Abstract
A study was undertaken to examine the effects of the heavy metals copper, lead, and zinc on biofilm and planktonic Pseudomonas aeruginosa. A rotating-disk biofilm reactor was used to generate biofilm and free-swimming cultures to test their relative levels of resistance to heavy metals. It was determined that biofilms were anywhere from 2 to 600 times more resistant to heavy metal stress than free-swimming cells. When planktonic cells at different stages of growth were examined, it was found that logarithmically growing cells were more resistant to copper and lead stress than stationary-phase cells. However, biofilms were observed to be more resistant to heavy metals than either stationary-phase or logarithmically growing planktonic cells. Microscopy was used to evaluate the effect of copper stress on a mature P. aeruginosa biofilm. The exterior of the biofilm was preferentially killed after exposure to elevated concentrations of copper, and the majority of living cells were near the substratum. A potential explanation for this is that the extracellular polymeric substances that encase a biofilm may be responsible for protecting cells from heavy metal stress by binding the heavy metals and retarding their diffusion within the biofilm.
Heavy metals are ubiquitous and persistent environmental pollutants that are introduced into the environment through anthropogenic activities, such as mining and smelting, as well as through other sources of industrial waste. In fact, over one-half the superfund sites in the United States are contaminated with at least one heavy metal (www.atsdr.cdc.gov). Heavy metals contaminate drinking water reservoirs and freshwater habitats and can alter macro- and microbiological communities (18, 24). The known mechanisms of heavy metal toxicity include inducing oxidative stress and interfering with protein folding and function (30).
Bacteria have developed a variety of resistance mechanisms to counteract heavy metal stress. These mechanisms include the formation and sequestration of heavy metals in complexes, reduction of a metal to a less toxic species, and direct efflux of a metal out of the cell (30-33). Pseudomonas aeruginosa is a ubiquitous, environmentally important microbe that may employ many resistance mechanisms, such as the mer operon that reduces toxic Hg2+ to volatile Hg0, which then diffuses out of the cell (33). However, in bacteria, efflux systems are a more common resistance mechanism for dealing with heavy metals. One such system is the cop system of Pseudomonas syringae, which contains the structural genes copABCD and is homologous to the pco system in Escherichia coli. The copB and copD genes are involved in the transport of copper across the membrane, while the products of the copA and copC genes are outer membrane proteins that bind Cu2+ in the periplasm, protecting the cell from copper (6, 36). Other types of efflux systems simply pump toxic metal ions out of the cell; these systems include the P-type ATPase cadA, which was first identified in Staphylococcus aureus and is found in other gram-positive bacteria, that pumps out Cd2+ and Zn2+ by using a phospho-aspartate intermediate (30, 32, 36).
The genetics and biochemistry of heavy metal resistance mechanisms have been carefully studied in free-swimming organisms; however, many bacteria in the environment exist in surface-attached communities called biofilms. Biofilm bacteria are usually embedded in an extracellular polymeric substance (EPS) matrix composed of polysaccharides, proteins, and nucleic acids (11, 34, 38, 44, 47). A hallmark trait of biofilms is increased resistance to antimicrobial agents compared to the resistance of free-swimming organisms (7, 13). A proposed mechanism that contributes to this increased resistance is binding and sequestration of antimicrobial agents by EPS components, such as negatively charged phosphate, sulfate, and carboxylic acid groups (17). Another factor that may contribute to the resistance of biofilms is that many antimicrobial agents target metabolically active cells. Biofilms are subject to a wide range of chemical gradients that result in decreased metabolic activity within the depths of a biofilm. In a recent study, Spoering and Lewis examined the relative effects of antimicrobial agents on stationary- and logarithmic-phase cells of P. aeruginosa and found that stationary-phase cells were more resistant to a variety of different antimicrobial agents (37). In the same study the researchers suggested that the resistance of biofilms to antimicrobial agents can be primarily attributed to the stationary phase or slow growth and the presence of a small resistant subpopulation of cells termed “persistors.”
Previous studies of biofilm and heavy metal interactions have mainly focused on the sorption of heavy metals. Several researchers have reported that biofilms are capable of removing heavy metal ions from bulk liquid (10, 16, 22, 25), and the use of biofilms to remove heavy metals from wastewater has been investigated (40, 45). Electron microscopy revealed that a P. aeruginosa biofilm was capable of sequestering heavy metals and that there was surface-associated precipitation of lanthanum by biofilm cells (23), while mercury-reducing Pseudomonas putida biofilms were found to accumulate elemental mercury on the exterior of the biofilms (41). Burkholderia cepacia biofilms on hematite and alumina surfaces were found to preferentially accumulate Pb2+ at concentrations higher than 1 μM, implying that the chemical nature of the attachment surface affects metal sequestration (39). Within a biofilm it has been found that EPS, specifically the polysaccharide components, binds heavy metals (5, 19-21, 27, 28).
In this study we sought to assess the effects of heavy metal toxicity on biofilm and free-swimming P. aeruginosa. The relative toxicities of the heavy metals zinc, copper, and lead for biofilm and free-swimming cells were examined. In addition, we compared the relative susceptibilities of logarithmic- and stationary-phase P. aeruginosa liquid cultures. Surprisingly, logarithmically growing P. aeruginosa was found to be more resistant than stationary-phase cells. However, biofilms were found to be less susceptible than free-swimming cultures. Our results also indicate that the exterior of a biofilm is preferentially killed after heavy metal treatment, which suggests that a biofilm is protected by sorption of heavy metals to the EPS matrix.
MATERIALS AND METHODS
Bacterial strains and media.
A wild-type laboratory strain of P. aeruginosa (PAO1), derived from the laboratory of Barbara Iglewski, was used for this study. A strain of PAO1 chromosomally tagged with the green fluorescent protein (GFP) was used to facilitate microscopy (13). PAO1 was grown in a modified version of minimal salts vitamin medium (MSV) at pH 6.5, which contained (per liter) 1 g of (NH4)2SO4, 0.06 g of MgSO4 · 7H2O, 0.06 g of CaCl2, 0.02 g of KH2PO4, 0.03 g of Na2HPO4 · 7H2O, 2.383 g of HEPES, 1 ml of 10 mM FeSO4, and 1 ml of a trace vitamin solution. The trace vitamin solution contained (per liter) 20 mg of biotin, 20 mg of folic acid, 50 mg of thiamine HCl, 50 mg of d-(+)-calcium pantothenate, 1 mg of vitamin B12, 50 mg of riboflavin, 50 mg of nicotinic acid, 100 mg of pyridoxine HCl, and 50 mg of p-aminobenzoic acid. For the variant MSVP, pyruvate (5.45 × 10−2 M) was added as the carbon source, and for the variant MSVG, glucose (5.55 × 10−3 M) was added as the carbon source. MOPSO-buffered saline (MOPSO) contained (per liter) 3.5 g of MOPSO (3-morpholino-2-hydroxypropanesulfonic acid) and 8.7 g of NaCl, and the pH was adjusted to 7.1 with HCl. The metals used in this study were ZnSO4, CuSO4, and Pb(NO3)2 (Sigma-Aldrich Co., St. Louis, Mo.). The antibiotic tobramycin was obtained from Sigma-Aldrich.
MIC determination.
For the MIC analysis we used a modified version of the NCCLS protocol (29), which was designed to minimize metal precipitation. In a 96-well microtiter plate, P. aeruginosa was subjected to an array of heavy metal concentrations in MSVP. The wells contained 100 μl of MSVP plus heavy metals at concentrations ranging from 0.06 to 4 mM for Zn, from 0.03 to 2 mM for Cu, and from 0.015 to 1 mM for Pb. Each well was inoculated with a 10-μl aliquot of a PAO1 culture in the late exponential phase with an optical density at 600 nm (OD600) of 0.3. Growth was monitored at OD600 every 2 h by using a microtiter plate spectrophotometer (EL800 universal microplate reader; Bio-Tek Instruments, Inc., Winooski, Vt.), and the MIC was determined after 56 h of growth at 37°C. Each experiment was performed in triplicate.
Rotating-disk biofilm reactor.
The relative heavy metal toxicities for biofilms and free-swimming P. aeruginosa were assayed by using a rotating-disk biofilm reactor (13). The reactor contained 250 ml of MSVP, and it was inoculated with an early-logarithmic-phase PAO1 culture and operated in batch mode for 24 h at room temperature. The reactor was then switched to chemostat mode with a dilution rate of 0.06 h−1 by using a peristaltic pump (Masterflex L/S; Cole-Parmer Instrument Co., Vernon Hills, Ill.). A rotating disk containing 18 identical polycarbonate coupons was added to the reactor after 24 h, and a biofilm was allowed to develop for another 24 h. Equal numbers of cells from biofilm and free-swimming cultures, as measured by viable plate counting, were then removed by taking an aliquot of the free-swimming culture from the reactor and removing the biofilm coupons from the rotating disk. The biofilm coupons were washed with MSVP or MOPSO-buffered saline to remove loosely attached cells. The samples were exposed to heavy metals at concentrations ranging from 0.015 to 225 mM for Zn, Cu, or Pb in either MSVP or MOPSO-buffered saline for 5 h at 37°C. After treatment, the samples were then placed in 900 μl of phosphate-buffered saline (PBS) (if treatment occurred in MSVP) or in 900 μl of MOPSO-buffered saline (if treatment occurred in MOPSO-buffered saline). The samples were then sonicated for 10 min at 38.5 to 40.5 Hz (Aquasonic ultrasonic cleaner; VWR Scientific Products, West Chester, Pa.) to remove the biofilm from the coupons and were vortexed to resuspend the cells. Sonication had a negligible effect on cell viability when viable plate counts were determined before and after sonication of samples (data not shown). The samples were serially diluted, and viable plate counting was performed. Biofilm and planktonic cell responses to heavy metal stress were compared by determining minimum biocidal concentrations (MBC). The MBC was the level of a heavy metal needed to completely kill a population, as determined by viable plate counting.
Cupric electrode experiment.
A cupric (Cu2+) electrode (Ion Plus; Thermo-Orion, Beverly, Mass.) was used to assess the concentrations of free Cu in MSVP and MOPSO-buffered saline. Cu(NO3)2 standards were prepared in distilled water by serial dilution to obtain concentrations between 0.001 and 10 mM. A standard curve was generated by determining the electrode potential with 20 ml of each standard and 0.4 ml of 5 M NaNO3, which kept the ionic strength constant for each standard. The concentration of free Cu in MSVP and MOPSO was measured by adding 0.4 ml of 5 M NaNO3 to 20 ml of MOPSO or MSVP and then incrementally adding aliquots of copper, measuring the electrode potential, and relating this measurement to the free Cu concentration. Free Cu concentrations in P. aeruginosa cultures were measured by adding 0.5 ml of 5 M NaNO3 to 25 ml of a culture containing 0.03 mM added Cu and then measuring the electrode potential.
Thermodynamic speciation prediction.
The speciation software Mineql (43) was used to model the speciation of Cu, Pb, and Zn in the different medium variations and buffers. Additional stability constants for Cu, Pb, Zn, and pyruvic acid were obtained from Martell and Smith (26). The theoretical speciation of the heavy metals in MSVP and MOPSO was determined, as were the concentrations of free metals and the predicted metal-containing complexes and precipitates.
Assessing the relative susceptibilities of logarithmic- and stationary-phase cells to metal stress.
Batch cultures of PAO1 were grown in Erlenmeyer flasks containing MSVG at 37°C. One-milliliter samples were taken during the logarithmic and stationary phases at the proper culture OD600. Stationary-phase samples were centrifuged at 12,000 × g for 5 min, washed, and resuspended in MSV to an OD600 of 0.03. Logarithmic-phase samples were centrifuged at 12,000 × g for 5 min and washed, and the OD600 was adjusted to 0.03 in MSVG. Equal numbers of stationary- and logarithmic-phase cells were then exposed to different concentrations of Cu, Pb, Zn, and tobramycin for 5 h at 37°C. No measurable growth occurred during incubation with the heavy metals and tobramycin, as the incubation time was less than the doubling time (6 h) for PAO1 in MSVG at 37°C. Samples were sonicated for 10 min in 900 μl of PBS, vortexed, and serially diluted in PBS, and viable plate counts were determined.
Flow cell biofilm culture system.
PAO1 biofilms were grown in a once-through continuous culture system (14) containing flow cells that were 40 by 4 by 1 mm and had a glass coverslip substratum (Fisher Premium; Fisher Scientific, Hanover Park, Ill.). The flow rate was maintained at 0.067 ml/min with a Watson Marlow 205S peristaltic pump (Watson Marlow Ltd., Falmouth, England). Initially, biofilm development of PAO1 tagged with GFP was assayed in MSVP at 30°C over time. Biofilms were examined by scanning confocal laser microscopy (SCLM) (Zeiss LSM 510; Carl Zeiss, Jena, Germany) by using a step size of approximately 0.5 μm in the z direction.
PAO1 biofilms grown in the flow cell system with MSVP were allowed to mature for 4 days at 30°C, at which point the biofilms were confluent. On day 4 of growth, biofilms were exposed to either 1 mM added Cu for 12 h or 64 mM added Zn for 24 h. The biofilms were stained by stopping the flow and injecting 0.3 ml of a stain composed of 0.01 μM SYTO9 and 0.03 μM propidium iodide (LIVE/DEAD BacLight viability stain; Molecular Probes Inc., Eugene, Oreg.). The biofilms were stained for 15 min in the dark. The biofilms were then examined by using SCLM.
Modification of COMSTAT to analyze LIVE/DEAD images.
The image analysis program COMSTAT (14) was modified to include a subroutine capable of analyzing images stained with the LIVE/DEAD BacLight viability stain. This subroutine recognizes the relative biomass that fluoresces green (live) and red (dead) at levels above a user-defined threshold value and reports the percentage of biomass that is alive and the percentage of biomass that is dead in each slice in a stack of images. The areas of the biomass fluorescing green and red represented the relative amounts of living and dead biomass in a biofilm.
RESULTS
Planktonic growth curves.
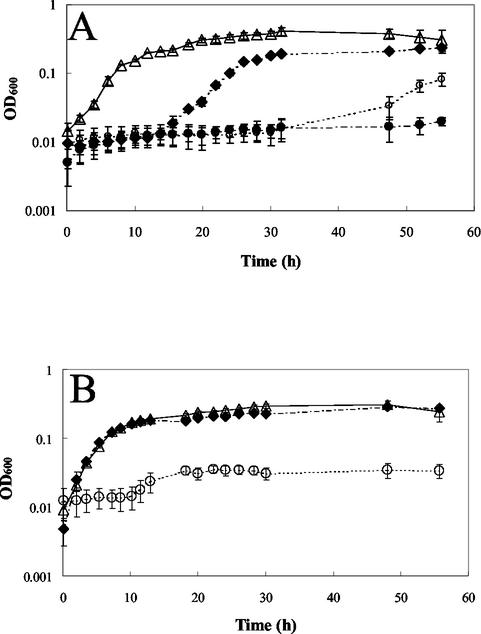
MSVP was selected from a variety of minimal growth media based on the fact that it minimized complexation and precipitation of heavy metals. In particular, special care was taken to minimize the concentration of phosphates in MSVP. The growth of PAO1 in liquid culture in the presence of increasing concentrations of heavy metals was analyzed. As the added concentration of heavy metal increased, the growth of cultures decreased. At added Cu concentrations below the MIC (2 mM), growth was observed (Fig. 1A). However, a significant lag phase was observed when copper was present. The duration of the lag phase increased as the copper concentration increased. No significant lag phase was observed for lead; in fact, there appeared to be a critical threshold concentration, 0.125 mM, below which there was growth similar to that of the no-lead control and above which there was no growth (Fig. 1B). PAO1 was found to be susceptible to the heavy metals in the following order: Zn < Cu < Pb (Table 1).
FIG. 1.
Planktonic PAO1 growth in MSVP at 37°C. (A) Growth in the presence of Cu. Symbols: ▵, no added Cu; ⧫, 0.06 mM added Cu; ○, 1 mM added Cu; •, 2 mM added Cu. (B) Growth in the presence of Pb. Symbols: ▵, no added Pb; ⧫, 0.03 mM added Pb; ○, 0.125 mM added Pb.
TABLE 1.
MICs and MBCs of heavy metalsa
| Cells | Medium | MIC (mM) of:
|
MBC (mM) of:
|
||||
|---|---|---|---|---|---|---|---|
| Cu | Zn | Pb | Cu | Zn | Pb | ||
| Planktonic | 2 | 8 | 0.125 | ||||
| Planktonic | MSVP | 0.125 | >64 | 0.3 | |||
| MOPSO | 0.01 | 2 | 0.25 | ||||
| Biofilm | MSVP | >6 | >64 | 0.8 | |||
| MOPSO | 6 | >64 | >0.4 | ||||
MICs were determined after 56 h, and MBCs were determined after 5 h by using the rotating-disk reactor.
Relative levels of resistance of free-swimming bacteria and biofilms to heavy metal stress.
A rotating-disk reactor was used to grow biofilm and free-swimming cultures of PAO1 in order to compare their susceptibilities to heavy metal stress. Biofilm P. aeruginosa cells were found to be more resistant than free-swimming cells when they were assayed in the growth medium MSVP (MBCs) (Table 1). However, at elevated Cu and Zn concentrations, precipitation interfered with the ability to determine the MBCs of biofilm cultures.
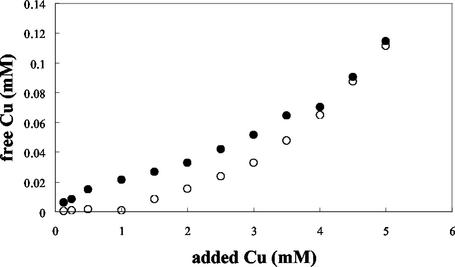
To further minimize precipitation, the heavy metal treatment was conducted in MOPSO-buffered saline (pH 7.1). Experiments with a Cu-specific electrode revealed that MOPSO-buffered saline formed fewer complexes and precipitates with heavy metals than MSVP, resulting in higher levels of Cu for similar added total Cu concentrations (Fig. 2). At low concentrations of added Cu, the free Cu concentration in MSVP changed very little until 1.5 mM, at which point the concentration began to increase linearly. In MOPSO, increases in the added Cu concentration from 0.125 to 4 mM resulted in linear increases in the free Cu concentration; however, there was still significant complexation of Cu (Fig. 2 and Table 2). A direct comparison of the free Cu concentrations in MOPSO and MSVP was conducted by using a cupric electrode and the speciation program Mineql. The predicted and measured free copper concentrations for MOPSO were about 1 × 10−6 to 6 × 10−6 M (Table 2). For MSVP the predicted concentration of free Cu was 2.25 × 10−6 M, and the measured concentration was 4.61 × 10−7 M. This discrepancy may have been due to complexation occurring with other components in MSVP not accounted for in the model. The Mineql software predicted the following complexation states of Cu in MOPSO: CuCl−, CuOH−, and Cu(OH)2(s). The complexes predicted to form in MSVP were primarily copper-pyruvate complexes, CuSO4, and CuHPO4 (Table 2). The concentrations of free Cu in cultures of P. aeruginosa grown in MSVP did not change over time as the pyruvate was consumed (data not shown).
FIG. 2.
Concentrations of free Cu in MSVP and MOPSO-buffered saline. Free Cu concentrations were measured with a Cu-specific electrode. A standard curve was prepared with known Cu concentrations ranging from 0.001 to 10 mM by relating the electrode potential to the concentration of free cationic Cu. Symbols: •, Cu concentration in MOPSO-buffered saline; ○, Cu concentration in MSVP.
TABLE 2.
Complexation of Cu in solutions containing 0.125 mM added Cua
| Culture medium | Measured Cu (M) | Predicted Cu (M) | Predicted complexation states |
|---|---|---|---|
| MSVP | 4.61 × 10−7 | 2.25 × 10−6 | CuSO4, CuHPO4, Cu-pyru- vate−, Cu-(pyruvate)2 |
| MOPSO | 6 × 10−6 | 1.3 × 10−6 | CuCl−, CuOH, CuOH2−,(s) |
Free Cu concentrations were measured with a cupric electrode. Predicted concentrations of Cu were determined by using the speciation program Mineql.
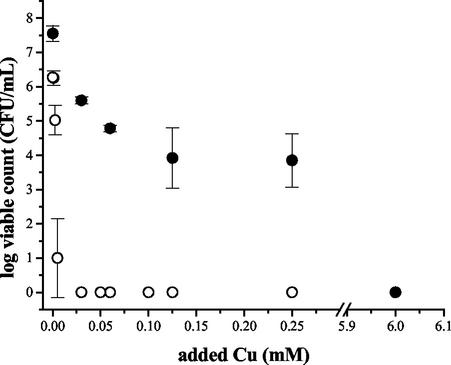
Heavy metal treatment of biofilm and free-swimming cultures in MOPSO also revealed the general trend that a P. aeruginosa biofilm was more resistant to Cu, Pb, and Zn than free-swimming cells (Table 1). With Cu treatment in MOPSO, the MBC of Cu for a PAO1 biofilm was 6 mM, while for the free-swimming cells it was only 0.01 mM (Fig. 3). These concentrations are lower than the MBCs in MSVP, in which the MBC of Cu for a PAO1 biofilm was >6 mM, while for the free-swimming cells it was 0.125 mM (Table 1).
FIG. 3.
MBCs for biofilm and free-swimming PAO1 cells in response to copper treatment in MOPSO-buffered saline. Biofilm and free-swimming cultures were grown in MSVP at room temperature by using the rotating-disk biofilm reactor. Samples of biofilm and free-swimming cells were harvested and then subjected to a range of Cu concentrations in MOPSO-buffered saline at 37°C for 5 h. Symbols: •, biofilm; ○, free-swimming cells.
Comparative levels of heavy metal resistance of stationary- and logarithmic-phase populations.
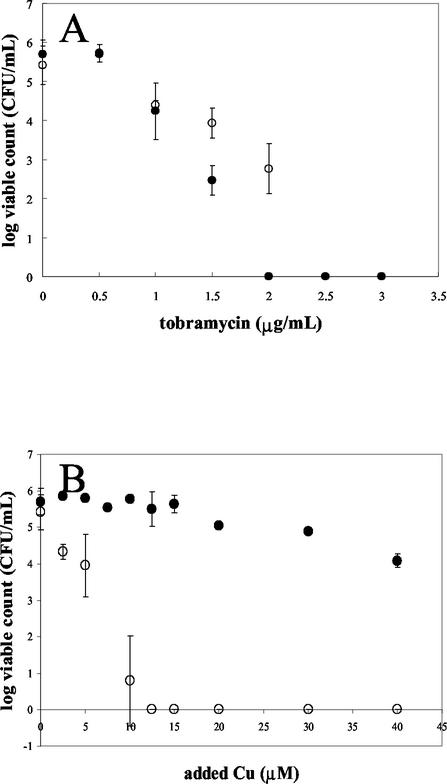
In order to compare the relative levels of resistance of PAO1 at different points in the growth curve, cells were grown in MSVG planktonically and harvested at different times. Logarithmic-phase cells were exposed to heavy metals for 5 h in MSVG to ensure that the cells remained in the logarithmic phase, while treatment of stationary-phase cells was performed in MSV without a carbon source to ensure that the cells remained in the stationary phase. Tobramycin, an antibiotic that targets metabolically active cells, was used as a control. MSVG and MSV were used because Cu-specific electrode studies and Mineql modeling showed that the free Cu concentrations in MSV and MSVG were similar, in contrast to the wide disparity in free Cu concentrations between MSVP and MSV due to the predicted complexation of metals with pyruvate (data not shown). As previously reported (37), stationary-phase cells exposed to tobramycin were less susceptible to tobramycin than logarithmically grown cells (Fig. 4A). However, the opposite trend was observed for cells exposed to copper; logarithmically grown cells were resistant to higher concentrations of added copper than stationary-phase PAO1 cells (Fig. 4B). Logarithmically grown cells were also more resistant to lead than stationary-phase cells (data not shown). Zinc-treated stationary-phase cells appeared to be more resistant to zinc than logarithmically grown cells (data not shown); however, this was difficult to evaluate due to complexation and precipitation.
FIG. 4.
Viability of stationary-phase and logarithmically grown cells subjected to tobramycin stress (A) and copper stress (B). Planktonic cultures were grown at 37°C, and then aliquots were harvested at the logarithmic and stationary phases. Stationary-phase cultures were treated in MSV without a carbon source for 5 h at 37°C, while logarithmic-phase cells were treated in MSVG. In panel A the stationary-phase and logarithmically grown cell points overlap at 2.5 and 3 μg of tobramycin per ml. Symbols: •, logarithmic-phase cells; ○, stationary-phase cells.
PAO1 biofilm development in MSVP.
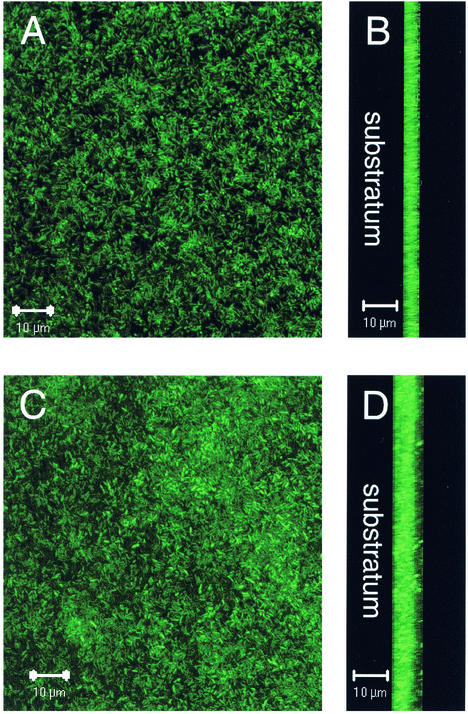
Biofilm development in the minimal medium MSVP was investigated over time by using a once-through continuous flow cell system (14). PAO1 tagged with GFP was used in order to visualize biofilm cells with SCLM. During the first day a monolayer of sparsely attached bacteria was seen, and by the second day there was more complete surface coverage (Fig. 5A). By the second day, the average biofilm thickness was 3.57 μm (Fig. 5B). By day 5, the biofilm had become densely packed and had an average depth of 5.26 μm (Fig. 5C and D). Although the biofilm on day 5 did contain a few clusters, it was primarily flat and had no structure.
FIG. 5.
Development of a PAO1 biofilm in MSVP at 30°C. (A and B) Micrographs taken after 2 days of growth. (A) Top down view; (B) saggital view with the substratum to the left. (C and D) Micrographs taken after 5 days of growth. (C) Top down view; (D) saggital view with the substratum to the left. The micrographs were taken with the SCLM by using a magnification of ×630.
Copper treatment of a PAO1 biofilm.
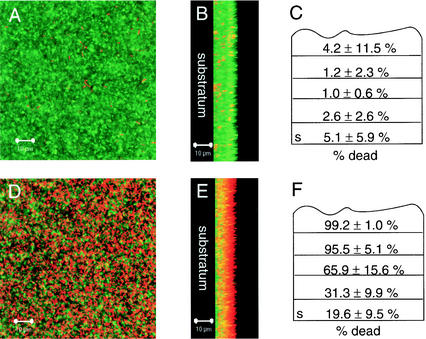
Nonfluorescent PAO1 was grown to obtain mature biofilms (4 days) by using the flow cell system. As expected, the appearance of these biofilms was similar to the appearance of the GFP-tagged PAO1 biofilm. The biofilms were then subjected to treatment with 1 mM copper added to the growth medium. After 12 h, the biofilms were exposed to the LIVE/DEAD BacLight viability stain. After staining, the biofilms were viewed with SCLM. The majority of cells in the untreated control biofilms were alive (Fig. 6A and B). For Cu-treated biofilms, there was an outer layer of cells that were primarily dead, with some living cells towards the substratum (Fig. 6D and E). This trend was also observed in cell clusters; cells at the exterior were dead, and cells buried in the depths of the biofilms were primarily alive. A similar trend for the distribution of live and dead cells was observed in a zinc-treated biofilm (data not shown).
FIG. 6.
Cu-treated and untreated PAO1 biofilms grown in MSVP at 30°C. (A and B) Micrographs of an untreated biofilm. (A) Top down view; (B) saggital view with the substratum to the left. (D and E) Micrographs of a biofilm treated with 1 mM added Cu for 12 h. (D) Top down view; (E) saggital view with the substratum to the left. Dead cells were stained red with propidium iodide, while live cells were stained green with SYTO9 by using the BacLight LIVE/DEAD viability stain. The micrographs were taken with the SCLM by using a magnification of ×630. (C and F) Quantification of live and dead biomasses as determined by using COMSTAT to estimate the percentage of dead cells as a function of the total biomass within an untreated biofilm (C) and a biofilm treated with 1 mM added copper for 12 h (F). The substratum layer is indicated by s. Averages were calculated for 9 to 12 confocal image stacks for each condition and divided into fifths to normalize for variation in the image stack size. The average depth of the biofilms was 10 μm.
COMSTAT evaluation of the biofilms verified the qualitative examination of the treated and untreated biofilms. Each biofilm image stack was divided into fifths, and the relative live and dead biomass was estimated for each fraction of the biofilm to normalize for varying thickness of separate image stacks. The outer layers of the copper-treated biofilms had increased percentages of dead biomass (99.2% for the outermost layer), and there were fewer dead cells in the interior (19.6%), while in untreated biofilms there were small percentages of dead cells throughout the biofilm (between 1.0 and 5.1%) (Fig. 6C and F). The difference between the top layers of the treated and untreated biofilms was found to be significant (P < 0.01), as was the difference between the top layer and the interior of the copper-treated biofilms (P < 0.01).
DISCUSSION
There has been significant effort directed towards understanding the biochemistry of heavy metal resistance mechanisms of free-swimming bacteria. However, little is known about biofilm communities and their ability to withstand heavy metals. In this study we focused on P. aeruginosa, a paradigm organism for studying biofilms, and examined its ability to withstand heavy metal stress in both liquid and biofilm cultures.
In this study we assayed heavy metal susceptibility in two ways: inhibition of growth (MIC) and toxicity (MBC). We found that free-swimming P. aeruginosa cells were susceptible to the heavy metals tested in the following order of susceptibility: Zn2+ < Cu2+ < Pb2+. The measured MICs of lead and zinc were lower than those previously reported for P. aeruginosa (24 to 48 mM for Zn and 15 mM for Pb) (8). The discrepancy between previously reported values and the values obtained in this study is most likely due to the complex rich growth medium (Mueller-Hinton broth) used by de Vicente et al. (8), which probably resulted in a high level of complexation between the metal cations and components of the growth medium. Our values were closer to those of Bender and Cooksey, who reported a MIC of CuSO4 of 100 μM for P. syringae (2). With both zinc and copper, P. aeruginosa exhibited increasing lags in growth after exposure to increasing concentrations of the heavy metals (Fig. 1A). This observation appeared to be due to selection for copper-resistant phenotypes. Cells transferred from cultures growing in the presence of 0.06 mM copper (Fig. 1A) to a new growth medium with the same concentration of copper exhibited no lag in growth. However, when this experiment was repeated by first transferring Cu-exposed cells to solid growth medium without Cu before samples were subcultured in liquid growth medium containing 0.06 mM Cu, the samples exhibited a lag in growth, as previously described (data not shown). This observation suggests that P. aeruginosa adopts a Cu-resistant phenotype. This trend was not observed in the case of lead, with which growth was not observed at concentrations greater than a specific threshold concentration (Fig. 1B).
An analysis of the MBC data revealed a slightly different trend than was observed with the MIC data, with toxicity increasing in the order Zn < Pb < Cu. The added heavy metal concentrations required for toxicity were all higher when they were measured in MSVP than when they were measured in MOPSO-buffered saline (Table 1). Presumably, this was due to complexation between the heavy metals and pyruvate in MSVP. While using MOPSO-buffered saline ameliorated some of the complexation problems that we had in assessing MBCs, we still had problems working with zinc since P. aeruginosa was resistant to high concentrations, including those at which visible precipitates were formed. In general, the amount of added metal required to kill the cells was greater than the amount required to inhibit growth. This was not observed in the case of copper. The amount of CuSO4 required to kill P. aeruginosa was significantly less than the amount required to inhibit growth. This might be explained by the nature of the MIC and MBC assays, which used different exposure times as well as different initial loads of cells.
The Cu-specific electrode data (Fig. 2) highlight the importance of the aqueous chemical environment in dictating the amount of free cation observed in solution. The low measured values for the free copper concentration relative to the added copper concentration were most likely due to complexation of the metal cation with components of the aqueous environment. A question that remains is the toxicity of free copper relative to the toxicity of copper in its major complexed states. A comparison of the MSVP and MOPSO data (Table 1 and Fig. 2) suggests that free metal cations may be the most toxic form. The MOPSO data showed that there was a direct relationship between the added copper concentration and the measured free copper concentration (Fig. 2). However, in MSVP, an initial plateau in the free copper level was observed until the added Cu concentration was approximately 1 mM, after which there appeared to be a linear relationship between the added and measured free copper concentrations. The initial plateau for the measured free copper concentration was probably due to complexation of copper ions with the pyruvate present in the growth medium. Indeed, the thermodynamics of the system as predicted by the Mineql software estimated that pyruvate-metal cation interactions should be a predominate complexation state for heavy metals in MSVP (Table 2). However, the free Cu concentrations in planktonic cultures grown in MSVP did not change significantly during growth as P. aeruginosa consumed pyruvate (data not shown).
The concentrations of added heavy metals used in this study do exceed many reported values for the total metal concentrations at contaminated sites (3, 9, 42). However, the amounts of a free ion and the different complexes that it forms are poorly understood in environmental systems. Understanding the toxicity of free metal ion in relation to the complexes that it forms and the relative concentrations of these species in natural and experimental systems is crucial.
The MBCs of free-swimming and biofilm cultures were compared by exposing equal numbers of cells to various heavy metal concentrations, using a rotating-disk reactor (13, 46). Biofilm cells were found to be more resistant than an equal number of free-swimming cells. The degree of increased resistance varied depending on the heavy metal. There was an approximately twofold increase in the resistance of lead-treated biofilms compared to the resistance of free-swimming cells in MOPSO, and there was an estimated 600-fold increase in resistance for copper-treated biofilms compared to the resistance of free-swimming cells (Table 1 and Fig. 3). These data are not surprising since P. aeruginosa biofilms have been reported to be more resistant to a variety of antimicrobial stresses than free-swimming cells (7, 13). Persistor cells, as described by Lewis et al. (4, 37), were observed in biofilms in which less than 1% of the original population was extremely difficult to kill with elevated concentrations of heavy metals.
The fact that stationary-phase bacteria are more resistant to antibiotics that target actively growing cells than logarithmically growing cells has been known for some time (12, 37). We compared the relative levels of resistance of equal numbers of logarithmically growing cells and stationary-phase cells to heavy metals at a variety of concentrations. The comparison was done by exposing the organisms for 5 h in MSV containing glucose (logarithmic culture) and in MSV lacking glucose (stationary-phase culture). Glucose was used instead of pyruvate to minimize complexation between the heavy metal cations and the carbon source in order to ensure that the cells in each medium (MSV and MSVG) were exposed to the same level of free metal cation. The doubling time of P. aeruginosa in the growth medium is 6 h, so viability was assayed after 5 h of exposure. In the case of copper or lead, logarithmically growing cells were found to be significantly more resistant than stationary-phase cells (Fig. 4B).
Our data suggest that slow growth is not a heavy metal resistance mechanism for P. aeruginosa. However, comparing the data generated by Spoering and Lewis (37) to our results is difficult since different biofilm culture systems were used and the initial numbers of bacteria subjected to treatment were not normalized. One possible explanation for why logarithmic-phase cells were observed to be more resistant than stationary-phase cells is that active heavy metal efflux mechanisms often require ATP, which is more abundant in actively growing cells. Interestingly, biofilms were more resistant than logarithmically growing cells, suggesting that distinct resistance mechanisms are employed by planktonic and biofilm populations for heavy metal resistance.
Development of P. aeruginosa biofilms was monitored by using flow cell technology in the MSVP growth medium (Fig. 5). This analysis showed that biofilms developed slowly and exhibited little structural heterogeneity. Viability staining was employed to visualize the spatial distribution of living and dead cells in heavy metal-treated biofilms (Fig. 6). In untreated biofilms, small numbers of dead cells were spread throughout the biofilms (Fig. 6A to C). However, in copper-treated biofilms there was a layer of dead cells at the exterior of each biofilm, and towards the substratum there were increasing numbers of live cells (Fig. 6D to F). A possible explanation of this finding is that cells at the biofilm-bulk liquid interface are exposed to the highest levels of heavy metal. This observation is also consistent with other studies indicating that penetration of certain antibiotics and Zn is retarded in biofilms (1, 15, 35). Presumably, most of the actively growing cells in a biofilm are located at the biofilm-bulk liquid interface, which should be the most resistant region of the biofilm, as predicted by the results shown in Fig. 4B. The data reinforce the idea that biofilms have a heavy metal resistance mechanism distinct from that of planktonically growing cells. One potential mechanism is complexation or sequestration of divalent metal cations by the EPS and cells at the biofilm-bulk liquid interface. EPS and polysaccharides isolated from EPS have been reported previously to bind heavy metals (19, 21, 27), in particular Cu (5, 20, 28). Complexation of heavy metals may result in a gradient in the biofilm, with the highest concentration at the periphery and the lowest concentration near the substratum. The distribution of dead cells in the copper-treated biofilm supports this explanation (Fig. 6D to F). However, it is not clear that the metal concentration within the biofilm had reached equilibrium in this system at the time that viability was assayed (12 h). A similar distribution of live and dead cells was seen at 24 h (data not shown); however, a more systematic analysis would have been necessary to determine when equilibrium was reached in the system.
Our findings suggest that biofilm and planktonic populations have distinct heavy metal resistance mechanisms. Understanding these mechanisms is important for understanding the microbial ecology of heavy metal-affected environments, as well as the basis of biofilm resistance to antimicrobial agents in general. In future work we will examine and compare the molecular components that contribute to heavy metal resistance in biofilm and planktonic populations.
Acknowledgments
Gail Teitzel is supported by grant CHE 9810378 from the Institute of Environmental Catalysis and by the National Science Foundation.
We thank Grant Balzer for assistance with SCLM, Jean-François Gaillard and Amy Dahl for assistance with working with heavy metals, and David Chopp for assistance with programming the BacLight subroutine in COMSTAT.
REFERENCES
- 1.Anderl, J. N., M. J. Franklin, and P. S. Stewart. 2000. Role of antibiotic penetration limitation in Klebsiella pneumoniae biofilm resistance to ampicillin and ciprofloxacin. Antimicrob. Agents Chemother. 44:1818-1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bender, C. L., and D. A. Cooksey. 1986. Indigenous plasmids in Pseudomonas syringae pv. tomato: conjugative transfer and role in copper resistance. J. Bacteriol. 165:534-541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Breault, R. F., J. A. Colman, G. R. Aiken, and D. McKnight. 1996. Copper speciation and binding by organic matter in copper-contaminated streamwater. Environ. Sci. Technol. 30:3477-3486. [Google Scholar]
- 4.Brooun, A., S. Liu, and K. Lewis. 2000. A dose-dependent study of antibiotic resistance in Pseudomonas aeruginosa biofilms. Antimicrob. Agents Chemother. 44:640-646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Christensen, B. E., J. Kjosbakken, and O. Smidsrød. 1985. Partial chemical and physical characterization of two extracellular polysaccharides produced by marine, periphytic Pseudomonas sp. strain NCMB 2021. Appl. Environ. Microbiol. 50:837-845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cooksey, D. A. 1994. Molecular mechanisms of copper resistance and accumulation in bacteria. FEMS Microbiol. Rev. 14:381-386. [DOI] [PubMed] [Google Scholar]
- 7.Costerton, J. W., P. S. Stewart, and E. P. Greenberg. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318-1322. [DOI] [PubMed] [Google Scholar]
- 8.de Vincente, A., M. Avilès, J. C. Codina, J. J. Borrego, and P. Romero. 1990. Resistance to antibiotics and heavy metals of Pseudomonas aeruginosa isolated from natural waters. J. Appl. Bacteriol. 68:625-632. [DOI] [PubMed] [Google Scholar]
- 9.Fergusson, J. E. 1990. The heavy elements: chemistry, environmental impact and health effects. Pergamon Press, New York, N.Y.
- 10.Ferris, F. G., S. Schultze, T. C. Witten, W. S. Fyfe, and T. J. Beveridge. 1989. Metal interactions with microbial biofilms in acidic and neutral pH environments. Appl. Environ. Microbiol. 55:1249-1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flemming, H.-C., and J. Wingender. 2001. Relevance of microbial extracellular polymeric substances (EPSs). Part I. Structural and ecological aspects. Water Sci. Technol. 43:1-8. [PubMed] [Google Scholar]
- 12.Gilbert, P. S., P. J. Collier, and M. R. Brown. 1990. Influence of growth rate on susceptibility to antimicrobial agents: biofilms, cell cycle, dormancy, and stringent response. Antimicrob. Agents Chemother. 34:1865-1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hentzer, M., G. M. Teitzel, G. J. Balzer, A. Heydorn, S. Molin, M. Givskov, and M. R. Parsek. 2001. Alginate overproduction affects Pseudomonas aeruginosa biofilm structure and function. J. Bacteriol. 183:5395-5401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heydorn, A., A. T. Nielsen, M. Hentzer, C. Sternberg, M. Givskov, B. K. Ersbøll, and S. Molin. 2000. Quantification of biofilm structures by the novel computer program COMSTAT. Microbiology 146:2395-2407. [DOI] [PubMed] [Google Scholar]
- 15.Hoyle, B. D., J. Alcantara, and J. W. Costerton. 1992. Pseudomonas aeruginosa biofilm as a diffusion barrier to piperacillin. Antimicrob. Agents Chemother. 36:2054-2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang, Y.-B., W.-H. Wang, and A. Peng. 2000. Accumulation of Cu(II) and Pb(II) by biofilms grown on particulate in aquatic systems. J. Environ. Sci. Health Part A Environ. Sci. Eng. 35:575-592. [Google Scholar]
- 17.Hunt, S. 1986. Diversity of biopolymer structure and its potential for ion-binding applications, p. 15-46. In H. Eccles and S. Hunt (ed.), Immobilisation of ions by bio-sorption. Ellis Horwood Ltd., West Sussex, United Kingdom.
- 18.Ivorra, N., S. Bremer, H. Guasch, M. H. S. Kraak, and W. Admiraal. 2000. Differences in the sensitivity of benethic microalgae to Zn and Cd regarding biofilm development and exposure history. Environ. Toxicol. Chem. 19:1332-1339. [Google Scholar]
- 19.Kaplan, D., D. Christiaen, and S. M. Arad. 1987. Chelating properties of extracellular polysaccharides from Chlorella spp. Appl. Environ. Microbiol. 53:2953-2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kazy, S. K., P. Sar, S. P. Singh, A. K. Sen, and S. F. D'Souza. 2002. Extracellular polysaccharides of a copper-sensitive and a copper-resistant Pseudomonas aeruginosa strain: synthesis, chemical nature and copper binding. World J. Microbiol. Biotechnol. 18:583-588. [Google Scholar]
- 21.Kim, S.-Y., J.-H. Kim, C.-J. Kim, and D.-K. Oh. 1996. Metal adsorption of the polysaccharide produced from Methlobacterium organophilum. Biotechnol. Lett. 18:1161-1164. [Google Scholar]
- 22.Labrenz, M., G. K. Druschel, T. Thomsen-Ebert, B. Gilbert, S. A. Welch, K. M. Kemner, G. A. Logan, R. E. Summons, G. De Stasio, P. L. Bond, B. Lai, S. D. Kelly, and J. F. Banfield. 2000. Formation of sphalerite (ZnS) deposits in natural biofilms of sulfate-reducing bacteria. Science 290:1744-1747. [DOI] [PubMed] [Google Scholar]
- 23.Langley, S., and T. J. Beveridge. 1999. Metal binding by Pseudomonas aeruginosa PAO1 is influenced by growth of the cells as a biofilm. Can. J. Microbiol. 45:616-622. [PubMed] [Google Scholar]
- 24.Lefcort, H., M. Q. Aguon, K. A. Bond, K. R. Chapman, R. Chaquette, J. Clark, P. Kornachuk, B. Z. Lang, and J. C. Martin. 2002. Indirect effects of heavy metals on parasites may cause shifts in snail species compositions. Arch. Environ. Contam. Toxicol. 43:34-41. [DOI] [PubMed] [Google Scholar]
- 25.Liehr, S. K., H.-J. Chen, and S.-H. Lin. 1994. Metals removal by algal biofilms. Water Sci. Technol. 30:59-68. [Google Scholar]
- 26.Martell, A. E., and R. M. Smith. 1977. Critical stability constants, vol. 3. Plenum Press, New York, N.Y.
- 27.McLean, R. J., D. Beauchemin, L. Clapham, and T. J. Beveridge. 1990. Metal-binding characteristics of the gamma-glutamyl capsular polymer of Bacillus licheniformis ATCC 9945. Appl. Environ. Microbiol. 56:3671-3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mittelman, M. W., and G. G. Geesey. 1985. Copper-binding characteristics of exopolymers from a freshwater-sediment bacterium. Appl. Environ. Microbiol. 49:846-851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.NCCLS. 2000. Minimum inhibitory concentration (MIC) interpretive standards (μg/ml) for Pseudomonas aeruginosa and other non-Enterobacteriaceae. NCCLS document M7-A5. NCCLS, Wayne, Pa.
- 30.Nies, D. H. 1999. Microbial heavy-metal resistance. Appl. Microbiol. Biotechnol. 51:730-750. [DOI] [PubMed] [Google Scholar]
- 31.Nies, D. H., and S. Silver. 1995. Ion efflux systems involved in bacterial metal resistances. J. Ind. Microbiol. 14:186-199. [DOI] [PubMed] [Google Scholar]
- 32.Nucifora, G., L. Chu, T. K. Misra, and S. Silver. 1989. Cadmium resistance from Staphylococcus aureus plasmid pI258 cadA gene results from a cadmium-efflux ATPase. Proc. Natl. Acad. Sci. USA 86:3544-3548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Outten, F. W., C. E. Outten, and T. O'Halloran. 2000. Metalloregulatory systems at the interface between bacterial metal homeostasis and resistance, p. 145-157. In G. Storz and R. Hengge-Aronis (ed.), Bacterial stress responses. ASM Press, Washington, D.C.
- 34.Platt, R. M., G. G. Geesey, J. D. Davis, and D. C. White. 1985. Isolation and partial chemical analysis of firmly bound exopolysaccharide from adherent cells of a freshwater sediment bacterium. Can. J. Microbiol. 31:675-680. [DOI] [PubMed] [Google Scholar]
- 35.Rose, F. L., and C. E. Cushing. 1970. Periphyton: autoradiography of zinc-65 adsorption. Science 168:576-577. [DOI] [PubMed] [Google Scholar]
- 36.Silver, S. 1996. Bacterial resistance to toxic metal ions—a review. Gene 179:9-19. [DOI] [PubMed] [Google Scholar]
- 37.Spoering, A. L., and K. Lewis. 2001. Biofilms and planktonic cells of Pseudomonas aeruginosa have similar resistance to killing by antimicrobials. J. Bacteriol. 183:6746-6751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sutherland, I. W. 2001. Exopolysaccharides in biofilms, flocs and related structures. Water Sci. Technol. 43:77-86. [PubMed] [Google Scholar]
- 39.Templeton, A. S., T. P. Trainor, S. J. Traina, A. M. Spormann, and G. E. Brown, Jr. 2001. Pb(II) distributions at biofilm-metal oxide interfaces. Proc. Natl. Acad. Sci. USA 98:11897-11901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.von Canstein, H., Y. Li, K. N. Timmis, W.-D. Deckwer, and I. Wagner-Döbler. 1999. Removal of mercury from chloralkali electrolysis wastewater by a mercury-resistant Pseudomonas putida strain. Appl. Environ. Microbiol. 65:5279-5284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wagner-Döbler, I., H. Lünsdorf, T. Lübbenhüsen, H. F. von Canstein, and Y. Li. 2000. Structure and species composition of mercury-reducing biofilms. Appl. Environ. Microbiol. 66:4559-4563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Webb, S. M., G. G. Leppard, and J.-F. Gaillard. 2000. Zinc speciation in a contaminated aquatic environment: characterization of environmental particles by analytic electron microscopy. Environ. Sci. Technol. 34:1926-1933. [Google Scholar]
- 43.Westall, J. C., J. L. Zachary, and F. M. M. Morel. 1976. MINEQL. Computer program of thermodynamic calculation. Technical note 18. RM Parsons Laboratory, Massachusetts Institute of Technology, Cambridge, Mass.
- 44.Whitchurch, C. B., T. Tolker-Nielsen, P. C. Ragas, and J. S. Mattick. 2002. Extracellular DNA required for bacterial biofilm formation. Science 295:1487.. [DOI] [PubMed] [Google Scholar]
- 45.White, C., and G. M. Gadd. 1998. Accumulation and effects of cadmium on sulphate-reducing bacterial biofilms. Microbiology 144:1407-1415. [DOI] [PubMed] [Google Scholar]
- 46.Whiteley, M., M. G. Bangera, R. E. Bumgarner, M. R. Parsek, G. M. Teitzel, S. Lory, and E. P. Greenberg. 2001. Gene expression in Pseudomonas aeruginosa biofilms. Nature 413:860-864. [DOI] [PubMed] [Google Scholar]
- 47.Whitfield, C. 1988. Bacterial extracellular polysaccharides. Can. J. Microbiol. 34:415-420. [DOI] [PubMed] [Google Scholar]