Abstract
Acute laminitis has been associated with the overgrowth of gram-positive bacteria within the equine hindgut, causing the release of factor(s) leading to ischemia-reperfusion of the digits. The products of fermentation which trigger acute laminitis are, as yet, unknown; however, vasoactive amines are possible candidates. The objectives of this study were to use an in vitro model of carbohydrate overload to study the change in populations of cecal streptococci and lactobacilli and to establish whether certain species of these bacteria were capable of producing vasoactive amines from amino acids. Cecal contents from 10 horses were divided into aliquots and incubated anaerobically with either corn starch or inulin (fructan; both at 1 g/100 ml). Samples were taken at 6-h intervals over a 24-h period for enumeration of streptococci, lactobacilli, and gram-negative anaerobes by a dilution method onto standard selective growth media. The effects of the antibiotic virginiamycin (1 mg/100 ml) and calcium hydrogen phosphate (CaHPO4; 0.3 g/100 ml) were also examined. Fermentation of excess carbohydrate was associated with increases in numbers of streptococci and lactobacilli (2- to 3.5-log unit increases; inhibited by virginiamycin) but numbers of gram-negative anaerobes were not significantly affected. A screening agar technique followed by 16S rRNA gene sequence analysis enabled the identification of 26 different bacterial strains capable of producing one or more vasoactive amines. These included members of the species Streptococcus bovis and five different Lactobacillus spp. These data suggest that certain bacteria, whose overgrowth is associated with carbohydrate fermentation, are capable of producing vasoactive amines which may play a role in the pathogenesis of acute laminitis.
Fermentation of carbohydrate in the hindgut has been recognized as one of the primary events associated with acute laminitis in the horse (14). The ability of the equine small intestine to digest starch may be overwhelmed if starch is present in excess (>0.4% body weight [35]), leading to its metabolism by cecal and colonic bacteria and the production of lactic acid. Fructans, the oligosaccharides present in grass which act as the storage form of carbohydrate, do not appear to be digested by the small intestine (21) and so are also substrates for fermentative bacteria. The variation of fructan levels in grasses under different climatic conditions has been suggested to account for the seasonal incidence of acute laminitis (A. C. Longland, A. J. Cairns, and M. O. Humphreys, Proc. 16th Equine Nutr. Physiol. Soc., p. 258-259, 1999).
Gram-positive bacteria have been implicated in the pathogenesis of laminitis in the carbohydrate overload model since they have been shown to overgrow in the cecum of horses given corn starch by stomach tube (16). This overgrowth, particularly of streptococci and lactobacilli, was associated with lactic acid accumulation and the death of gram-negative bacteria leading to endotoxin release, both of which were postulated to be the cause of this condition (15, 31). Although endotoxin causes widespread inflammatory effects in the horse, on its own it does not induce laminitis (D. M. Hood, Proc. Am. Assoc. Equine Pract., vol. 41, p. 245-247, 1995). Another theory, put forward by Mungall et al. (33), suggests that exotoxins produced by Streptococcus bovis may induce enzymatic degradation of the lamellar basement membrane. However, the primary factor(s) which trigger the clinical syndrome of acute laminitis remain to be fully elucidated.
Amines, vasoactive compounds produced by the decarboxylation of amino acids by bacteria (24, 37), may also be capable of triggering changes seen in the developmental phase of acute laminitis. These compounds may be produced by gram-positive bacteria including streptococci and lactobacilli (18, 28) as well as gram-negative species and are present in high concentrations in the normal equine cecum (5). Furthermore, concentrations of a number of amines were found to be significantly increased when excess amounts of starch or fructans were added to cecal contents incubated in vitro (5). The effects of these compounds, should they gain access to the peripheral circulation at sufficient concentrations, may include digital vasoconstriction, due to direct and indirect interactions with serotonin (5-hydroxytryptamine) and adrenoreceptors (S. R. Bailey, Y. Berhane, C. M. Marr, and J. Elliott, Abstract, J. Vet. Intern. Med. 15:374, 2001; Y. Berhane, S. B. Bailey, and J. Elliott, Abstract, Br. J. Pharmacol. 135:291P, 2002). Serotonin is a particularly potent vasoconstrictor of equine digital arteries and veins (4, 6). Although not universally accepted, digital lamellar ischemia followed by reperfusion injury is thought to be the sequence of events leading to acute equine laminitis (22, 23).
Virginiamycin, a streptogramin antibiotic, has been used to prevent starch overload-induced laminitis, by preventing the overgrowth of gram-positive bacteria which ferment carbohydrate and produce lactic acid (40). It is not known whether it is the inhibition of lactate production per se and/or inhibition of the formation and absorption of other bacterial products which mediates this protective effect. Furthermore, dietary calcium in the form of CaHPO4 has been shown to modulate inulin-induced cecal fermentation in the rat, partly by control of luminal pH (36), but has not been investigated in other species in this regard.
The present study describes the changes in populations of streptococci, lactobacilli, and gram-negative anaerobes in equine cecal contents, using an in vitro model of carbohydrate overload. The effects of virginiamycin and CaHPO4 on these bacterial populations were also studied. The changes in pH and concentrations of vasoactive amines recorded in these experiments have been reported elsewhere (5). Isolates of streptococci and lactobacilli obtained from these experiments were then screened for their ability to decarboxylate certain amino acids to produce vasoactive amines, and they were subsequently identified by 16S rRNA gene sequence analysis.
MATERIALS AND METHODS
Anaerobic incubation of cecal contents.
Equine cecal contents were obtained from adult horses which had been euthanized for reasons other than gastrointestinal disease, and put into sterile containers flushed with nitrogen gas, with care being taken to preserve anaerobic conditions. The contents were chilled to approximately 4°C for transport to the laboratory. All of the incubation experiments were carried out under oxygen-free conditions, at 37°C (Mk 3 anaerobic work station [Don Whitley Scientific Ltd., Basingstoke, Hampshire, United Kingdom] gassed with 80% nitrogen, 10% hydrogen, and 10% carbon dioxide).
The cecal contents were strained to remove the coarser fibrous material (over 0.5 cm in length) and then divided into 100-ml aliquots. In the first set of investigations, either corn starch (1 g/100 ml) or inulin (1 g/100 ml) was added to separate aliquots, with a third aliquot incubated without added carbohydrate to act as a control. Preliminary studies were undertaken to examine the effects of different carbohydrate concentrations on cecal fermentation, and 1 g/100 ml was found to be sufficient to give maximal effects. Cecal contents were incubated for 24 h at 37°C while being agitated (orbital shaker; Jencons-PLS Ltd., Leighton Buzzard, Bedfordshire, United Kingdom), as previously described (5), and during this time the pH of the fluid in each aliquot was measured at 2-h intervals to establish that carbohydrate fermentation was occurring.
Samples were taken from the cecal contents at 0, 6, 12, 18, and 24 h for enumeration of those streptococci, lactobacilli, and gram-negative anaerobes which would grow on standard selective media. For growth of streptococci, a Columbia blood agar with a streptococcus-selective supplement containing colistin sulfate and oxolinic acid (final concentrations, 5 and 2.5 mg/500 ml medium, respectively; Oxoid Ltd., Basingstoke, Hampshire, United Kingdom) was used. For the growth of lactobacilli, LAMVAB agar was used, made in accordance with the methods of Hartemink et al. (19). This was a medium with low pH and containing vancomycin to inhibit the growth of other gram-positive bacteria such as enterococci. In initial studies, three other selective media were also tested: de Man, Rogosa, and Sharpe agar (MRS) (12); Rogosa agar (38); and tomato juice agar (10). For growth of gram-negative anaerobes, Wilkins-Chalgren anaerobe agar (44) was used with a gram-negative anaerobe-selective supplement containing vancomycin and nalidixic acid (final concentrations, 10 and 2.5 μg/ml of medium, respectively; Oxoid Ltd.).
Bacterial numbers were determined by a dilution method, adapted from a technique originally used by Miles and Misra (30). Serial dilutions of cecal contents in sterile reduced physiological saline (0.5 g of cysteine hydrochloride/liter, pH 6.7) from 1:103 to 1:1012 (vol/vol) were transferred to each of the selective media in triplicate. Following 2 to 3 days of incubation under anaerobic conditions at 37°C, the number of CFU were counted where they numbered between 3 and 30 for a particular dilution. The mean of the triplicate determinations was then used to calculate the number of CFU per ml of cecal contents. Cecal contents from 10 horses were used in these investigations, and data were expressed as the geometric means ± standard errors of the means (SEM) and plotted against time.
In a second set of studies, virginiamycin (10 μg/ml) or calcium hydrogen phosphate (CaHPO4; 0.3 g/100 ml) was added to aliquots of cecal contents to which either starch or inulin had been added (both 1 g/100 ml). Other aliquots, to which 1 g of carbohydrate/100 ml or no carbohydrate had been added, acted as positive and negative controls, respectively. The cecal contents were incubated for 24 h, and samples were taken for determination of bacterial numbers as before. Cecal contents taken from four different animals were used in these experiments, and changes in bacterial numbers over the 24-h period were expressed as the geometric mean ± SEM.
Determination of decarboxylase activity in streptococci and lactobacilli.
Colonies of streptococci and lactobacilli, taken from cecal contents from four different horses and incubated for 24 h with either starch or inulin (both at1 g/100 ml), were grown on streptococcus-selective, LAMVAB or MRS agars. Approximately 100 colonies, representing a range of morphologies, were subcultured and stored in Microbank tubes (Prolab Diagnostics, South Wirral, Cheshire, United Kingdom) at −80°C until required.
Decarboxylase activity in bacterial isolates was assessed using the methods of Bover-Cid and Holzapfel (8). Prior to the screening assays, isolates were grown in an activation medium consisting of MRS broth containing 5 mg of pyridoxal-5-phosphate/100 ml and 100 mg of each of the five amino acids (l-isomers of phenylalanine, tryptophan, tyrosine, isoleucine, and valine)/100 ml. The isolates were incubated anaerobically at 37°C and subcultured 5 to 10 times at 24-h intervals to maximize decarboxylase enzyme activity. Isolates were then transferred onto 24-well plates containing decarboxylase screening agars (8), each containing only one of the above-mentioned amino acids, plus bromcresol purple pH indicator. Following 72 h of incubation, a color change in the medium from brown to purple, representing a pH rise, was considered a positive result. A high-pressure liquid chromatography (HPLC) method (previously described [5]) was used to confirm the presence of the corresponding amines (phenylethylamine, tryptamine, tyramine, 3-methyl butylamine, and isobutylamine) in several representative samples of activation broth in which decarboxylase-positive bacteria had been subcultured.
Identification of decarboxylase positive isolates.
Those bacterial isolates producing a positive color change in the presence of one or more of the amino acids in the screening media were further identified. After ensuring that the streptococci and lactobacilli were pure strains of catalase-negative gram-positive cocci or rods, respectively, the isolates were compared according to their profile of carbohydrate metabolism. This was achieved using the Rapid Strep and the API50 CH kits (both from BioMerieux UK Ltd., Basingstoke, Hampshire, United Kingdom), carried out according to the manufacturer's instructions. These tests were not used to obtain a definitive identification of the isolates, but where several isolates were found to have the same profile of carbohydrate metabolism, it was assumed that they were the same species, and only one of the isolates was used for further typing.
The representative samples of bacterial isolates identified as being positive for decarboxylase activity were then identified using sequence data from the first 500 bp of the 16S rRNA gene, performed by NCIMB Ltd., Aberdeen, United Kingdom (41). The sequence obtained for each isolate was identified by database comparison (BLAST search) using the GenBank nucleotide database (3).
Statistical analyses.
All statistical comparisons were performed using GraphPad Prism, version 3.00, for Windows (GraphPad Software, San Diego, Calif.).
The effects of the two carbohydrate sources on bacterial numbers were compared to the controls by means of two-way (repeated-measures) analysis of variance followed by Bonferroni's post hoc test. The effects of the inhibitors, virginiamycin and calcium phosphate, were assessed at the 24-h time point, comparing the results obtained in the presence of the inhibitors to the positive and negative controls using one-way analysis of variance with Fisher's multiple comparison. In all cases, significance was accepted at P < 0.05.
Materials.
All selective growth media and supplements were obtained from Oxoid Ltd., apart from the ingredients for the LAMVAB agar and decarboxylase screening medium, which were obtained from Merck Ltd., Lutterworth, Leicestershire, United Kingdom. Virginiamycin, CaHPO4 and amino acids were obtained from the Sigma Chemical Company, Poole, Dorset, United Kingdom.
RESULTS
Effect of carbohydrate on bacterial numbers.
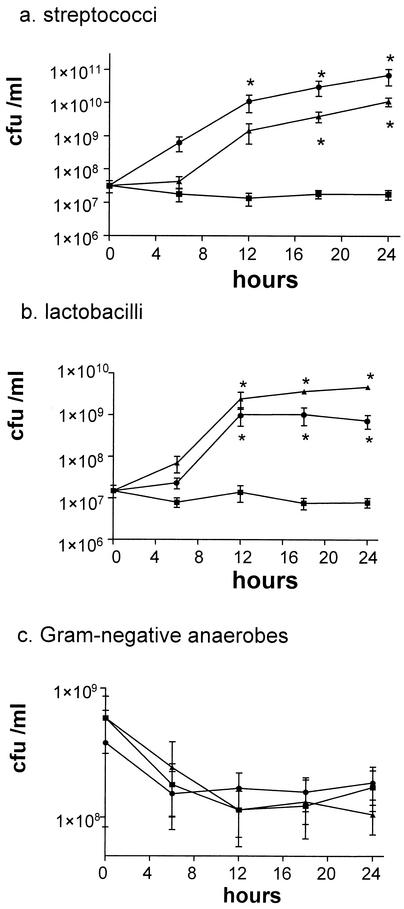
LAMVAB medium was chosen for the isolation and enumeration of lactobacilli, as the growth of some acid-tolerant streptococci from the equine cecum was not prevented on the other media tested. The numbers of streptococci, lactobacilli, and gram-negative anaerobic bacteria did not vary significantly in the control aliquots over the duration of the 24-h experimental period. The addition of starch or inulin resulted in a significant increase in the numbers of streptococci and lactobacilli over the 24-h period of incubation, by factors of 2 to 3.5 log units (Fig. 1a and b) and caused a significant drop in pH (1.5 ± 0.2 and 1.7 ± 0.3 pH units in the presence of starch and inulin, respectively). The numbers of gram-negative anaerobic bacteria were not significantly affected by carbohydrate overload (Fig. 1c).
FIG. 1.
Effects of carbohydrate overload on numbers of streptococci (a), lactobacilli (b), and gram-negative anaerobes (c) in equine cecal contents incubated anaerobically in vitro. Cecal contents were divided into aliquots and incubated for 24 h with the inclusion of either inulin (1 g/100 ml; •) or corn starch (1 g/100 ml; ▴) or without added carbohydrate (control; ▪). Bacterial numbers in each aliquot at 6-h intervals were determined by serial dilution of cecal contents in sterile reduced physiological saline plated onto selective growth medium. Each value represents the geometric mean ± SEM (error bars) of estimates taken from 10 separate experiments. *, significant difference compared with control values (two-way repeated measures analysis of variance with Bonferroni's post hoc test).
Effect of inhibitors on bacterial numbers.
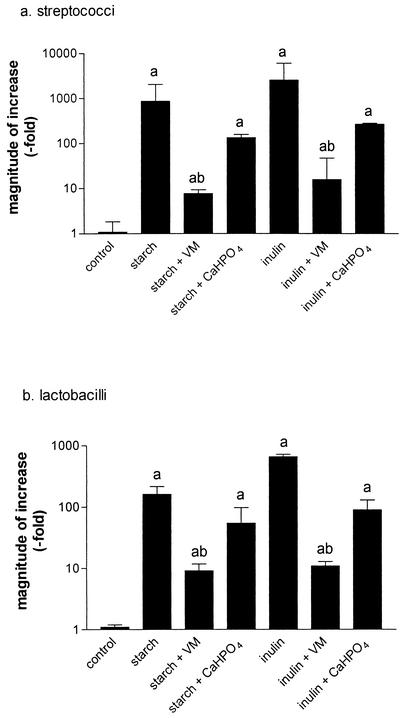
The effects of virginiamycin (10 μg/ml) and calcium hydrogen phosphate (0.3 g/100 ml) are shown in Fig. 2a and b, respectively. Virginiamycin (10 μg/ml) significantly inhibited the increase in numbers of streptococci induced by starch and inulin by 98.4 and 97.8%, respectively, and inhibited the increase in lactobacilli by 96.6 and 98.4%, respectively (Fig. 2). Calcium hydrogen phosphate (0.3 g/100 ml) had no significant effect on bacterial numbers, although there appeared to be a slight inhibitory trend.
FIG. 2.
Effects of virginiamycin and calcium hydrogen phosphate on numbers of Streptococcus (a) and Lactobacillus (b) organisms in equine cecal contents induced by incubation with starch or inulin. Aliquots of cecal contents were incubated with carbohydrate in the presence or absence of virginiamycin (VM) (1 mg/100 ml) or calcium hydrogen phosphate (CaHPO4) (30 mg/100 ml). Bacterial numbers were measured at 0 and 24 h and expressed as CFU/milliliter, and the magnitude of the change over this period was calculated by dividing the number of bacteria present at 24 h by the initial figure (geometric mean + SEM [error bars] of estimates taken from four separate experiments). Abbreviations atop error bars: a, significant differences compared with control; b, significant difference compared to starch or inulin alone (one-way analysis of variance with Fisher's multiple comparison.)
Identification of amino acid decarboxylase-producing strains of streptococci and lactobacilli.
Approximately 100 different isolates of streptococci and lactobacilli from four separate experiments were screened for the ability to decarboxylate one or more of the five amino acids incorporated into the screening agar medium. A total of 26 isolates produced a color change indicative of decarboxylase activity. HPLC analysis of five samples of activation broth in which these isolates had been subcultured showed increased production of the relevant amine(s) in the region of 1.5- to 10-fold.
A further screening of the decarboxylase-positive isolates, using the Rapid Strep or API50CH kits, demonstrated that the 26 isolates could be placed in 10 different groups on the basis of substrate fermentation. 16S rRNA gene sequencing and subsequent species identification was performed on representative isolates of these 10 fermentation groups.
The species of streptococci and lactobacilli identified by this method (including BLAST search score) are shown in Table 1, combined with the results of the decarboxylase screening assay. The species identified included Streptococcus bovis and five different Lactobacillus spp., most strains of which were from the species Lactobacillus mucosae. One isolate contained a sequence most closely matched with that of Lactobacillus salivarius, although the similarity of the sequence compared with that previously published was only 89.61%.
TABLE 1.
Conversion of amino acids to amines by strains isolated from equine cecal contents incubated with starch or inulina
| Bacterial species | BLAST search score (%) | Conversion of amino acidb
|
||||
|---|---|---|---|---|---|---|
| Tryptophan | Tyrosine | Phenylalanine | Isoleucine | Valine | ||
| Streptococcus bovis | 100c | + | + | − | − | − |
| Streptococcus bovis | + | − | + | + | − | |
| Streptococcus bovis | 100d | + | − | + | − | − |
| Streptococcus bovis | + | − | − | + | − | |
| Lactobacillus mucosae | 100e | − | + | − | − | − |
| Lactobacillus mucosae | + | + | + | − | + | |
| Lactobacillus mucosae | − | + | − | − | − | |
| Lactobacillus mucosae | 100e | − | + | − | − | − |
| Lactobacillus mucosae | + | + | + | + | + | |
| Lactobacillus mucosae | + | + | + | + | + | |
| Lactobacillus mucosae | 99.78e | + | − | − | − | − |
| Lactobacillus mucosae | + | − | − | − | − | |
| Lactobacillus mucosae | − | − | − | + | + | |
| Lactobacillus mucosae | + | − | + | − | − | |
| Lactobacillus mucosae | + | − | + | + | + | |
| Lactobacillus mucosae | 99.77e | + | − | − | + | + |
| Lactobacillus mucosae | + | − | + | + | + | |
| Lactobacillus mucosae | + | − | + | + | − | |
| Lactobacillus mucosae | + | − | + | + | + | |
| Lactobacillus mucosae | + | − | + | − | − | |
| Lactobacillus reuteri | 99.79f | − | + | + | − | + |
| Lactobacillus reuteri | + | − | + | + | + | |
| Lactobacillus salivariusj | 89.61g | − | + | − | − | − |
| Lactobacillus salivarius | + | − | + | − | − | |
| Lactobacillus delbruekii | 98.95h | + | − | − | + | − |
| Lactobacillus fermentum | 97.92i | + | − | + | − | − |
Bacterial strains were screened for their ability to decarboxylate each amino acid (either tryptophan, tyrosine, phenylalanine, isoleucine, or valine) using a pH-sensitive screening medium (see text).
Symbols: +, visible colour change in the medium; −, negative result. Following substrate fermentation analysis of bacteria producing positive results, representative strains were identified by 16S rRNA gene sequencing.
AF104109 version GI: 4324443.
AF396921 version GI: 15011526.
AF126738 version GI: 5163336.
AB017358 version GI: 4519574.
AF420311 version GI: 15987824.
AY050173 version GI: 15982695.
AF302117 version GI: 10732798.
Bacterial species whose sequence most closely matched that of L. salivarius.
Subjectively, the most intense color changes were produced by the decarboxylation of tyrosine by isolates belonging to the species L. mucosae and Lactobacillus reuteri plus the isolates with similarities to L. salivarius, although there was no distinct species-related pattern to the amino acids which were metabolized.
DISCUSSION
It has been shown that a wide range of potentially vasoactive amines occur at relatively high concentrations within the cecal contents of horses and that the concentrations of phenylethylamine and isoamylamine in particular were increased under conditions providing excess carbohydrate in vitro (5). We therefore postulated that the carbohydrate-fermenting bacteria, Streptococcus spp. and Lactobacillus spp., might, at least in part, be responsible for their production. The present study confirmed that numbers of streptococci and lactobacilli markedly increased in this model system following the addition of fermentable carbohydrate. Certain strains of these bacteria were shown to be capable of producing one or more amines which may be of pathological importance due to their vasoactive properties.
Excessive amounts of fermentable carbohydrate (corn starch), administered orally to experimental horses, has been shown to cause lactic acid production within the cecum, associated with an overgrowth of gram-positive bacteria, particularly Streptococcus spp. and Lactobacillus spp. (15, 16). These changes often lead to the development of acute laminitis some 36 to 48 h later (14). Similarly, inulin (a naturally occurring form of fructan carbohydrate of a type similar to that found in grass) has also been used to initiate this condition (C. Pollitt, personal communication), and it has been suggested recently that fructans may be responsible for initiating pasture-induced laminitis (Longland et al., Proc. 16th Equine Nutr. Physiol. Soc.).
The in vitro model used in the present study was similar to those in other studies examining rumen contents from cattle and sheep, in which an excess of fermentable carbohydrate will also cause toxicity and acidosis (2, 13). Indeed, the bacterial flora of the equine cecum shows many similarities with that of the bovine rumen (26, 27). Fructans have also been shown to cause fermentation in the ceca of monogastric animals such as the rat (36), due to the fact that such oligosaccharides are not digestible in the mammalian small intestine.
The conditions under which the cecal contents were incubated did not lead to significant changes in pH or in the numbers of the bacterial species of interest in the control aliquots over the duration of the experimental period. The addition of starch or fructans to the cecal contents resulted in a marked fall in pH over the time course of the experiments (5), the magnitude of which is consistent with the cecal pH changes due to lactic acid production demonstrated in vivo when horses have been administered large amounts of starch (15, 31).
The fermentation of starch or fructans added to cecal contents was associated with marked increases (2 to 3.5 log units) in numbers of streptococci and lactobacilli. The increase in numbers of streptococci and lactobacilli in the present study is consistent with the results of those in vivo experiments (16). In those studies by Garner et al., however, the numbers of gram-negative anaerobic bacteria fell following the administration of carbohydrate, thought to be due to lactic acid production causing their death and subsequent release of endotoxin. The numbers of gram-negative anaerobic bacteria counted in cecal contents from the present study did not decline significantly, although it may be that more-prolonged exposure to conditions of low pH might overcome their apparent pH tolerance and result in their decline. The total number of gram-negative anaerobic bacteria isolated in the present study was in line with a previous report enumerating equine cecal bacteria (25).
The rate of increase of gram-positive bacterial numbers correlated with the fall in pH and the increase in concentrations of phenylethylamine and isoamylamine previously described (5), and it is known that gram-positive bacteria, such as streptococci and lactobacilli, have the ability to produce amines from amino acids (8, 18). It should be noted however, that concentrations of these and other amine compounds were already high in cecal contents from normal horses relative to their potential effects on equine digital blood vessels (Berhane et al., Abstr., Br. J. Pharmacol. 135:291P; Bailey et al., J. Vet. Int. Med. 15:374, 2001). Therefore, it should not be concluded that these particular gram-positive bacteria were the sole source of production of these compounds. Indeed, many other groups of bacteria, including groups of gram-negative bacteria such as the enterobacteria, contain the decarboxylase enzymes necessary to produce amines (17). Nevertheless, the marked increase in the numbers of streptococci and lactobacilli associated with carbohydrate fermentation suggests that these bacteria may be of great significance in the production of vasoactive amines under these conditions.
The streptogramin antibiotic, virginiamycin, inhibited the increases in numbers of streptococci and lactobacilli associated with carbohydrate excess in the present study, as it did the production of phenylethylamine and isoamylamine (5). It did not appear to decrease numbers of streptococci or lactobacilli to levels below the numbers observed in control aliquots, however. Virginiamycin was included at a concentration of 10 μg/ml, based upon the concentration shown to have effects on rumen fermentation in vitro (29) and its MIC for S. bovis and Lactobacillus spp. cultured from the rumen (34). Orally administered virginiamycin moderates rumen fermentation in vivo (11), and in the horse, this antibiotic has been marketed in Australia as Founderguard, an oral formulation for the prevention of laminitis. This product has been shown to suppress lactic acid production in the hind gut of horses caused by grain overload (40).
As a comparison to virginiamycin, the effects of calcium hydrogen phosphate (CaHPO4) were also investigated. Dietary calcium in the form of CaHPO4 has been shown to ameliorate inulin-induced cecal fermentation in the rat, partly by control of luminal pH (36). Insoluble CaPi did not otherwise affect microbial activity. In our model, CaHPO4 significantly limited the fall in pH associated with fermentation of both starch and inulin by equine cecal contents (5). There was no significant effect on bacterial numbers in the present study, however, although a trend was seen. Taken together, these results confirm that the significant effects of virginiamycin on amine production were due to specific antimicrobial properties, rather than being indirect through the moderation of pH.
The selective culture media for the growth of the three groups of bacteria examined in this study appeared to show good sensitivity, with LAMVAB agar providing better selective growth of cecal lactobacilli than MRS and Rogosa agars. Twenty-six strains of lactobacilli and streptococci from four different animals gave positive reactions for decarboxylase activity in the presence of one or more of the amino acids when grown on the decarboxylase screening agar. Several different amino acids were examined in this procedure, corresponding to the production of different vasoactive amines, since there is substrate selectivity among the different amino acid decarboxylase enzymes (7). Interestingly, once the bacterial species had been identified by 16S rRNA gene sequence analysis, it became clear that there was no species-specific pattern to the combination of amino acids converted. This finding has previously been reported by Bover-Cid et al. (9), who also noted that decarboxylase activity in lactobacilli tended to be dependent on strain rather than species.
Of the four isolates of Streptococcus spp. identified as having the ability to decarboxylate one or more of the amino acids tested, all were found by sequence analysis to be of the species S. bovis (43). S. bovis has been implicated in the pathogenesis of acute laminitis in the horse, being one of the principal species found to overgrow in the ceca of horses given corn starch to experimentally induce this condition (1). In addition, a recent paper by Mungall et al. (33), in which supernatants of bacterial broth in which S. bovis had been grown were added to hoof lamellar explants, described that laminar separation occurred as a result of matrix metalloproteinase activation. The present study therefore provides another possible means by which S. bovis may play a role in causing laminitis.
The majority of strains of lactobacilli able to decarboxylate one or more of the added amino acids in the present study belonged to the species L. mucosae. This is a relatively new species, initially isolated from the pig intestine (39), and not previously described in the horse hindgut. It is, however, closely related to other species such as L. reuteri and Lactobacillus fermentum (39), which were also found to have decarboxylase activity in the present study. These species, together with L. salivarius and Lactobacillus delbruekii, have all been shown to be responsible for the production of amines in various fermented foodstuffs (8, 42). The strong positive color change for the decarboxylation of tyrosine by a number of strains of lactobacilli may reflect the extent to which these bacteria produce tyramine (32). However, this method was not considered to be of quantitative value, since the concentration of tyramine in some samples of activation broth measured by HPLC appeared to be no greater than that of other amines.
In summary, this study has demonstrated that the addition of starch or fructan carbohydrates to equine cecal contents was associated with a marked increase in the numbers of streptococci and lactobacilli over 24 h, some of which were capable of decarboxylating certain amino acids to produce amine compounds. Their growth was markedly inhibited by virginiamycin, but not by calcium hydrogen phosphate. The vasoactive properties of some of these amines suggest that their absorption from the cecum into the circulation of the horse, following fermentation of excess carbohydrate, could trigger changes in blood flow to the digit and therefore could be involved in the pathogenesis of acute laminitis. Additional studies are warranted to investigate further the role of vasoactive amines in this disease and the conditions favoring their production in the equine hindgut.
Acknowledgments
This work was supported by the Waltham Centre for Pet Nutrition.
We thank Maggie Bushnell for her technical assistance.
REFERENCES
- 1.Al Jassim, R. A. M., and J. B. Rowe. 1999. Better understanding of acidosis and its control. Rec. Adv. Anim. Nutr. Aust. 12:91-97. [Google Scholar]
- 2.Allison, M. J., I. M. Robinson, R. W. Dougherty, and J. A. Bucklin. 1975. Grain overload in cattle and sheep: changes in microbial populations in the caecum and rumen. Am. J. Vet. Res. 36:181-185. [PubMed] [Google Scholar]
- 3.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bailey, S. R., and J. Elliott. 1998. Plasma 5-hydroxytryptamine constricts equine digital blood vessels in vitro: implications for the pathogenesis of acute laminitis. Equine Vet. J. 30:124-130. [DOI] [PubMed] [Google Scholar]
- 5.Bailey, S. R., A. N. Rycroft, and J. Elliott. 2002. Production of amines in an in vitro model of carbohydrate overload. J. Anim. Sci. 80:2656-2662. [DOI] [PubMed] [Google Scholar]
- 6.Baxter, G. M., R. E. Laskey, R. L. Tackett, J. N. Moore, and D. Allen. 1989. In vitro reactivity of digital arteries and veins to vasoconstrictive mediators in healthy horses and horses with early laminitis. Am. J. Vet. Res. 50:508-517. [PubMed] [Google Scholar]
- 7.Boeker, E., and E. Snell. 1972. Amino acid decarboxylases, p. 217. In P. Boyer (ed.), The enzymes, vol. VI, 3rd ed. Academic Press, New York, N.Y.
- 8.Bover-Cid, S., and W. H. Holzapfel. 1999. Improved screening procedure for biogenic amine production by lactic acid bacteria. Int. J. Food Microbiol. 53:33-41. [DOI] [PubMed] [Google Scholar]
- 9.Bover-Cid, S., M. Hugas, M. Izquierdo-Pulido, and M. C. VidalCarou. 2001. Amino acid-decarboxylase activity of bacteria isolated from fermented pork sausages Int. J. Food Microbiol. 66:185-189. [DOI] [PubMed] [Google Scholar]
- 10.Briggs, M. 1953. An improved medium for lactobacilli. J. Dairy Res. 20:36-40. [Google Scholar]
- 11.Coe, M. L., T. G. Nagaraja, Y. D. Sun, N. Wallace, E. G. Towne, K. E. Kemp, and J. P. Hutcheson. 1999. Effect of virginiamycin on ruminal fermentation in cattle during adaptation to high concentrate diet and during an induced acidosis. J. Anim. Sci. 77:2259-2268. [DOI] [PubMed] [Google Scholar]
- 12.de Man, J. C., M. Rogosa, and E. M. Sharpe. 1960. A medium for the cultivation of lactobacilli. J. Appl. Bacteriol. 23:130-135. [Google Scholar]
- 13.Demeyer, D. I., D. Fiedler, and K. G. DeGraeve. 1996. Attempted induction of reductive acetogenesis into the rumen fermentation in vitro. Reprod. Nutr. Dev. 36:233-240. [DOI] [PubMed]
- 14.Garner, H. E., J. R. Coffman, A. W. Hahn, and C. Salem. 1975. Equine laminitis of alimentary origin: an experimental model. Am. J. Vet. Res. 36:441-445. [PubMed] [Google Scholar]
- 15.Garner, H. E., D. P. Hutcheson, J. R. Coffman, A. W. Hahn, and C. Salem. 1977. Lactic acidosis: a factor associated with equine laminitis. J. Anim. Sci. 45:1037-1041. [DOI] [PubMed] [Google Scholar]
- 16.Garner, H. E., J. N. Moore, J. H. Johnson, L. Clark, J. F. Amend, L. G. Tritschler, J. F. Coffman, R. F. Sprouse, D. P. Hutcheson, and C. A. Salem. 1978. Changes in the caecal flora associated with the onset of laminitis. Equine Vet. J. 10:249-252. [DOI] [PubMed] [Google Scholar]
- 17.Ghenghesh, K. S., and D. B. Drucker. 1989. Gas liquid chromatography of amines produced by the enterobacteriaceae. Braz. J. Med. Biol. Res. 22:653-665. [PubMed] [Google Scholar]
- 18.Golovnya, R. V., I. L. Zhuravleva, and S. G. Kharatyan. 1969. Gas chromatographic analysis of amines in volatile substances of Streptococcus lactis. J. Chromatogr. 44:262-268. [DOI] [PubMed] [Google Scholar]
- 19.Hartemink, R., V. R. Domenech, and R. M. Rombouts. 1997. LAMVAB—a new selective medium for the isolation of lactobacilli from faeces. J. Microbiol. Methods 29:77-84. [Google Scholar]
- 20.Hartemink, R., and F. M. Rombouts. 1999. Comparison of media for the detection of bifidobacteria, lactobacilli and total anaerobes from faecal samples. J. Microbiol. Methods 36:181-192. [DOI] [PubMed] [Google Scholar]
- 21.Hoffman, R. M., J. A. Wilson, D. S. Kronfeld, W. L. Cooper, L. A. Lawrence, D. Sklan, and P. A. Harris. 2001. Hydrolyzable carbohydrates in pasture, hay, and horse feeds: direct assay and seasonal variation. J. Anim. Sci. 79:500-506. [DOI] [PubMed]
- 22.Hood, D. M., D. A. Grosenbaugh, M. B. Mostafa, S. J. Morgan, and B. Thomas. 1993. The role of vascular mechanisms in the development of acute equine laminitis. J. Vet. Int. Med. 7:228-234. [DOI] [PubMed] [Google Scholar]
- 23.Hood, D. M., L. P. Wagner, and G. W. Brumbaugh. 2001. Evaluation of hoof wall surface temperature as an index of digital vascular perfusion during the prodromal and acute phases of carbohydrate-induced laminitis in horses. Am. J. Vet. Res. 62:1167-1171. [DOI] [PubMed] [Google Scholar]
- 24.Hurst, W. J. 1990. A review of HPLC methods for the determination of selected biogenic amines in foods. J. Liquid Chromatogr. 13:1-23. [Google Scholar]
- 25.Julliand, V., A. de Vaux, L. Millet, and G. Fonty. 1999. Identification of Ruminococcus flavefaciens as the predominant cellulotytic bacterial species of the equine cecum. Appl. Environ. Microbiol. 65:3738-3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kern, D. L., L. L. Slyter, J. M. Weaver, E. C. Leffel, and G. Samuelson. 1973. Pony caecum vs. steer rumen: the effect of oats and hay on the microbial ecosystem. J. Anim. Sci. 37:463-469. [DOI] [PubMed] [Google Scholar]
- 27.Kern, D. L., L. L. Slyter, E. C. Leffel, J. M. Weaver, and R. R. Oltjen. 1974. Ponies vs. steers: microbial and chemical characteristics of intestinal ingesta. J. Anim. Sci. 38:559-563. [DOI] [PubMed] [Google Scholar]
- 28.Leuschner, R. G. K., and W. P. Hammes. 1999. Formation of biogenic amine in mayonnaise, herring and tuna fish salad by lactobacilli. Int. J. Food Sci. Nutr. 50:159-164. [DOI] [PubMed] [Google Scholar]
- 29.Marounek, M., V. Skrivanova, and J. Simunek. 1995. The effect of virginiamycin on rumen fermentation in vitro after adaptation of donors to the inoculum. Vet. Med. (Prague) 40:129-132. [PubMed] [Google Scholar]
- 30.Miles, A. A., and S. S. Misra. 1938. The estimation of the bactericidal power of the blood. J. Hyg. Camb. 38:732.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moore, J. N., H. E. Garner, J. N. Berg, and R. F. Sprouse. 1979. Intracaecal endotoxin and lactate during the onset of equine laminitis: a preliminary report. Am. J. Vet. Res. 40:722-723. [PubMed] [Google Scholar]
- 32.Moreno-Arribas, V., S. Torlois, A. Joyeux, A. Bertrand, and A. Lonvaud-Funel. 2000. Isolation, properties and behaviour of tyramine-producing lactic acid bacteria from wine. J. Appl. Microbiol. 88:584-593. [DOI] [PubMed] [Google Scholar]
- 33.Mungall, B. A., M. Kyaw-Tanner, and C. C. Pollitt. 2001. In vitro evidence for a bacterial pathogenesis of equine laminitis. Vet. Microbiol. 79:209-223. [DOI] [PubMed] [Google Scholar]
- 34.Nagaraga, T. G., and M. B. Taylor. 1987. Susceptibility and resistance of ruminal bacteria to antimicrobial feed additives. Appl. Environ. Microbiol. 53:1620-1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Potter, G. D., F. F. Arnold, D. D. Householder, D. H. Hansen, and K. M. Brown. 1992. Digestion of starch in the small or large intestine of the equine. Pferdeheilkunde 1:107-111. [Google Scholar]
- 36.Remesy, C., M. A. Levrat, L. Gamet, and C. Demigne. 1993. Caecal fermentations in rats fed oligosaccharides (inulin) are modulated by dietary calcium level. Am. J. Physiol. 264:G855-G862. [DOI] [PubMed] [Google Scholar]
- 37.Rice, S. L., and P. E. Koehler. 1976. Tyrosine and histidine decarboxylase activities of Pediococcus cerevisiae and Lactobacillus species and the production of tyramine in fermented sausages. J. Milk Food Technol. 38:256-258. [Google Scholar]
- 38.Rogosa, M., J. A. Mitchell, and R. F. Wiseman. 1951. A selective medium for the isolation and enumeration of oral and fecal lactobacilli. J. Bacteriol. 62:132-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roos, S., F. Karner, L. Axelsson, and H. Jonsson. 2000. Lactobacillus mucosae sp. nov., a new species with in vitro mucus-binding activity isolated from pig intestine. Int. J. Syst. E vol. Microbiol. 50:251-258. [DOI] [PubMed] [Google Scholar]
- 40.Rowe, J. B., D. W. Pethick, and M. J. Lees. 1994. Prevention of acidosis and laminitis associated with grain feeding in horses. J. Nutr. 124:S2742-S2744. [DOI] [PubMed]
- 41.Sanger, F., S. Nicklen, and A. R. Coulson. 1977. DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. 74:5463-5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Straub, B. W., M. Kicherer, S. M. Schilcher, and W. P. Hammes. 1995. The formation of biogenic amines by fermentation organisms. Z. Lebensm. Unters. Forsch. 201:79-82. [DOI] [PubMed] [Google Scholar]
- 43.Whitehead, T. R., and M. A. Cotta. 2000. Development of molecular methods for identification of Streptococcus bovis from human and ruminal origins. FEMS Microbiol. Lett. 182:237-240. [DOI] [PubMed] [Google Scholar]
- 44.Wilkins, T. D., and S. Chalgren. 1976. Medium for use in antibiotic susceptibility testing of anaerobic bacteria. Antimicrob. Agents Chemother. 10:926-928. [DOI] [PMC free article] [PubMed] [Google Scholar]