Abstract
Fermentation of fructooligosaccharides (FOS) and other oligosaccharides has been suggested to be an important property for the selection of bacterial strains used as probiotics. However, little information is available on FOS transport and metabolism by lactic acid bacteria and other probiotic bacteria. The objectives of this research were to identify and characterize the FOS transport system of Lactobacillus paracasei 1195. Radiolabeled FOS was synthesized enzymatically from [3H]sucrose and purified by column and thin-layer chromatography, yielding three main products: glucose (G) α-1,2 linked to two, three, or four fructose (F) units (GF2, GF3, and GF4, respectively). FOS hydrolysis activity was detected only in cell extracts prepared from FOS- or sucrose-grown cells and was absent in cell supernatants, indicating that transport must precede hydrolysis. FOS transport assays revealed that the uptake of GF2 and GF3 was rapid, whereas little GF4 uptake occurred. Competition experiments showed that glucose, fructose, and sucrose reduced FOS uptake but that other mono-, di-, and trisaccharides were less inhibitory. When cells were treated with sodium fluoride, iodoacetic acid, or other metabolic inhibitors, FOS transport rates were reduced by up to 60%; however, ionophores that abolished the proton motive force only slightly decreased FOS transport. In contrast, uptake was inhibited by ortho-vanadate, an inhibitor of ATP-binding cassette transport systems. De-energized cells had low intracellular ATP concentrations and had a reduced capacity to accumulate FOS. These results suggest that FOS transport in L. paracasei 1195 is mediated by an ATP-dependent transport system having specificity for a narrow range of substrates.
The ability of specific dietary substances to influence the gastrointestinal microflora by increasing the population of beneficial bacteria has attracted considerable research attention. These so-called prebiotic substances are defined as food ingredients that are neither hydrolyzed nor adsorbed in the stomach or gastrointestinal tract but rather confer beneficial effects on the host by selectively stimulating the growth of desirable bacteria in the colon (14). Some investigators have suggested that the prebiotic hypothesis, if true, could be “one of the most important stories to emerge in nutrition and gut microbiology since the turn of the century” (24).
Among the prebiotic substances that have been studied, the most heavily researched are the disaccharide lactulose; the oligosaccharides, especially fructooligosaccharides (FOS) and galactooligosaccharides; and various polysaccharides, including inulin and various starch-based materials (2, 8, 9, 14, 21, 33, 41). By definition, these substances are resistant to hydrolytic enzymes and are ordinarily fermented by a narrow range of colonic bacteria (9, 14, 29). Importantly, among the bacteria that metabolize prebiotic oligosaccharides are strains of Lactobacillus and Bifidobacterium spp., organisms that are generally considered to be desirable members of the colonic microbiota (12, 19, 26). Numerous in vitro and in vivo studies have shown that these bacteria are stimulated or enriched in the presence of FOS or other oligosaccharides (3-6, 13, 17, 25, 32, 34, 39).
Recently, we screened 28 strains of lactic acid bacteria (LAB) and related bacteria for the ability to ferment FOS and showed that 12 of 16 strains of Lactobacillus and 7 of 8 strains of Bifidobacterium (19) fermented FOS. In contrast, eight strains of Escherichia coli, Salmonella spp., and other enteric bacteria were unable to use FOS as a sole carbon source (H. Kaplan and R. W. Hutkins, unpublished data). The FOS mixture used in this study was purified to remove the simple sugars from a commercially available material (Nutraflora; GTC Nutrition Company, Westminster, Colo.) that was made via enzymatic synthesis from sucrose (15, 16). It consisted of a glucose monomer (G) α-1,2 linked to two or more β-2,1-linked fructosyl units (F) to yield 1-kestose (GF2), nystose (GF3), and 1F-fructofuranosyl nystose (GF4). Growth experiments with several of the FOS-fermenting strains revealed that only the GF2 and GF3 fractions were consumed and that GF4 was not metabolized. However, the metabolic basis for FOS metabolism and, specifically, the means by which these sugars are transported by LAB has not been determined. Although transport systems for various trisaccharides were described for other gram-positive bacteria, including Clostridium thermocellum and Streptococcus mutans, there are no reports describing such systems in LAB (31, 36). Therefore, the objectives of this study were to determine how FOS transport occurs in the FOS-fermenting strain Lactobacillus paracasei 1195 to assess the specificity of the FOS transport system and to identify the energy source used to drive FOS transport.
MATERIALS AND METHODS
Organism and growth conditions.
L. paracasei 1195 was obtained from the University of Nebraska Department of Food Science and Technology Culture Collection. This strain was previously classified as Lactobacillus plantarum but was reclassified as L. paracasei based on ribotyping and 16S RNA sequence analyses. Cells were maintained in MRS broth (Difco, Inc., Ann Arbor, Mich.) supplemented with 0.05% (wt/vol) l-cysteine. Cells were routinely incubated at 37°C in an anaerobic chamber (Forma Scientific, Marietta, Ohio) containing an atmosphere of 5% CO2, 10% H2, and 85% N2 for 12 h. For most experiments, MRS-FOS broth was prepared by adding 2% (wt/vol) FOS (GTC Nutrition Company) to MRS. The FOS solution was filter sterilized prior to addition to the autoclaved MRS broth. The commercial FOS contains 4 to 5% free glucose, fructose, and sucrose; therefore, in some experiments, purified FOS was prepared by removing these sugars by charcoal column chromatography (19).
Synthesis of radiolabeled FOS.
Radiolabeled FOS was synthesized enzymatically from fructose [1-3H(N)]sucrose (10.2 Ci/mmol; Sigma Chemical Co., St. Louis, Mo.) by following previously described procedures (16, 37). Briefly, 1.0 ml of a 50% (wt/vol) [3H]sucrose solution (225 mCi) was incubated with 1.25 U of purified fructosyltransferase (FFase) in 50 mM sodium acetate buffer (pH 5.5) for 24 h at 55°C. The FFase was obtained from Aspergillus niger strain ATCC 20611 (15, 16), which was kindly provided by Yasuhito Tashiro, Bio Science Laboratories, Meiji Seika Kaisha, Ltd., Tokyo, Japan. The reaction was terminated by boiling the mixture for 5 min. Sugars in the mixture were then separated by preparative thin-layer chromatography (TLC). The reaction mixture and standards for glucose, fructose, sucrose, and FOS were spotted on 20- by 20-cm TLC silica gel plates (Whatman Ltd., Kent, United Kingdom). The plates were twice developed in an acetic acid-chloroform-water (7:5:1) solvent. Bands for the standard sugars were visualized by spraying plates with ethanolic 50% H2SO4, followed by heating for 5 min at 150°C. For the reaction mixture, horizontal sections (ca. 1 to 2 cm) for each lane were scraped from the plates and eluted with water and the debris was removed by centrifugation. The supernatant mixtures were lyophilized (FTS Systems, Inc., Stone Ridge, N.Y.), and the purity of the [3H]FOS was confirmed by TLC. Based on the Rf values for each FOS species, the approximate composition of the pooled [3H]FOS mixture was 29% GF2, 67% GF3, 3% GF4, and 1% glucose, fructose, and sucrose combined.
Sugar uptake assays.
Cells from exponentially grown cultures were harvested by centrifugation (10,000 × g at 4°C for 15 min). Pellets were washed twice with 50 mM potassium phosphate buffer containing 5 mM MgCl2 (pH 6.5) and resuspended in the same buffer to an optical density (at 625 nm) between 0.8 and 1.0. Transport assays were started by the addition of a 30.0 mM [3H]FOS (104 dpm/nmol) working solution to cell suspensions to give a final FOS concentration of 1 mM. At various times, duplicate 1.0-ml portions of the reaction mixture were removed and centrifuged (12,000 × g for 2 min) through silicon oil (18). The radioactivity in the supernatant and in the cell pellets was determined by liquid scintillation counting (model LS 3801 apparatus; Beckman Instruments, Fullerton, Calif.). Transport experiments were also done in the presence of competing saccharides added at 100-fold excess 1 min prior to the addition of 1 mM [3H]FOS. Rates were determined from duplicate samples taken after 30 and 60 s. Results are given as averages of duplicate samples from two independent experiments.
To identify the accumulated FOS species, 8.0 ml of cells was resuspended and incubated with [3H]FOS as described above and after 10 min, five 1.0-ml portions of the reaction mixture were removed and centrifuged through silicon oil. Pooled pellets were resuspended in 1.0 ml of 5% butanol and vigorously vortexed for 5 min. Debris was removed by centrifugation, and the sugars in the supernatant were separated by TLC as described above. Sections comigrating with each FOS fraction (GF2, GF3, and GF4) were scraped from the plates, resuspended in water, and eluted. The concentration of each FOS species was determined based on the calculated specific activity and the intracellular volume.
The effect of ionophores and metabolic inhibitors on FOS transport was determined by treating individual cell suspensions (grown and prepared as described above) with 0.02 mM nigericin, 0.02 mM valinomycin, nigericin plus valinomycin (0.02 mM each), 0.05 mM monensin, 10 mM iodoacetate, or 10 mM sodium fluoride (NaF) or by leaving suspensions untreated. Stock concentrations of inhibitors were dissolved in either ethanol (for suspensions containing nigericin, valinomycin, or monensin) or water (for suspensions containing iodoacetate or NaF). Preliminary experiments demonstrated that ethanol addition had no effect on transport. All cell suspensions were incubated with each agent for 10 min at room temperature prior to the addition of [3H]FOS. Average rates were determined, as described above, from three independent experiments.
In experiments in which ortho-vanadate was used, cells were grown in MRS-FOS medium supplemented with 50 mM l-arginine to induce enzymes of the arginine deiminase pathway. Pellets were washed twice with 50 mM 2-(N-morpholino)ethanesulfonic acid (MES) containing 5 mM MgCl2 (pH 6.5) and resuspended to an optical density of approximately 25. To de-energize cells, suspensions were incubated with 10 mM 2-deoxyglucose for 60 min at 37°C. Cells were subsequently washed twice and then resuspended in the same buffer to an optical density between 0.8 and 1.0. De-energized cell suspensions were preenergized for 10 min with 50 mM arginine in 50 mM MES buffer with or without 5 mM ortho-vanadate (22).
The intracellular pH and membrane potentials were determined based on the accumulation ratio of [14C]benzoate and [3H]tetraphenylphosphonium bromide (TPP), respectively (20), and the proton motive force (PMF) was calculated from the Nernst equation. Triplicate samples were used, and all experiments were replicated twice. The intracellular volume was estimated by the differences in the accumulation of 3H2O and [3H]polyethylene glycol (20). Radiolabeled [3H]TPP was purchased from American Radiolabeled Chemicals, Inc. (St. Louis, Mo.); all other isotopes were purchased from New England Nuclear.
Enzyme assays.
Log-phase cells were harvested by centrifugation (10,000 × g at 4°C for 20 min). The supernatant was saved, and the pellets were washed twice with phosphate buffer (pH 6.5) and resuspended in the same buffer. The cells were disrupted by using a Mini-BeadBeater (BioSpec Products, Inc., Bartlesville, Okla.) with 0.1 mm-diameter beads by using five 10-s pulses at 5,000 rpm. Debris was removed by centrifugation. The β-fructofuranosidase assay mixtures consisted of 0.2 ml of either supernatant or cell extracts and 0.8 ml of 50 mM potassium phosphate buffer containing either 2% (wt/vol) purified FOS, sucrose, or inulin (average degree of polymerization of 35). The mixtures were incubated at 37°C for 60 min, and the reaction was stopped by boiling for 10 min. The concentrations of free fructose and glucose in the samples were determined by using a fructose, glucose, sucrose enzyme kit (R-Biopharm Inc., Marshall, Mich.). Hydrolysis activities were expressed as nanomoles of fructose released per minute per milligram of protein.
Other measurements.
The ATP concentration was determined by the method of Waite et al. (38), with the following modifications. Log-phase cells were washed and resuspended in 50 mM morpholinepropanesulfonic acid (MOPS) buffer, pH 6.5, to an optical density (at 625 nm) of 0.02. At time zero, FOS was added to cells at a 1 mM final concentration and the mixture was incubated at 22°C. After 10 min, a 50-μl sample was removed and placed in a Filtravette (New Horizon Diagnostics, Columbia, Md.) and 50 μl of buffer was added. Cells collected on the filter were immediately used for intracellular ATP measurements by adding 50 μl of nucleotide-releasing agent for bacteria (Lumac B.V., Landgraaf, The Netherlands) and 50 μl of luciferin-luciferase reagent (New Horizon Diagnostics). Cells and reagents were rapidly aspirated three times, and the Filtravette was placed in a model 3550 microluminometer (New Horizon Diagnostics). Light emission was integrated over 10 s, and results were recorded as relative light units. Standard curves were prepared by serial dilution of an ATP standard solution in 50 mM MOPS buffer, pH 6.5.
Phosphotransferase activity in permeabilized cells was determined by an NADH-coupled spectrophotometric enzyme assay as described previously (7). Protein concentrations of cell extracts were determined by the method of Lowry et al. (23). All chemicals, unless noted, were from Sigma Chemical Co.
RESULTS
FOS uptake by L. paracasei 1195.
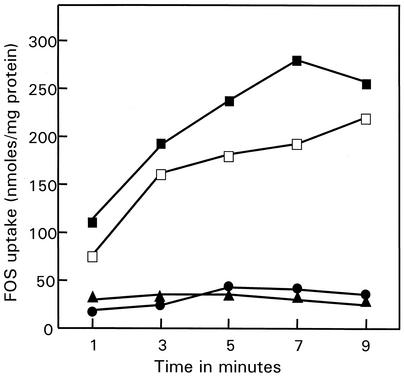
Cells of L. paracasei 1195 grown on FOS or sucrose, washed, and resuspended in buffer accumulated [3H]FOS when assayed immediately after harvest (Fig. 1). No accumulation was observed for either glucose- or fructose-grown cells.
FIG. 1.
FOS transport by L. paracasei 1195. Log-phase cells grown in MRS broth containing FOS (▪), sucrose (□), glucose (▴), or fructose (•) were harvested, washed twice, and resuspended in potassium phosphate buffer. The reactions were started by the addition of 1 mM [3H]FOS.
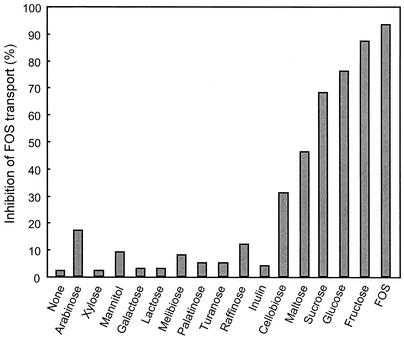
The effect of competing sugars and sugar analogs on [3H]FOS uptake was determined by measuring the initial rate of FOS accumulation in the presence of the test compounds. As shown in Fig. 2, transport rates were reduced in the presence of glucose, fructose, or sucrose (76, 87, and 68%, respectively) added at 100 times the [3H]FOS concentration. Uptake of [3H]FOS was completely inhibited by a 100-fold excess of nonlabeled FOS. In contrast, the disaccharides maltose and cellobiose caused moderate inhibition (46 and 31%, respectively) and none of the other sugars tested in this experiment had an inhibitory effect. The sucrose analogs palatinose (6-O-β-d-glucopyranosyl-d-fructofuranose) and turanose (3-O-β-d-glucopyranosyl-d-fructofuranose) did not affect FOS uptake.
FIG. 2.
Inhibition of FOS transport by competitive sugars in L. paracasei 1195. Experiments were performed as described in the text. The control FOS uptake rate was 101 nmol per min per mg (dry weight) of cells.
Energetics of the FOS transport system.
To identify the energy source used to fuel FOS transport, uptake assays in the presence of ionophores and metabolic inhibitors were performed. The effects of these agents on the PMF and on intracellular ATP concentrations were also determined. Uptake of FOS was generally reduced by both of the metabolic inhibitors tested (Table 1). Iodoacetate, an inhibitor of the glycolytic enzyme glyceraldehyde-3-phosphate dehydrogenase (GADPH), reduced the FOS uptake rate by 62% and decreased the intracellular ATP level by 97%. This large reduction in ATP formation was also associated with only a modest decrease in PMF. Sodium fluoride, another inhibitor of substrate level phosphorylation, reduced the uptake of FOS by 36% and that of ATP by 71%. The ionophores nigericin, valinomycin, and monensin had little effect on FOS uptake and intracellular ATP, despite rather large decreases in the PMF. Greater reductions in FOS uptake rates, PMF, and ATP concentrations were observed in the presence of nigericin plus valinomycin. In general, the ability of cells to transport FOS was dependent less on the PMF and more on the intracellular availability of ATP.
TABLE 1.
Effect of metabolic inhibitors on FOS transport rates in L. paracasei 1195
| Treatment | % Inhibition | pMF (mV)b | [ATP] (mM)c |
|---|---|---|---|
| Controla | 0 | −153 ± 4 | 2.03 |
| Nigericin | 29 | −52 ± 3 | 1.68 |
| Valinomycin | 5 | −44 ± 5 | 1.56 |
| Nigericin + valinomycin | 42 | −24 ± 2 | 0.85 |
| Monensin | 15 | −69 ± 4 | 1.58 |
| Iodoacetate | 62 | −92 ± 1 | 0.06 |
| NaF | 36 | −90 ± 2 | 0.58 |
The control rate was 101 nmol of FOS accumulated per min per mg (dry weight) of cells.
Averages ± standard deviations (n = 6).
Intracellular concentrations.
The presence of an FOS-specific phosphoenolpyruvate-dependent phosphotransferase system (PTS) in L. paracasei 1195 was tested by measuring the formation of pyruvate in reaction mixtures containing cell extracts, FOS, and phosphoenolpyruvate. No PTS activity was observed (data not shown), nor could we detect phosphorylated FOS species (data not shown).
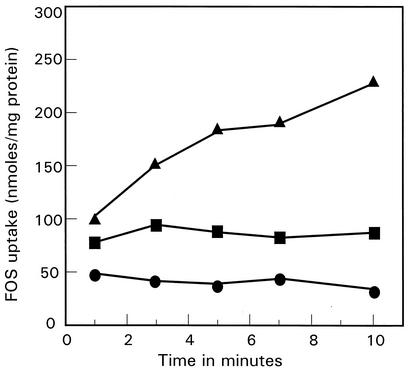
To investigate further the nature of the energy source of FOS uptake, cells were starved in order to deplete ATP. Preliminary experiments indicated that cells maintained high levels of ATP and could still transport FOS, even after prolonged incubation (up to 4 h) in buffer at 37°C. However, it was possible to de-energize cells and deplete ATP by incubating cell suspensions in buffer containing the nonmetabolizable sugar 2-deoxyglucose. De-energized cells could be reenergized by the addition of arginine, which generates ATP via the arginine deiminase pathway. In these cells, the intracellular ATP concentrations increased from 0.76 mM (in the de-energized cells) to 3.43 mM. The arginine deiminase pathway is not affected by ortho-vanadate, an inhibitor of glycolytic substrate level phosphorylation (40); thus, it was possible to add vanadate to arginine-treated cells without affecting the ability of cells to make ATP. Vanadate-treated cells energized with arginine had an intracellular ATP concentration of 3.00 mM. When FOS uptake experiments were performed with the de-energized, ATP-depleted cells, FOS was not accumulated; however, arginine-energized cells accumulated FOS to high levels (Fig. 3). Importantly, when ortho-vanadate was added to the energized cells, uptake was abolished. These findings indicate that FOS uptake requires ATP and that the inhibition of FOS transport by vanadate must be due to inhibition of the transport system directly.
FIG. 3.
Effect of vanadate on FOS uptake by arginine-energized cells of L. paracasei 1195. Cells were grown in MRS broth supplemented with 50 mM l-arginine to induce enzymes of the arginine deiminase pathway. Cells were harvested, washed, and de-energized by incubation with 2-deoxyglucose for 40 min at 37°C (▪). A portion of the cells were then energized with 50 mM arginine either with (•) or without (▴) 5 mM vanadate. Uptake assays were started by the addition of 1 mM [3H]FOS.
FOS hydrolysis activity of L. paracasei 1195.
Extracellular FOS hydrolysis activity was absent in L. paracasei 1195 cells regardless of the sugars used as a growth substrate. In contrast, FOS hydrolysis activity, presumably due to a β-fructofuranosidase, was detected in cell extracts for all growth substrates but was much higher during growth on sucrose, FOS, and inulin than during that on fructose and glucose (Table 2). Interestingly, the sucrose-hydrolyzing activity in the cell extracts, although somewhat lower than when FOS was the substrate, mirrored that of FOS hydrolysis in that activities were induced by sucrose, FOS, or inulin and repressed by glucose or fructose.
TABLE 2.
Hydrolysis of FOS, sucrose, and inulin by cell extracts of L. paracasei 1195a
| Growth substrate | FOS | Sucrose | Inulin |
|---|---|---|---|
| Glucose | 6 ± 2.4 | 4 ± 0.7 | 4 ± 1.7 |
| Fructose | 27 ± 4.2 | 30 ± 4.2 | 25 ± 4.9 |
| Sucrose | 112 ± 2.8 | 85 ± 2.1 | 26 ± 1.4 |
| Inulin | 142 ± 4.9 | 113 ± 4.1 | 24 ± 3.5 |
| FOS | 165 ± 4.2 | 106 ± 3.5 | 49 ± 3.5 |
Activities are expressed as nanomoles of fructose released per minute per milligram of protein (means ± standard deviations; n = 4).
When the FOS and inulin hydrolysis products were analyzed, only fructose initially appeared in the reaction mixture (data not shown). Although a small amount of glucose was eventually detected, the fructose concentration after 60 min was more than three times higher than the glucose concentration. Not surprisingly, equal concentrations of free glucose and fructose were produced by sucrose-, FOS-, and inulin-grown cell extracts when sucrose was used as the substrate. None of the cells extracts could hydrolyze palatinose, turanose, or levan.
Recovery of internal sugars in L. paracasei 1195.
Although cells accumulated [3H]FOS, it was not immediately apparent whether FOS was taken up intact or hydrolyzed extracellularly just before transport. Therefore, cells were incubated with [3H]FOS and separated from the incubation buffer by centrifugation and the accumulated material was extracted and then separated by TLC. The intracellular and extracellular concentrations of individual FOS moieties were calculated based on the specific activity of each fraction. The results showed that all three FOS species were present in the intracellular extracts; however, GF2 was the most abundant form of FOS, having an intracellular concentration more than 10 times the outside concentration (Table 3). Although glucose, fructose, and sucrose were also detected in the intracellular extracts, it is likely that they were produced from FOS via intracellular hydrolysis during the 10-min incubation period. Indeed, when toluene-permeabilized cells were incubated with [3H]FOS, the glucose, fructose, and sucrose concentrations increased as GF2 and GF3 decreased (data not shown).
TABLE 3.
Intracellular accumulation of FOS by L. paracasei 1195
| FOS species | [In]/[out] ratioa |
|---|---|
| GF4 | 0.3 |
| GF3 | 1.4 |
| GF2 | 10.8 |
| Sucrose | 1.8 |
| Glucose + fructose | 3.5 |
Based on the intracellular and extracellular concentrations of each FOS or sugar moiety.
DISCUSSION
Despite significant commercial interest in using oligosaccharides as prebiotic substrates, little is known about the physiological basis for how these oligosaccharides are metabolized by LAB and related bacteria. Previously, we showed that the ability to ferment FOS was a property shared by several species of bifidobacteria and lactobacilli and that the GF2 and GF3 fractions were preferentially metabolized (19). Although the means by which FOS and other oligosaccharides are hydrolyzed by LAB have been studied (13, 27, 32), there have been no reports describing oligosaccharide transport by these bacteria. The experiments reported here provide the first description of an FOS transport system in a LAB.
FOS transport and hydrolysis activities are inducible and specific.
FOS was transported at high rates in L. paracasei 1195 cells grown previously on FOS or sucrose but up to fivefold less in glucose- or fructose-grown cells. Similarly, FOS hydrolysis activity was always highest in extracts from cells obtained during growth on FOS, sucrose, or inulin. Collectively, these results indicate that both the FOS uptake and hydrolysis systems are induced by sucrose or higher oligosaccharides and repressed by products of their hydrolysis. Uptake of FOS by induced cells was also competitively inhibited by the presence of excess glucose, fructose, or sucrose in the assay mixture, indicating that these sugars may be transported by the FOS transport system. However, since excess unlabeled FOS did not the inhibit uptake of either [14C]glucose, [14C]fructose, or [14C]sucrose (data not shown), it appears that separate uptake systems for the latter sugars likely exist.
Two other glucose-containing disaccharides, cellobiose and maltose, also inhibited FOS uptake to a lesser extent. However, other mono- and disaccharides, specifically, the sucrose analogs palatinose and turanose, the trisaccharide raffinose, and the fructose polymer inulin, had no effect on FOS uptake rates. These data suggest that the FOS uptake system in L. paracasei 1195 is specific for a narrow range of substrates, although the presence of glucose or fructose residues appears to be less critical than the actual structure of the substrate. Rather, it appears that the main requirement for the uptake system is that the substrate be a β-fructose or β-type sugar linked to α-glucose. Turanose and palatinose did not inhibit FOS transport because, even though they are both glucose-fructose disaccharides, they differ in the fructose linkage type and do not contain β-type linkage as in sucrose.
Substrate size is apparently also important, since relatively little GF4 was accumulated (Table 3). These results must be qualified, however, since the mixed [3H]FOS substrate contained only 3% GF4, and it is possible that the low [in]/[out] ratio of 0.3 was due not to the substrate preference but rather to the higher concentrations of GF2 and GF3 in the reaction mixture. However, these results are consistent with those from our previous report, which showed that the GF4 moiety of FOS was not consumed during growth on FOS, even after the GF2 and GF3 fractions had been mostly depleted (19). Moreover, the substrate competition assays revealed that inulin, which has the same molecular structure of FOS, only with many more fructose monomers, had little affinity for the FOS transport system.
The intracellular FOS hydrolysis activity was also inducible. Only extracts obtained from cells grown previously on FOS, sucrose, or inulin were able to hydrolyze FOS; no activity was observed for glucose- or fructose-grown cells. Other substrates, including melibiose, raffinose, and levan, were not hydrolyzed (data not shown). Interestingly, some FOS hydrolysis activity was detected in the cell supernatants from the inulin-grown cells, demonstrating that an extracellular inulinase that is capable of hydrolyzing sucrose and FOS was produced. The inulinase, however, is likely distinct from the FOS-hydrolyzing enzyme, since this activity was absent in the FOS-grown cells.
Examination of the FOS and inulin hydrolysis products revealed that fructose was the most abundant product, although a small amount of glucose was detected. This suggests that the enzyme responsible for hydrolysis is likely an exo-β-fructofuranosidase that is capable of hydrolyzing FOS and inulin. However, the observed 3/1 product ratio (fructose-glucose) after FOS hydrolysis and the activity on sucrose indicates that the enzyme can also hydrolyze the α-1,2 bond between fructose and glucose.
FOS uptake is mediated via an ATP-dependent system.
LAB accumulate sugars by either secondary active transport (mainly the PMF), the PTS, or an ATP-mediated system. Uptake of FOS by L. paracasei 1195 was only partially affected (<30% inhibition) by nigericin, valinomycin, or monensin, ionophores that dissipate pH gradients, membrane potentials, or sodium ion gradients, respectively. This result indicates that FOS uptake was not mediated by one of these components of the PMF. Furthermore, when the PMF was abolished by the addition of valinomycin plus nigericin, uptake still occurred. Although all of the ionophores did reduce FOS uptake, the observed inhibition was likely due to the ability of these agents to deplete ATP pools as a secondary, indirect effect, caused when cells use ATP-dependent pumps or transporters in an attempt to reestablish ion gradients (1). We conclude from these experiments with ionophores, therefore, that FOS uptake can proceed in the absence of an electrochemical ion gradient and that FOS uptake is not mediated by a PMF.
No PTS activity was observed when using FOS as a substrate. Also, examination of the intracellular material accumulated after a 10-min incubation with [3H]FOS revealed that none of the labeled products had been phosphorylated. These results show that FOS uptake does not occur by a PTS. In addition, we noted that neither fructose, sucrose, nor glucose appeared in the supernatants during the FOS transport assays, indicating that efflux of the intracellular hydrolysis products did not occur.
Uptake of FOS by L. paracasei 1195 was inhibited by agents that block substrate level phosphorylation and ATP synthesis. In particular, iodoacetate depleted nearly all of the ATP reserves and caused the greatest reduction of FOS uptake (62%). Although cells were able to maintain ATP reserves while held in buffer in the absence of a fermentable sugar, it was possible to deplete more than 50% of the ATP stores by incubating cells in the presence of the nonmetabolizable glucose analog 2-deoxyglucose. That these ATP-depleted cells were impaired in their ability to transport FOS further suggested that uptake requires ATP.
The ability of L. paracasei 1195 to accumulate FOS was also sensitive to ortho-vanadate. This agent has been shown to be an effective inhibitor of ATP-binding cassette (ABC) transport systems (11). Vanadate, an analogue of inorganic phosphate, mimics the gamma phosphate of ATP and binds to ADP during the transition state of ATP hydrolysis (6). Thus, in ABC-driven transport systems, vanadate inhibits ATPase activity. According to this model, then, the substrate/permease-substrate binding-protein complex is trapped by vanadate such that substrate transport is prevented (11, 35). Our findings indicate that the inhibition of FOS transport by ortho-vanadate is due to inhibition of the transport system itself and that the FOS transport system most likely belongs to the ABC superfamily.
Although transport systems for FOS in LAB have not been previously reported, in the oral streptococcus S. mutans, a binding-protein-dependent multiple sugar metabolism (MSM) transport system was described that transported the trisaccharides raffinose and isomaltotriose and the disaccharide melibiose (31). The msm genes coding for this system are organized as a cluster typical of ABC operons in that it contains genes coding for ATP- and substrate-binding proteins, and two membrane-spanning domains (28, 31). Cvitkovitch et al. (10) provided evidence that the transport of sugars by the MSM system in Streptococcus mutans was regulated by a PTS that modulates both expression and transport activity. A similar system, encoded for by the raf gene cluster, was found to be involved in raffinose transport in Streptococcus pneumoniae (30). The raf system, however, is specific for raffinose, whereas the MSM system has broader specificity, transporting at least three different substrates. In contrast, sugar competition assays indicated that raffinose had little affinity for the FOS transport system described here. Nonetheless, although the genetic basis for how L. paracasei 1195 metabolizes oligosaccharides has not yet been established, biochemical and physiological data support the presence of an inducible and specific ATP-dependent FOS transport system that is functionally similar to the MSM system in the related organism S. mutans.
Acknowledgments
This research was supported by grants from Dairy Management, Inc., and the U.S. Department of Agriculture-Midwest Advanced Food Manufacturing Alliance.
We thank Yasuhito Tashiro for supplying us with fructosyltransferase, Greg Siragusa for lending us the luminometer, and the GTC Nutrition Company for providing FOS. We also thank Lisa Durso for critical review of the manuscript.
Footnotes
Paper no. 13835, Journal Series, Nebraska Agricultural Experiment Station, Lincoln.
REFERENCES
- 1.Ames, G. F., and A. K. Joshi. 1990. Energy coupling in bacterial periplasmic permeases. J. Bacteriol. 172:4133-4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bird, A. R., I. L. Brown, and D. L. Topping. 2000. Starches, resistant starches, the gut microflora and human health. Curr. Issues Intest. Microbiol. 1:25-37. [PubMed] [Google Scholar]
- 3.Boehm, G., M. Lidestri, P. Casetta, J. Jelinek, F. Negretti, B. Stahl, and A. Marini. 2002. Supplementation of a bovine milk formula with an oligosaccharide mixture increases counts of faecal bifidobacteria in preterm infants. Arch. Dis. Child Fetal Neonatal. Ed. 86:F178-F181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bouhnik, Y., B. Flourie, L. D'Agay-Abensour, P. Pochart, G. Gramet, M. Durand, and J. C. Rambaud. 1997. Administration of transgalacto-oligosaccharides increases fecal bifidobacteria and modifies colonic fermentation metabolism in healthy humans. J. Nutr. 127:444-448. [DOI] [PubMed] [Google Scholar]
- 5.Bouhnik, Y., K. Vahedi, L. Achour, A. Attar, J. Salfati, P. Pochart, P. Marteau, B. Flourie, F. Bornet, and J. C. Rambaud. 1999. Short-chain fructo-oligosaccharide administration dose-dependently increases fecal bifidobacteria in healthy humans. J. Nutr. 129:113-116. [DOI] [PubMed] [Google Scholar]
- 6.Chen, J., S. Sharma, F. A. Quiocho, and A. L. Davidson. 2001. Trapping the transition state of an ATP-binding cassette transporter: evidence for a concerned mechanism of maltose transport. Proc. Natl. Acad. Sci. USA 98:1525-1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Christensen, D. P., and R. W. Hutkins. 1994. Glucose uptake by Listeria monocytogenes Scott A and inhibition by pediocin JD. Appl. Environ. Microbiol. 60:3870-3873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collins, M. D., and G. R. Gibson. 1999. Probiotics, prebiotics, and synbiotics: approaches for modulating the microbial ecology of the gut. Am. J. Clin. Nutr. 69:1052S-1057S. [DOI] [PubMed] [Google Scholar]
- 9.Crittenden, R. G. 1999. Prebiotics, p. 141-156. In G. W. Tannock (ed.), Probiotics: a critical review. Horizon Scientific Press, Wymondham, Norfolk, United Kingdom.
- 10.Cvitkovitch, D. G., D. A. Boyd, and I. R. Hamilton. 1995. Regulation of sugar transport via the multiple sugar metabolism operon of Streptococcus mutans by the phosphoenolpyruvate phosphotransferase system. J. Bacteriol. 177:5704-5706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davidson, A. L. 2002. Mechanism of coupling of transport to hydrolysis in bacterial ATP-binding cassette transporters. J. Bacteriol. 184:1225-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fuller, R. 1989. Probiotics in man and animals. J. Appl. Bacteriol. 66:365-378. [PubMed] [Google Scholar]
- 13.Gibson, G. R., E. R. Beatty, X. Wang, and J. H. Cummings. 1995. Selective stimulation of bifidobacteria in the human colon by oligofructose and inulin. Gastroenterology 108:975-982. [DOI] [PubMed] [Google Scholar]
- 14.Gibson, G. R., and M. B. Roberfroid. 1995. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J. Nutr. 125:1401-1412. [DOI] [PubMed] [Google Scholar]
- 15.Hidaka, H., M. Hirayama, and N. Sumi. 1988. A fructooligosaccharide-producing enzyme from Aspergillus niger ATCC 20611. Agric. Biol. Chem. 52:1181-1187. [Google Scholar]
- 16.Hirayama, M., N. Sumi, and H. Hidaka. 1989. Purification and properties of a fructooligosaccharide-producing β-fructofuranosidase from Aspergillus niger ATCC 20611. Agric. Biol. Chem. 53:667-673. [Google Scholar]
- 17.Hopkins, M. J., J. H. Cummings, and G. T. Macfarlane. 1998. Inter-species differences in maximum specific growth rates and cell yields of bifidobacteria cultured on oligosaccharides and other simple carbohydrate sources. J. Appl. Microbiol. 85:381-386. [Google Scholar]
- 18.Hutkins, R. W., and E. R. Kashket. 1987. Phosphotransferase activity in Clostridium acetobutylicum from acidogenic and solventogenic phases of growth. Appl. Environ. Microbiol. 51:1121-1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaplan, H., and R. W. Hutkins. 2000. Fermentation of fructooligosaccharides by lactic acid bacteria and bifidobacteria. Appl. Environ. Microbiol. 66:2682-2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kashket, E. R., A. G. Blanchard, and W. C. Metzger. 1980. Proton motive force during growth of Streptococcus lactis cells. J. Bacteriol. 158:238-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kolida, S., K. Tuohy, and G. R. Gibson. 2002. Prebiotic effects of inulin and oligofructose. Br. J. Nutr. 87:S193-S197. [DOI] [PubMed] [Google Scholar]
- 22.Kunji, E. R. S., E. J. Smid, R. Plapp, B. Poolman, and W. N. Konings. 1993. Di-tripeptides and oligopeptides are taken up via distinct transport mechanisms in Lactococcus lactis. J. Bacteriol. 175:2052-2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lowry, O. H., N. J. Rosebrough, A. L. Farr, and R. J. Randall. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193:265-275. [PubMed] [Google Scholar]
- 24.Macfarlane, G. T., and J. H. Cummings. 1999. Probiotics and prebiotics: can regulating the activities of intestinal bacteria benefit health? Br. Med. J. 318:999-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McBain, A. J., and G. T. Macfarlane. 1997. Investigation of bifidobacterial ecology and oligosaccharide metabolism in a three-stage compound continuous culture system. Scand. J. Gastroenterol. 32:32S-40S. [DOI] [PubMed] [Google Scholar]
- 26.McKellar, R. C., and H. W. Modler. 1989. Metabolism of fructo-oligosaccharides by Bifidobacterium spp. Appl. Microbiol. Biotechnol. 31:537-541. [Google Scholar]
- 27.McKellar, R. C., H. W. Modler, and J. Mullin. 1993. Characterization of growth and inulinase production by Bifidobacterium spp. on fructooligosaccharides. Bifidobact. Microflora 12:75-86. [Google Scholar]
- 28.McLaughlin, R. E., and J. J. Ferretti. 1996. The multiple-sugar metabolism (msm) gene cluster of Streptococcus mutans is transcribed as a single operon. FEMS Microbiol. Lett. 140:261-264. [DOI] [PubMed] [Google Scholar]
- 29.Oku, T., T. Tokunaga, and N. Hosoya. 1984. Nondigestibility of a new sweetener, “neosugar,” in the rat. J. Nutr. 114:1574-1581. [DOI] [PubMed] [Google Scholar]
- 30.Rosenow, C., M. Maniar, and J. Trias. 1999. Regulation of the α-galactosidase activity in Streptococcus pneumoniae: characterization of the raffinose utilization system. Genome Res. 9:1189-1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Russell, R. R. B., J. Aduse-Opoku, I. C. Sutcliffe, L. Tao, and J. J. Ferretti. 1992. A binding protein-dependent transport system in Streptococcus mutans responsible for multiple sugar metabolism. J. Biol. Chem. 267:4631-4637. [PubMed] [Google Scholar]
- 32.Rycroft, C. E., M. R. Jones, G. R. Gibson, and R. A. Rastall. 2001. A comparative in vitro evaluation of the fermentation properties of prebiotic oligosaccharides. J. Appl. Microbiol. 91:878-887. [DOI] [PubMed] [Google Scholar]
- 33.Sako, T., K. Matsumoto and R. Tanaka. 1999. Recent progress on research and applications of non-digestible galacto-oligosaccharides. Int. Dairy J. 9:69-80. [Google Scholar]
- 34.Sghir, A., J. M. Chow, and R. I. Mackie. 1998. Continuous culture selection of bifidobacteria and lactobacilli from human faecal samples using fructooligosaccharide as selective substrate. J. Appl. Microbiol. 85:769-777. [DOI] [PubMed] [Google Scholar]
- 35.Sharma, S., and A. L. Davidson. 2000. Vanadate-induced trapping of nucleotide by the purified maltose transport complex requires ATP hydrolysis. J. Bacteriol. 182:6570-6576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Strobel, H. J., F. C. Caldwell, and K. A. Dawson. 1995. Carbohydrate transport by the anaerobic thermophile Clostridium thermocellum LQRI. Appl. Environ. Microbiol. 61:4012-4015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tokunaga, T., T. Oku, and N. Hosoya. 1989. Utilization and excretion of a new sweetener fructooligosaccharide (neosugar), in rats. J. Nutr. 119:553-559. [DOI] [PubMed] [Google Scholar]
- 38.Waite, B. L., G. R. Siragusa, and R. W. Hutkins. 1998. Bacteriocin inhibition of two glucose transport systems in Listeria monocytogenes. J. Appl. Microbiol. 84:715-721. [DOI] [PubMed] [Google Scholar]
- 39.Wang, X., and G. R. Gibson. 1993. Effects of the in vitro fermentation of oligofructose and inulin by bacteria growing in the human large intestine. J. Appl. Bacteriol. 75:373-380. [DOI] [PubMed] [Google Scholar]
- 40.Yokota, A., M. Veenstra, P. Kurdi, H. W. van Veen, and W. N. Konings. 2000. Cholate resistance in Lactococcus lactis is mediated by an ATP-dependent multispecific organic anion transporter. J. Bacteriol. 182:5196-5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yun, J. W. 1996. Fructooligosaccharides—occurrence, preparation, and application. Enzyme Microb. Technol. 19:107-117. [Google Scholar]