig. 4c shows that 3 nmol of dimeric GST-Hsp90 forms complexes with 30 pmol of GR in 50 mM NaCl, as tested by using GST-Hsp90 pull-down experiments and GST alone as a negative control (not shown). At a low salt concentration, ≈50% of the GR added, previously activated to a DNA-binding form by ligand treatment in vivo, was complexed to GST-Hsp90, and this complex formation was inhibited by higher salt concentrations. This result suggests that GR may interact in vivo with Hsp90 not only before but also after activation by the specific hormone.
DISCUSSION
Hsp90 and a cohort of chaperones including immunophilins and p23, participate, by means of formation of complexes, in the proper maturation of steroid receptors to a form that binds specific ligands with high affinity. The chaperone machinery built around Hsp90 also seems able to maintain the receptor “poised” until the ligand binds (4, 8). Although it was demonstrated that a decreased level of Hsp90 dramatically impaired signal transduction by steroid receptors (9), the influence of an increased level of Hsp90 on steroid-dependent responses is not known.
High Hsp90 Levels Attenuate the Induction by Glucocorticosteroids of the MMTV Promoter Activity.
Here, we report that an increased Hsp90 cellular concentration was able to negatively modulate the response to glucocorticosteroid at the level of a specific reporter gene, and this effect was more pronounced when Hsp90 was nuclear targeted (Hsp90NLS).
Since Hsp90NLS, like Hsp90WT, conferred viability, normal growth, and responsiveness to glucocorticosteroids in yeast, the negative modulation observed in the murine 34i cell line was not related to a toxic effect of the transfected constructs. Moreover, when a constitutive reporter was used, no effect of overexpression of Hsp90WT or Hsp90NLS was found, thus excluding a nonspecific mechanism. The partial inhibition of the response to glucocorticosteroids was strikingly related to the level of expression of Hsp90 and was not found in cell lines unable to express a high level of exogenous Hsp90WT or Hsp90NLS. It should be noted that after transfection of Hsp90WT or Hsp90NLS, the cellular Hsp90 level increases from 1 to ≈10% of the cytosol proteins in transfected cells (23). In particular, Hsp90NLS, which is ≈50% nuclear before cell fractionation, contributes to an increase in nuclear Hsp90 concentration of ≈100-fold. Thus, overexpression of Hsp90WT increases ≈10-fold the cytoplasmic Hsp90/GR ratio, whereas Hsp90NLS overexpression, which induces a shift of ≈50% of cytoplasmic GR to the nuclear compartment, results in ≈10-fold increase of the cytoplasmic Hsp90/GR ratio and ≈30-fold increase of the nuclear one. Consequently Hsp90 that, probably by its chaperone activity, is a positive modulator of steroid hormone action, may also act as a negative modulator when Hsp90/receptor ratio is increased, especially in the nuclear compartment.
GR Activated to a DNA-Binding Form Interacts with Hsp90.
Concerning the mechanisms underlying this negative modulation, Hsp90 may interfere at different steps leading to transcriptional activation by liganded GR. An excess of Hsp90 may shift the equilibrium between free GR and GR–Hsp90 complexes toward an associated state. In this case, Hsp90 would act as an inhibitor of the hormone-dependent transformation and activation of the receptor. However, the fact that the attenuation of the hormonal response by Hsp90NLS, differently from that found with other Hsp90 mutants selected for impaired responses to steroids (31), is detected only at saturating level of hormone, when GR and Hsp90 are maximally dissociated, suggests that the high level of Hsp90 affects GR function after receptor activation to a DNA-binding form. This hypothesis has been confirmed by the results of EMSA experiments showing that, after GR activation by hormone in vivo, the binding of salt-extracted GR to GRE is in part inhibited by Hsp90 and, most interestingly, that Hsp90 favors the dissociation of preformed GR–GRE complexes. Analogous results have been reported with ER binding to ERE, showing that an ≈10-fold molar excess of Hsp90 over ER accelerates the ER off-rate from ERE (18). These results are not in contradiction with other reports suggesting that equimolar Hsp90/ER or Hsp90/MyoD ratios facilitate the binding of both transcription factors to their cognate consensus DNA sequences (18, 32, 33).
The fact that, differently from wild-type receptors, the binding of LBD-deleted receptors to their consensus sequences is almost unaffected by Hsp90 strongly suggests that steroid receptors, already activated to a DNA-binding form by ligand treatment in vivo, could interact with Hsp90. Indeed, activated ER was able to reform complexes with Hsp90 (18). Here, by GST-Hsp90 pull-down experiments, we showed that activated GR interacted at 4°C with Hsp90 with an efficiency that was inversely dependent on salt concentration. However, this result does not exclude the contribution of other components of the nuclear extracts to the GR–GST-Hsp90 interaction.
The same interaction between preactivated GR and Hsp90 may also take place in vivo and play a role in the dissociation of GR from GRE as well as in GR recycling. It is believed that GR recycling by the Hsp90-based chaperone machinery occurs only in the cytoplasm (25). However, our findings suggest that Hsp90 may form complexes with activated GR in the nucleus, and therefore the attenuation of the response to glucocorticosteroid by Hsp90NLS may be the consequence of an inefficient receptor recycling, because of some imbalance between Hsp90 and other cooperating chaperones in the nuclear compartment.
The suggestion that Hsp90 may participate at different steps of the activation–deactivation pathway of steroid receptor is reminiscent of a regulatory model in which Hsp90 influences the interconversion between monomeric inactive and trimeric active heat shock factor 1 (HSF1) in Xenopus oocytes (34).
In conclusion, we propose that the Hsp90 chaperone may modulate the amplitude of responses to steroids even once the pathway has been activated. A positive modulation will be the result of an optimal Hsp90/steroid receptor ratio, whereas abnormally low or high ratios will negatively interfere with steroid-dependent responses. The physiological relevance of the effects obtained with Hsp90NLS consists in the fact that some Hsp90 exists in the nucleus of all cells whether or not they are a target for steroids. This level, which may vary according the cell or tissue type, increases in low cell density culture (J.D.-L., unpublished observations) or after heat shock (35); such increased nuclear Hsp90 levels may be responsible for protection of steroid-induced responses when Hsp90/steroid receptor ratio is optimal or for attenuation of the responses when this ratio is abnormal.
Acknowledgments
We thank J. F. Savouret for the PR50 cell line, K. Rajkowski for helpful comments on this manuscript, N. Jibard and F. Delahaye for technical assistance, and L. Outin and J. C. Lambert for the preparation of figures. This work was supported by the Institut National de la Santé et de la Recherche Médicale and by grants from the Fondation pour la Recherche Médicale and the Association pour la Recherche sur le Cancer to M.-G.C. In addition, K.I.K. and I.B. were supported by the Institut Electricité Santé and by the Société de Secours des Amis des Sciences; X.M., by the Lalor Foundation; and A.C., by the Ligue Nationale contre le Cancer.
ABBREVIATIONS
- LBD
ligand-binding domain
- Hsp90WT
heat shock protein of 90 kDa, wild-type
- NLS
nuclear localization signal
- GR
glucocorticosteroid receptor
- hGR
human GR
- GRE
glucocorticosteroid response element
- ER
estrogen receptor
- ERE
estrogen response element
- β-gal
β-galactosidase
- TA
triamcinolone acetonide
- EMSA
electrophoretic mobility-shift assay
- GST
glutathione S-transferase
- MMTV
mouse mammary tumor virus
References
- 1.Tsai M J, O’Malley B W. Annu Rev Biochem. 1994;63:451–486. doi: 10.1146/annurev.bi.63.070194.002315. [DOI] [PubMed] [Google Scholar]
- 2.Mangelsdorf D J, Thummel C, Beato M, Erlich P, Schütz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, Evans R M. Cell. 1995;83:835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beato M, Herrlich P, Schütz G. Cell. 1995;83:851–857. doi: 10.1016/0092-8674(95)90201-5. [DOI] [PubMed] [Google Scholar]
- 4.Pratt W B, Toft D O. Endocr Rev. 1997;18:306–360. doi: 10.1210/edrv.18.3.0303. [DOI] [PubMed] [Google Scholar]
- 5.Pratt W B, Jolly D J, Pratt D V, Hollenberg S M, Giguere V, Cadepond F, Schweitzer-Groyer G, Catelli M-G, Evans R M, Baulieu E-E. J Biol Chem. 1988;263:267–273. [PubMed] [Google Scholar]
- 6.Bresnick E H, Dalman F C, Sanchez E R, Pratt W B. J Biol Chem. 1989;264:4992–4997. [PubMed] [Google Scholar]
- 7.Cadepond F, Schweizer-Groyer G, Segard-Maurel I, Jibard N, Hollenberg S M, Giguere V, Evans R M, Baulieu E-E. J Biol Chem. 1991;266:5834–5841. [PubMed] [Google Scholar]
- 8.Bohen S P, Kralli A, Yamamoto K R. Science. 1995;268:1303–1304. doi: 10.1126/science.7761850. [DOI] [PubMed] [Google Scholar]
- 9.Picard D, Khursheed B, Garabedian M J, Fortin M G, Lindquist S, Yamamoto K R. Nature (London) 1990;348:166–168. doi: 10.1038/348166a0. [DOI] [PubMed] [Google Scholar]
- 10.Stebbins C E, Russo A A, Schneider C, Rosen N, Hartl F U, Pavletich N P. Cell. 1997;89:239–250. doi: 10.1016/s0092-8674(00)80203-2. [DOI] [PubMed] [Google Scholar]
- 11.Prodromou C, Roe S M, O’Brien R, Ladbury J E, Piper P W, Pearl L H. Cell. 1997;90:65–75. doi: 10.1016/s0092-8674(00)80314-1. [DOI] [PubMed] [Google Scholar]
- 12.Panaratou B, Prodromou C, Roe S M, O’Brien R, Ladbury J E, Piper P W, Pearl L H. EMBO J. 1998;17:4829–4836. doi: 10.1093/emboj/17.16.4829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Whitesell L, Cook P. Mol Endocrinol. 1996;10:705–712. doi: 10.1210/mend.10.6.8776730. [DOI] [PubMed] [Google Scholar]
- 14.Segnitz B, Gehring U. J Biol Chem. 1997;272:18694–18701. doi: 10.1074/jbc.272.30.18694. [DOI] [PubMed] [Google Scholar]
- 15.Nathan D F, Lindquist S. Mol Cell Biol. 1995;15:3917–3925. doi: 10.1128/mcb.15.7.3917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joab I, Radanyi C, Renoir J M, Buchou T, Catelli M-G, Binart N, Mester J, Baulieu E-E. Nature (London) 1984;308:850–853. doi: 10.1038/308850a0. [DOI] [PubMed] [Google Scholar]
- 17.Catelli M-G, Binart N, Jung-Testas I, Renoir J M, Baulieu E-E, Feramisco J R, Welch W J. EMBO J. 1985;4:3131–3135. doi: 10.1002/j.1460-2075.1985.tb04055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sabbah M, Radanyi C, Redeuilh G, Baulieu E-E. Biochem J. 1996;314:205–213. doi: 10.1042/bj3140205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Picard D, Salser S J, Yamamoto K R. Cell. 1988;54:1073–1080. doi: 10.1016/0092-8674(88)90122-5. [DOI] [PubMed] [Google Scholar]
- 20.Scherrer L C, Picard D, Massa E, Harmon J M, Simons S S, Yamamoto K R, Pratt W B. Biochemistry. 1993;32:5381–5386. doi: 10.1021/bi00071a013. [DOI] [PubMed] [Google Scholar]
- 21.Gasc J M, Renoir J M, Faber L E, Delahaye F, Baulieu E-E. Exp Cell Res. 1990;186:362–367. doi: 10.1016/0014-4827(90)90317-4. [DOI] [PubMed] [Google Scholar]
- 22.Renoir J M, Radanyi C, Jung-Testas I, Faber L E, Baulieu E-E. J Biol Chem. 1990;265:14402–14406. [PubMed] [Google Scholar]
- 23.Kang K I, Devin J, Cadepond F, Jibard N, Guiochon-Mantel A, Baulieu E-E, Catelli M-G. Proc Natl Acad Sci USA. 1994;91:340–344. doi: 10.1073/pnas.91.1.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meng X, Devin J, Sullivan W P, Toft D, Baulieu E-E, Catelli M-G. J Cell Sci. 1996;109:1677–1687. doi: 10.1242/jcs.109.7.1677. [DOI] [PubMed] [Google Scholar]
- 25.DeFranco D B, Ramakrishnan C, Tang Y. J Steroid Biochem Mol Biol. 1998;65:51–58. doi: 10.1016/s0960-0760(97)00177-5. [DOI] [PubMed] [Google Scholar]
- 26.Gouilleux F, Sola B, Couette B, Richard-Foy H. Nucleic Acids Res. 1991;19:1563–1569. doi: 10.1093/nar/19.7.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sikorski R S, Boeke J D. Methods Enzymol. 1991;194:302–318. doi: 10.1016/0076-6879(91)94023-6. [DOI] [PubMed] [Google Scholar]
- 28.Devin-Leclerc J, Meng X, Delahaye F, Leclerc P, Baulieu E-E, Catelli M-G. Mol Endocrinol. 1998;12:842–854. doi: 10.1210/mend.12.6.0121. [DOI] [PubMed] [Google Scholar]
- 29.Segard-Maurel I, Rajkowski K, Jibard N, Schweizer-Groyer G, Baulieu E-E, Cadepond F. Biochemistry. 1996;35:1634–1642. doi: 10.1021/bi951369h. [DOI] [PubMed] [Google Scholar]
- 30.Radanyi C, Renoir J M, Sabbah M, Baulieu E-E. J Biol Chem. 1989;264:2568–2573. [PubMed] [Google Scholar]
- 31.Bohen S P, Yamamoto K R. Proc Natl Acad Sci USA. 1993;90:11424–11428. doi: 10.1073/pnas.90.23.11424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Inano K, Curtis S W, Korach K S, Omata S, Horigome T. J Biochem. 1994;116:759–766. doi: 10.1093/oxfordjournals.jbchem.a124593. [DOI] [PubMed] [Google Scholar]
- 33.Shaknovich R, Shue G, Kohtz D S. Mol Cell Biol. 1992;12:5059–5068. doi: 10.1128/mcb.12.11.5059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Adnan A, Bharadwaj S, O’Carroll R, Ovsenek N. Mol Cell Biol. 1998;18:4949–4960. doi: 10.1128/mcb.18.9.4949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Collier N C, Schlessinger M J. J Cell Biol. 1986;103:1495–1507. doi: 10.1083/jcb.103.4.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]