Abstract
Terminal restriction fragment length polymorphism (T-RFLP) analysis was conducted on the 16S rRNA genes of the bacterial communities colonizing the epithelial surfaces of the terminal ilea of open conventionally housed mice in an institutional small-animal facility. Polymeric-immunoglobulin-receptor-deficient (pIgR−/−) mice that were unable to secrete antibodies across mucosal surfaces were cohoused with normal and otherwise genetically identical wild-type (C57BL/6) mice for 4 weeks. If secretory antibodies played a role in modeling the gastrointestinal microbiota, C57BL/6 mice would have had a more distinct and uniform microbiota than their pIgR−/− cage mates. The T-RFLP profiles of the bacterial communities were compared by using Sorensen's pairwise similarity coefficient, a newly developed weighted pairwise similarity coefficient, and on the basis of Shannon's and Simpson's diversity indices. No systematic differences were observed between the dominant components of the mucosa-associated bacterial communities of the terminal ileal walls of the two types of mice, indicating that secretory antibodies do not control the composition of this microbiota. Similar analyses of experiments conducted at two different times, between which the bacterial community composition of the mouse colony in the small-animal facility appeared to have changed, showed that differences could have been detected, had they existed.
Colonization of the mucosal surfaces of the gastrointestinal (GI) tract of mammals begins at birth with a succession of microorganisms, until a relatively stable, climax, GI microbial community is established (2, 10, 38-40). In mature animals, the number of microbial cells in the GI tract outnumbers the number of eukaryotic cells in the body, and the GI microbiota plays an important role in the host's life and health (2, 10). In addition to producing nutrients, the GI microbiota plays an important role in protection against disease. The dense microbial population occupies many attachment sites on the epithelial cell layer lining the mucosal surface of the GI tract, limiting the opportunities for colonization of the GI tract by pathogens (48). The resident microbial population is also antagonistic toward invaders by releasing antimicrobial compounds, including fermentation end products, and its protective role against enteric pathogens has been demonstrated (9, 16, 17). The GI microbiota contributes to the development of the systemic and mucosal immune systems and the physiology of the GI tract. Germfree mice have low levels of circulating and secreted antibody, but after conventionalization with a normal GI microbiota the development of the immune system and normal gut morphology commences, and antibodies are secreted in large amounts at mucosal surfaces (45).
The rate of antibody secretion at mucosal surfaces reaches 66 mg per kg of body weight of mammals per day and is considered to be the first specific line of immune defense (22, 32). Of the secreted antibody isotypes (immunoglobulin A [IgA], IgG and IgM), IgA is the most abundant and is predominantly produced by B1 lymphocytes residing in the lamina propria in a T-cell-independent pathway (1, 24, 28). However, lower levels of antibody are also produced by B2 lymphocytes in a conventional CD4+-T-cell-dependent pathway (28).
Large amounts of secretory immunoglobulin (sIg) are produced, but its major function has not been completely resolved. Studies with disease models have provided conflicting evidence for protection against mucosal pathogens (3, 15, 35; T. K. Uren and R. A. Strugnell, unpublished data), whereas evidence supporting its function in trafficing antigens or microorganisms out of the lamina propria back into the intestinal lumen is compelling (20, 37). It has also been proposed that sIg may act as a neutralizing “blanket” to prevent the translocation of antigens derived from the GI tract across the epithelial cell layer lining the mucosal surface in the first place (1, 19, 50). sIg has also been suggested to model the microbiota of the GI tract (4, 10, 32, 38). There is certainly an interaction of the GI microbiota with the mucosal immune system and subsequent coating of the microbiota with sIg (29, 49).
Studies of the role of sIg in maintaining or determining the structure of the commensal microbial community of the GI tract (6, 18, 31) have most recently examined the microbiota of gnotobiotic μMT mice possessing a simple-defined GI microbiota (31). Mice were transferred from a contained environment to conventional housing at weaning, and no overgrowth of this microbiota by exogenous microorganisms was observed (31). However, μMT mice have subsequently been shown to secrete variable but significant amounts of sIgA (30), casting doubts on the results of these experiments (4, 30). In contrast, more recent studies (18, 43) have shown that the colonization patterns of the segmented filamentous bacteria (SFB), as-yet-uncultivated but characteristic organisms found predominantly in the terminal ileum of young animals (7, 43), are influenced by the actions of the immune system. SFB are able to persist for longer in severe combined immunodeficient (SCID) mice, which are deficient in T and B cells, and therefore deficient in sIg (18). The persistence of SFB in SCID mice implies that the mucosal immune system may control the colonization of at least one component of the GI microbiota. sIg has been shown to interfere with bacterial adherence to the epithelium (31) and therefore has the ability to control the access of bacteria to the intestinal epithelium. Given the recent findings of a possible role of sIg in controlling colonization of SFB in SCID mice (18), and the problems associated with previous models studying the effect of sIg on the GI microbiota, we have revisited the effect of sIg on the GI microbiota in sIg-deficient mice exposed to an environment with the potential for unlimited microbial colonization.
We have examined the impact of sIg on the composition of the GI microbiota by comparing the 16S rRNA genes of bacterial communities in the terminal ileum of cohoused, open-conventional C57BL/6 and polymeric-immunoglobulin-receptor-deficient (pIgR−/−) mice. The use of a culture-independent technique (terminal restriction fragment length polymorphism [T-RFLP]) enables the entire bacterial community to be studied, without the problems associated with low culturability from complex microbial communities (27, 36). pIgR−/− mice express a truncated pIgR that is unable to bind immunoglobulin for secretion across mucosal surfaces (19, 42, 47). Therefore, pIgR−/− mice are deficient in all isotypes of sIg but have a genetic background that is otherwise identical to wild-type C57BL/6 mice (47). If sIg plays a significant role in modeling the commensal microbiota, open housing will result in changes to the microbiota in sIg-deficient animals, whereby otherwise transient organisms may become established as microbiota colonizing the epithelium of the GI tract.
MATERIALS AND METHODS
Mice.
C57BL/6 and C57BL/6-pIgR−/− (hereafter referred to as pIgR−/−) mice were used in the present study. A description of the construction and characterization of the immunological phenotype of pIgR−/− mice has been published elsewhere (47). Briefly, C57BL/6 embryonic stem cells were transfected in vitro with a recombination cassette carrying neomycin resistance (kindly supplied by F. Johansen, University of Oslo). Mutant embryonic stem cells were selected by neomycin resistance and analyzed by Southern blotting for mutation of the pIgR locus (19). Embryonic stem cells carrying the neomycin cassette inserted into the pIgR locus were microinjected into developing C57BL/6 embryos. These embryos were then implanted into pseudopregnant mice. Heterozygous offspring (i.e., pIgR+/−) were detected by Southern blot as previously described (19) and bred to homozygosity (i.e., pIgR−/−). The phenotype of these pIgR−/− mice was very similar to that described by others (19, 42).
To eliminate any potential effects that may be caused by separate housing, 6-week-old C57BL/6 and pIgR−/− specific-pathogen-free female mice were cohoused in groups of 4 for 4 weeks under conventional conditions prior to commensal microbiota analysis at 10 weeks of age. Cages contained individual animals from four different litters. Animals housed under these conventional conditions were susceptible to colonization by microorganisms from the normal small-animal facility environment. Two experiments were conducted, 3 months apart.
Phenotyping.
The phenotypes of pIgR−/− and C57BL/6 mice were confirmed by enzyme-linked immunosorbent assay (ELISA) of immunoglobulin levels in serum, saliva, and feces. Saliva was collected after intraperitoneal injection of 200 μl of carbachol (20 μg/ml in phosphate-buffered saline [PBS]; 1.9 mM KH2PO4, 8.1 mM Na2HPO4, and 150 mM NaCl at pH 7.4). Whole blood was collected from the retro-orbital plexus by using heparinized capillary tubes, and serum was separated by low-speed centrifugation. Fresh fecal samples were collected, and all samples were stored at −20°C until analysis. Immunoglobulin was extracted from feces by using the method described by Bromander et al. (5). ELISA was conducted with a 96-well Maxisorp immunoplates (Nunc A/S, Kamstrupp, Denmark) coated overnight with 50 μl of unconjugated goat anti-mouse IgA (5 μg/ml; Sigma, St. Louis, Mo.) in PBS. Initial dilutions in PBS of 1/200, 1/5, and 1/50 were made of serum, saliva, and fecal preparations, respectively, and serial 1-in-3 dilutions in PBS were made across the plate. Plates were then incubated for 2 h at 37°C before washing them five times with PBS containing 0.01% (vol/vol) Tween 20. Bound mouse IgA was measured by adding sheep anti-mouse immunoglobulin antibody conjugated to horseradish peroxidase (Silenus Laboratories, Hawthorn, Victoria, Australia). Plates were incubated for 2 h at 37°C and washed five times with PBS containing 0.01% (vol/vol) Tween 20. Conjugate antibody bound to mouse IgA was detected by the addition of 100 μl of substrate solution (25 mM citric acid, 50 mM Na2HPO4, 0.22 mM immunopure o-phenylenediamine dihydrochloride [Pierce, Rockford, Ill.], 16.6 mM H2O2; pH 5.2). Color development was stopped, after 10 min, by the addition of 30 μl of 2.5 M H2SO4, and the absorbance at 492 nm was read on a Labsystems Multiskan plate reader (Thermolabsystems, Helsinki, Finland). The amount of IgA in each sample was estimated from a standard curve prepared by using purified mouse IgA hybridoma protein (ICN Biomedicals, Costa Mesa, Calif.). Mean and standard deviation values were calculated from the individual titers from six mice (serum and feces) or five mice (saliva). The presence of immunoglobulin in bile was measured by Western blot. Bile was removed from the gallbladder of mice sacrificed by CO2 inhalation, with a syringe and fine-gauge needle, avoiding contamination with blood. The proteins in 20 μl of bile were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis according to the method of Laemmli et al. (25) as described by Dunstan et al. (13). Western blot analyses were carried out as described by Dunstan et al. (13) except that bound antibody was detected with sheep anti-mouse horseradish peroxidase-conjugated antibody (Silenus) diluted 1/1,000 in PBS.
Extraction of DNA from ileum.
After sacrifice of mice by CO2 inhalation, a 1-cm section of terminal ileum was immediately removed 2 cm from the ileocecal junction by using a sterile scalpel blade. The section was opened, and lumenal debris was removed by four successive washes, each one in 1 ml of PBS. Adherent bacteria were removed from the intestinal wall by homogenization for 20 s at 2,500 rpm in 1 ml of PBS by using a Mini-Beadbeater (BioSpec Products, Inc., Bartlesville, Okla.). The samples, containing PBS, bacterial cells, and the ileal segment, were then pulsed for 5 s in a microcentrifuge, and the supernatant containing released bacterial cells was removed for DNA extraction. Cells were harvested from the supernatant by centrifugation for 15 min at 16,000 × g and then resuspended in 0.5 ml of T50E (10 mM Tris [adjusted to pH 8.0 with HCl] plus 50 mM EDTA [adjusted to pH 8.0 with NaOH]). A total of 200 mg of sterile 0.1-mm-diameter glass beads (BioSpec) was added, and the cells were lysed by shaking in the Mini-Beadbeater for 10 s at 5,000 rpm. No intact cells were observed by phase-contrast microscopy after this step. Debris was pelleted by centrifugation for 5 min at 16,000 × g, and the supernatant, containing the DNA, was removed and placed in a fresh tube. The beads were washed with the addition of 0.5 ml of T50E to remove residual DNA and pelleted by centrifugation, and the supernatants containing DNA were pooled. Proteins in the pooled samples were digested for 2 h at 56°C with 0.5 mg of protease (Qiagen, Clifton Hill, Victoria, Australia) per 1.5-ml sample. The protease-treated samples were then extracted successively with equal volumes of Tris-buffered phenol (pH 8.0; Sigma, Castle Hill, New South Wales, Australia), Tris-buffered phenol-chloroform-isoamyl alcohol (25:24:1 [vol/vol/vol]), and finally chloroform/isoamylalcohol (24:1 [vol/vol]). DNA was precipitated from the T50E, after the addition of NaCl to a final concentration of 0.5 M and 3 volumes of 100% ethanol, by incubation at 4°C for 16 h and then pelleted by centrifugation for 20 min at 16,000 × g. DNA was then washed with 70% (vol/vol) ethanol, pelleted by centrifugation, and finally resuspended in 10 μl of T10E (10 mM Tris [adjusted to pH 8.0 with HCl] plus 1 mM EDTA [adjusted to pH 8.0 with NaOH]). Agarose gel electrophoresis was performed to assess DNA quality, and DNA was visualized by staining in the gel with ethidium bromide (0.1 μg/ml) and UV transillumination. DNA was quantified by using the precision molecular mass standard (Bio-Rad Laboratories, Hercules, Calif.) and Kodak 1D imaging software (Kodak Scientific Imaging Systems, New Haven, Conn.).
Generation of T-RFLP profiles.
All PCRs were performed in 0.2 ml tubes in a PCRsprint thermocycler (Hybaid, Ashford, Middlesex, United Kingdom) in a final volume of 100 μl. Each reaction contained 30 ng of DNA, 1 mM MgCl2, the high-pressure liquid chromatography-purified oligonucleotide primers 1492r (5′-GGYTACCTTGTTACGACTT-3′) and FAM27f (5′-GAGTTTGATCMTGGCTCAG-3′) (Geneworks Pty, Ltd., Adelaide, South Australia, Australia) at final concentrations of 1 μM each, PCR buffer (Qiagen), 2 U of Taq DNA polymerase (Qiagen), and 50 μM concentrations of each deoxynucleoside triphosphate (Promega, Annandale, New South Wales, Australia). The primer FAM27f was purchased labeled at the 5′ terminus with 6-carboxyfluorescein (6-FAM). Taq DNA polymerase and deoxynucleoside triphosphates were added to the reactions after an initial 5-min denaturation step at 94°C. The PCR amplification was 25 cycles of 94°C for 15 s, 50°C for 15 s, and 72°C for 60 s, followed by a final extension step of 50°C for 1 min and 72°C for 6 min. Unincorporated primers and reaction components were then removed by using an UltraClean PCR cleanup column (Geneworks). The DNA recovered was quantified by agarose gel electrophoresis as described above. The purified PCR products were digested with HaeIII (New England Biolabs, Inc., Beverly, Mass.) for 2 h at 37°C. Each digest contained 3 U of HaeIII, buffer 2 (New England Biolabs), and purified DNA (75 ng) in a final volume of 25 μl. The digested product was precipitated as described above, and dried pellets were resuspended in 10 μl of distilled water. An aliquot of 2.5 μl of the resuspended product was added to 0.3 μl of Genescan 500 Rox size standard (Applied Biosystems, Foster City, Calif.) and then 0.4 μl of Dextran Blue (50 mg per ml of 25 mM EDTA, pH 8.0 with NaOH) and 1.8 μl of formamide were added, before denaturation at 95°C for 3 min. A 1-μl portion of this was separated by using a 377 DNA sequencer (Applied Biosystems) at the Australian Genome Research Facility (Parkville, Victoria, Australia).
Analysis of T-RFLP profiles.
Each sample analyzed generated a profile consisting of terminal restriction fragments (T-RFs) that each had a size or fragment length (in nucleotides) and an area (reflecting the amount of 6-FAM label detected). The raw data set of peak areas and fragment lengths obtained from the Genotyper software (Applied Biosystems) was first compiled so that all unique T-RFs in the entire data set of all of the profiles to be analyzed were included in each T-RFLP profile in that data set. To do this, T-RFs that were present in at least one sample were added to the data set of all profiles that lacked that T-RF, and an area of zero was assigned to that T-RF if it had not been detected. A threshold area value was then used to remove small peaks that may be detected purely as a result of the amount of DNA applied to the separation gels (see Results for more information). To do this, the area that each T-RF contributed was calculated as a proportion of the total area for all T-RFs in that profile. These proportions were then assigned to the appropriate T-RFs as a relative area. T-RFs that contributed less than a designated threshold percentage (0 to 8%, as detailed in Results) were reassigned a value of zero. These edited data sets were then subjected to further analysis.
A Sorensen's pairwise similarity coefficient, Cs, was calculated for each pair of T-RFLP profiles within a complete edited data set as follows: Cs = 2j/(a + b), where j is the number of T-RFs with relative areas of greater than zero common to the two profiles being compared within an edited data set, and a and b are the number of T-RFs with a relative area of greater than zero in each of the two profiles. A weighted similarity coefficient, Cw, was calculated for each pair of T-RFLP profiles within an edited data set by using the empirically derived formula:
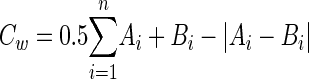 |
where Ai and Bi are the relative areas of the ith T-RF in the two profiles being compared, and n is the total number of T-RFs in that edited data set.
The Sorensen's pairwise similarity coefficients and the weighted similarity coefficients were converted to distance values, Es and Ew, respectively, where Es = 1 − Cs and Ew = 1 − Cw.
A least-squares method (14) was used to derive dendrograms expressing graphically the similarities of all T-RFLP profiles with all others in an edited data set from a matrix of Es or Ew values. These calculations were implemented in the ANGIS software package (26).
Shannon's diversity index, H, was calculated for each T-RFLP profile within each edited data set as follows:
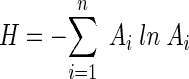 |
where Ai is the relative area of the ith T-RF with an area of greater than zero, and n is the total number of T-RFs with an area of greater than zero in that T-RFLP profile.
Simpson's diversity index, D, was calculated for each T-RFLP profile within each edited data set as follows:
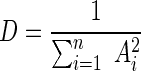 |
where Ai is the relative area of the ith T-RF with a value of greater than zero, and n is the total number of T-RFs with an area of greater than zero in that T-RFLP profile.
The significance of the differences between the mean values of H, D, Es, and Ew for different subsets within an edited data set was tested by using Student t test implemented in the Excel software package (Microsoft Corp., Redmond, Wash.). Regression coefficients were derived from linear fits to the data by using CricketGraph (Computer Associates International, Inc., Islandia, N.Y.). Principle component analysis was performed by using the NetMul multivariate analysis system (44).
RESULTS
Mouse phenotype.
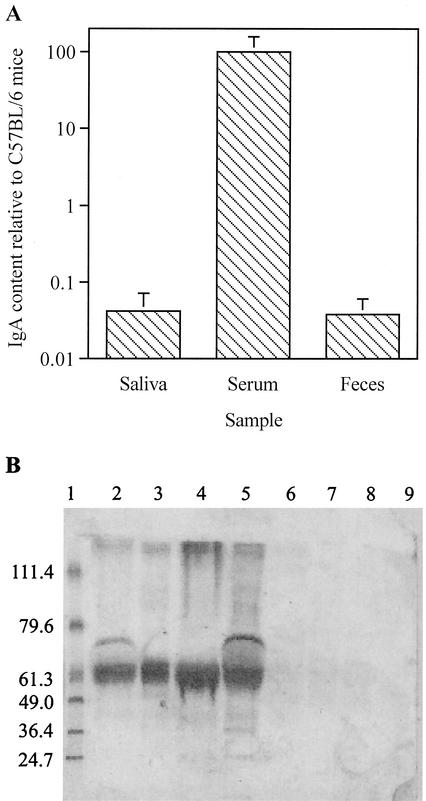
pIgR−/− mice were generated on a C57BL/6 background by using a neomycin-resistant targeting vector (19) that disrupted exon 3 of the locus encoding the pIgR (47). Secretory antibodies were absent from mucosal secretions of pIgR−/−, as reported previously (19). C57BL/6 mice had IgA levels in the saliva (6.2 ± 3.3 μg ml−1), serum (251 ± 106 μg ml−1), and feces (239 ± 62 μg g−1) that were comparable to those expected for normal mice (19, 42). pIgR−/− mice displayed much lower levels of IgA in saliva and feces, but much higher levels in serum (Fig. 1A). In addition, immunoglobulins could not be detected in the bile of pIgR−/− mice (Fig. 1B).
FIG. 1.
(A) Mean IgA contents in saliva and serum (in micrograms/milliliter) and in feces (in micrograms/g) of five (saliva) or six (feces and serum) pIgR−/− mice relative to the levels in the same number of C57BL/6 mice. Vertical bars indicate an interval of one standard deviation from the mean. (B) Western blot showing presence of immunoglobulin heavy chain in bile from C57BL/6 (lanes 2 to 5) and pIgR−/− (lanes 6 to 9) mice. BenchMark prestained molecular weight markers (Gibco-BRL/Life Technologies, Mount Waverley, Victoria, Australia) and their molecular masses (in kilodaltons) are shown on the left of the figure (lane 1).
T-RFLP reproducibility and analysis.
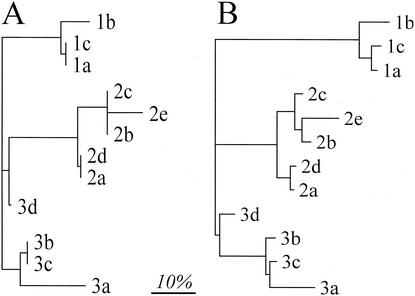
Samples of ileal community DNA from 3 mice (samples 1 and 2 = pIgR−/−; sample 3 = C57BL/6) were used as templates in PCR. The reproducibility of restriction enzyme digests was tested by performing three replicate digests on the PCR product amplified from DNA sample 1. The reproducibility of the PCR itself was examined by performing five replicate PCRs with DNA from each of samples 2 and 3 as a template and then digesting each of these independently. One T-RFLP profile generated from sample 3 was not included in the analysis because insufficient product DNA was present. A threshold was applied to remove all T-RFs that contributed <5% to the total area of all T-RFs (reasoning explained below). A distinct grouping of replicate samples was observed by using two methods of data analysis, Sorensen's similarity coefficient (Fig. 2A) and a weighted similarity coefficient (Fig. 2B). These results suggested that individual PCRs and restriction digests were reproducible and that a 5% threshold did not affect this reproducibility. Regardless of the analysis, the replicate digests on the same PCR product yielded less variability (mean Es = 0.05, range = 0.00 to 0.08; mean Ew = 0.09, range = 0.03 to 0.12) than the combination of independent PCR and digestion observed with sample 2 (mean Es = 0.07, range = 0.00 to 0.17; mean Ew = 0.11, range = 0.01 to 0.17) and sample 3 (mean Es = 0.13, range = 0.00 to 0.27; mean Ew = 0.15, range = 0.04 to 0.23).
FIG. 2.
Grouping of T-RFLP profiles generated from the ilea of mice, based on Sorensen's similarity coefficient (A) and a weighted similarity coefficient (B), both applied at a 5% threshold. The numbers 1 to 3 refer to different DNA templates, and the letters a to e refer to different replicates (see the text for details). The matrices of similarity coefficients were transformed to distances and the dendrograms constructed by using a least-squares algorithm (14). The scale bar represents a 10% difference.
Profiling of the GI microbiota in C57BL/6 and pIgR−/− mice.
We generated T-RFLP profiles of the 16S rRNA genes of the bacterial communities extracted from the terminal ilea of 16 C57BL/6 and 15 pIgR−/− mice. DNA extraction from the ilea of some mice was unsuccessful, and results from these analyses are therefore missing from the final data set. Common and unique T-RFs were present in the profiles. Common T-RFs were found equally in both C57BL/6 or pIgR−/− phenotypes, whereas unique T-RFs were not more prevalent in either the C57BL/6 or pIgR−/− mice (Table 1). The contribution of some T-RFs that were present in the majority of mice to the total amount of FAM-labeled digested product varied from <5 to 52%, but this variation was as great within animals of the same phenotype as it was between animals of different phenotypes.
TABLE 1.
Summary of unedited T-RFLP profiles from pIgR−/− and C57BL/6 micea
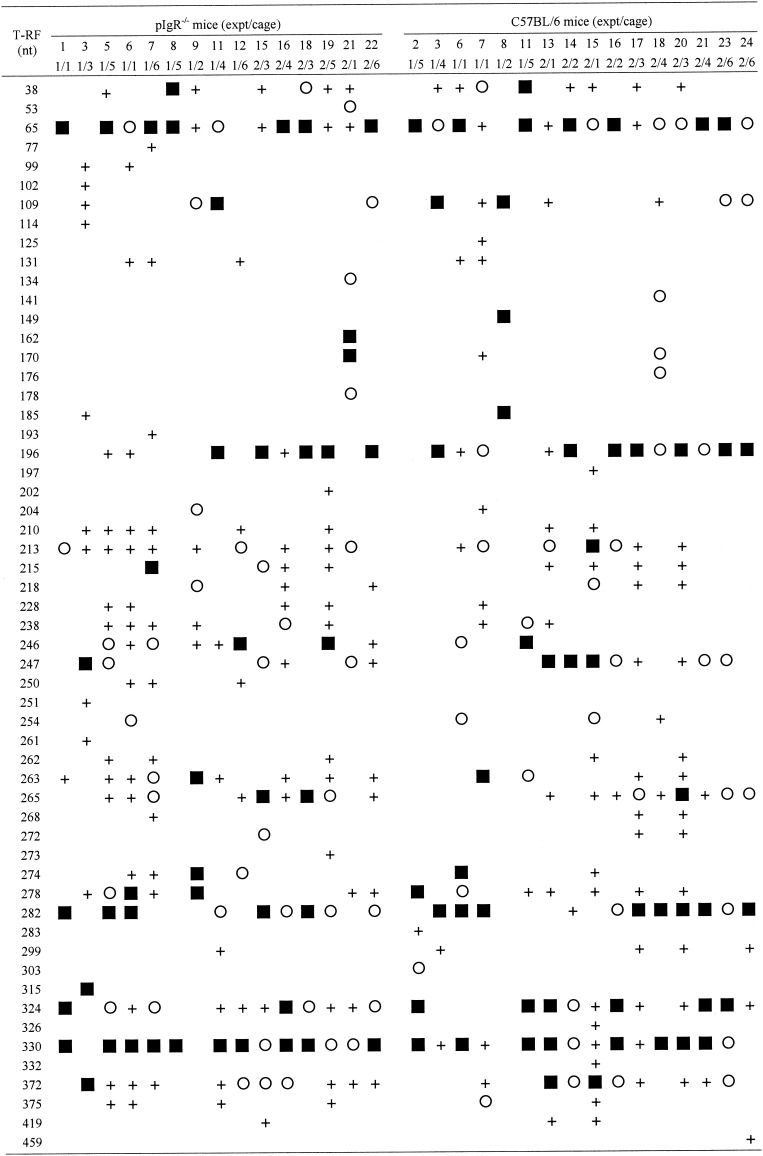
T-RFs constituting >10% (▪), 5 to 10% (○), or < 5% (+) of the total 6-FAM-labeled product were as indicated.
Standardization of T-RFLP profiles.
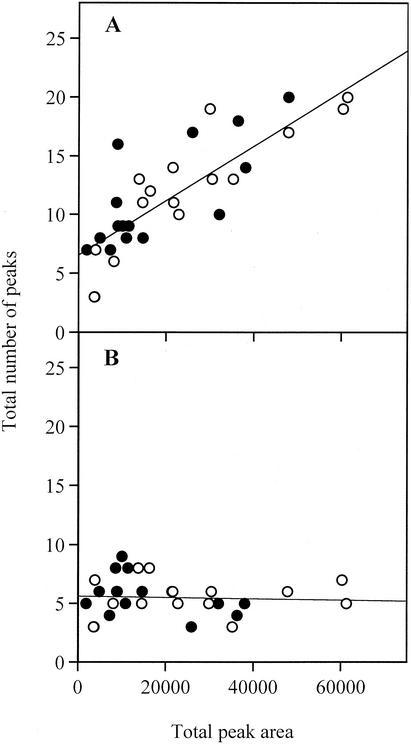
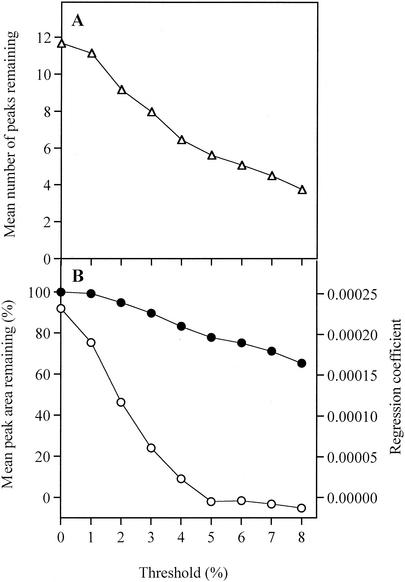
When the data set derived from the 31 T-RFLP profiles was examined, it was observed that the total number of T-RFs in each profile increased as the amount of labeled product in the profile increased (Fig. 3A). The relative proportion of the labeled digested product that each T-RF constituted, as part of a single profile from a single mouse ileum, was calculated by expressing the area under the peak corresponding to each T-RF as a percentage of the total labeled product on the profile, i.e., the sum of the areas for all of the T-RFs in a profile. We observed that the “new” T-RFs seen when large amounts of labeled product were analyzed almost universally constituted a small fraction of the total area of all T-RFs, as expected since the detection limit remained constant. To avoid the possibility that the amount of labeled product inadvertently biased the comparative analysis of the profiles, we examined the effect of applying a threshold peak area value to the data sets. All T-RFs that constituted less than a set percentage of the total area of a profile were removed prior to comparative analysis. At a threshold of 5%, the effect of area on the number of T-RFs per profile was minimal (Fig. 3B), while still leaving sufficient T-RFs to analyze the microbial community (Fig. 4A). After the application of a 5% threshold, the remaining T-RFs constituted on average 78% of the original labeled product in the profile. At this threshold, the regression coefficient between total labeled product in each profile and the number of T-RFs was approximately zero, indicating that the effect of the total amount of labeled product is much reduced compared to that observed when no threshold or smaller threshold values were applied (Fig. 4B).
FIG. 3.
Relationship between the amount of labeled product in a T-RFLP profile, expressed as total peak area (fluorescence units), and the number of peaks, if the entire data set of T-RFs is used (A) and if the T-RFs that constitute <5% of the total peak area are eliminated from the data set (B). Symbols: •, profiles from C57BL/6 mice; ○, profiles from pIgR−/− mice.
FIG. 4.
Effect of applying different thresholds on the T-RFLP data sets, calculated for 31 T-RFLP profiles. T-RFs that contributed less than the threshold to the total peak area were eliminated from the data set. (A) The elimination of smaller T-RFs by the application of the threshold results in a lower number of peaks remaining in the analysis. (B) This resulted in a decreasing correlation (measured as a regression coefficient) between the remaining total peak area and the remaining number of peaks (○) while not so dramatically affecting the remaining total peak area itself (•).
sIg does not model the GI community composition.
The GI microbiota of pIgR−/− mice was compared with that of C57BL/6 mice, and distance matrices were constructed by using the Sorensen's similarity coefficient and the weighted similarity coefficient. The composition of the bacterial microbiota of the terminal ileal bacterial communities of individual animals was observed to vary greatly from that of other individuals (Table 2). This variability was as great among animals of the same phenotype as it was among animals of different phenotypes (Table 2). Some mice had terminal ileal microbiota that generated T-RFLP profiles with no T-RFs constituting >5% of the total that were common to other profiles (Table 1), resulting in Es and Ew values of 1.00 at a 5% threshold (Table 2). The two different methods of analysis produced different distance values (Es and Ew) between any two samples. For example, by using the Sorensen's similarity coefficient, mice pIgR−/− [12] and C57BL/6 [21] were 69% different. When the same two samples were examined by using the weighted similarity coefficient, this difference rose to 86%. Examining the raw T-RFLP data, it can be seen that these two mice shared a common T-RF of 330 bp (Table 1), but the abundance of this T-RF varied, contributing 43% in pIgR−/− [12] and 6% in C57BL/6 [21] (data not shown). Therefore, the mice with large stoichiometric differences in the contribution that common T-RFs made to the total were found to be less alike by using the weighted similarity coefficient than with Sorensen's similarity coefficient.
TABLE 2.
Means and ranges of diversity analyses, at a 5% threshold, of T-RFLP profiles from ilea of mice by phenotype and experiment
| Mouse group or expt | n |
Es |
Ew |
Mean H | Mean D | ||
|---|---|---|---|---|---|---|---|
| Mean | Range | Mean | Range | ||||
| All mice | 31 | 0.63 | 0.09-1.00 | 0.72 | 0.22-1.00 | 1.52 | 4.15 |
| All pIgR−/− | 15 | 0.65 | 0.09-1.00 | 0.74 | 0.24-1.00 | 1.55 | 4.30 |
| All C57BL/6 | 16 | 0.62 | 0.14-1.00 | 0.71 | 0.22-1.00 | 1.49 | 4.02 |
| All expt 1 | 15 | 0.70 | 0.10-1.00 | 0.77 | 0.23-1.00 | 1.41 | 3.84 |
| All expt 2 | 16 | 0.50 | 0.10-1.00 | 0.62 | 0.23-1.00 | 1.63 | 4.45 |
| pIgR−/− expt 1 | 9 | 0.70 | 0.27-1.00 | 0.78 | 0.40-1.00 | 1.45 | 4.04 |
| pIgR−/− expt 2 | 6 | 0.57 | 0.23-0.87 | 0.65 | 0.29-0.93 | 1.71 | 4.68 |
| C57BL/6 expt 1 | 6 | 0.76 | 0.40-1.00 | 0.81 | 0.33-1.00 | 1.34 | 3.53 |
| C57BL/6 expt 2 | 10 | 0.48 | 0.14-1.00 | 0.62 | 0.24-1.00 | 1.58 | 4.31 |
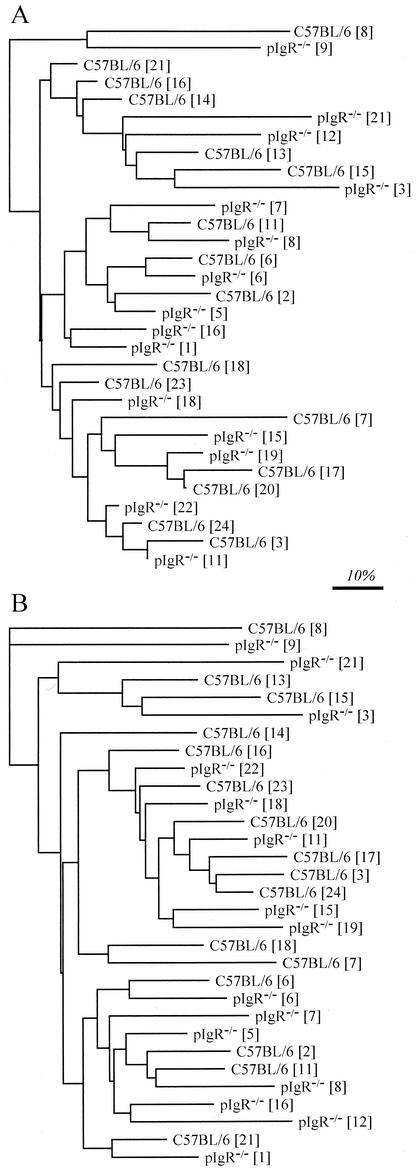
The distances between each sample were represented graphically by constructing a dendrogram based on the Sorensen's similarity coefficients of pairwise comparisons of T-RFLP profiles. No distinct pattern of clustering between the pIgR−/− and C57BL/6 mice was observed at either a 0% (not shown) or a 5% threshold (Fig. 5A). When multiple independently amplified and digested T-RFLP profiles generated from these DNA samples were included in the analyses, the replicates grouped most closely with each other (data not shown). There was no statistical support for segregation of T-RFLP profiles between C57BL/6 and pIgR−/− mice (Table 3).
FIG. 5.
Grouping of T-RFLP profiles generated from the ilea of mice, based on Sorensen's similarity coefficient (A) and a weighted similarity coefficient (B), both applied at a 5% threshold. The matrices of similarity coefficients were transformed to distances, and dendrograms were constructed by using a least-squares algorithm (14). The scale bar represents a 10% difference. The numbers in brackets refer to individual mice (see Table 1).
TABLE 3.
Comparison by Student t test of compositional and diversity analyses of T-RFLP profiles, at a 5% threshold, from the ilea of mice
| Mouse group comparison |
P |
|||
|---|---|---|---|---|
| Es | Ew | H | D | |
| All pIgR−/− vs all C57BL/6 | 0.237 | 0.211 | 0.567 | 0.491 |
| pIgR−/− expt 1 vs C57BL/6 expt 1 | 0.355 | 0.642 | 0.497 | 0.383 |
| pIgR−/− expt 2 vs C57BL/6 expt 2 | 0.198 | 0.720 | 0.335 | 0.505 |
| All expt 1 vs all expt 2 | <0.001 | <0.001 | 0.034 | 0.127 |
| pIgR−/− expt 1 vs pIgR−/− expt 2 | 0.069 | 0.066 | 0.048 | 0.242 |
| C57BL/6 expt 1 vs C57BL/6 expt 2 | <0.001 | 0.006 | 0.168 | 0.205 |
The T-RFLP profiles obtained from the different animals were also compared by using a weighted coefficient that took into account T-RF abundance (peak area). The pIgR−/− and C57BL/6 mice did not group separately, whether a 0% (not shown) or a 5% threshold was applied (Fig. 5B), and statistical analysis again indicated that there was no difference in the GI bacterial microbiota between the pIgR−/− and C57BL/6 phenotypes (Table 3). However, there did appear to be a strong grouping of mice based on experiment date, whether the threshold was 0% (not shown) or 5% (Table 3), indicating a general change in the composition of the GI microbiota in the small-animal facility between the two experiments, conducted 3 months apart.
Principle component analysis did not reveal any segregation of the profiles by mouse phenotype or by experiment date (data not shown).
Diversity analyses.
Shannon's (H) and Simpson's (D) diversity indices were calculated for each of the T-RFLP profiles at both 0% (not shown) and 5% peak thresholds (Table 3). No significant differences were apparent between the means of either of these diversity indices for pIgR−/− and C57BL/6 mice. When the mice from experiments 1 and 2 (conducted 3 months apart) were compared, no difference was apparent by using Simpson's index. Shannon's index indicated that the diversity of the microbiota in the ilea of mice in experiment 1, regardless of phenotype, was lower than in experiment 2 (Table 3).
DISCUSSION
sIg is produced constitutively in mice possessing a normal GI microbiota but does not appear to be absolutely necessary for protection against an array of mucosal pathogens (38; Uren and Strugnell, unpublished). sIg production is associated with energy expenditure and protein loss and so must fulfill some role, but exactly what this is remains an intriguing question. It is well established that there is an interaction between sIg and the commensal microbiota, but we demonstrate in the present study that secreted antibodies do not appear to have a role in controlling the composition of the bacterial community able to colonize the ileum.
Experimental design.
pIgR−/− mice were first reported several years ago (19, 42) and are deficient in sIg resulting from loss of pIgR function. These animals, with comparisons made to wild-type C57BL/6 mice, are ideal models for assessing the role of sIg independently of other genetic influences. The phenotypes of the pIgR−/− and C57BL/6 mice used in the present study were confirmed by ELISAs of IgA levels in saliva, serum, and feces and by Western blot analysis of total immunoglobulins in bile. Compared to C57BL/6 mice, pIgR−/− mice were deficient in sIg in the saliva, feces, and bile but had increased levels of IgA in serum, presumably as a result of accumulation due to the interrupted secretion pathway.
Offspring acquire maternal commensal microbiota during birth and before weaning (38). It is well established that the GI tract is then subject to successive microbial colonizations, before a stable GI microbiota is established (2, 40). Therefore, it is vital that the ages of the animals used in comparative microbiota studies are closely matched. The pIgR−/− mice used in the present study were mature (10 weeks old) and had been cohoused with age-matched C57BL/6 mice in equal numbers for 4 weeks prior to analysis of the GI microbiota. The commensal microbiota of the mice were analyzed at this age to ensure that a mature and stable microbiota was being studied. Cohousing of pIgR−/− and C57BL/6 mice for 4 weeks allowed the effect of sIg to be analyzed independently of any cage effects that might occur if mice with different genotypes were housed separately and for any effects due to phenotype on the commensal bacterial community of the ilea of pIgR−/− mice to become apparent. Four weeks should allow any invading organisms acquired from cage mates to become established as members of the epithelial microbiota. This is also sufficient time for specific antibodies to be generated after antigen challenge (13, 34), which should allow C57BL/6 mice, but not pIgR−/− mice, to clear any invading microbiota introduced after cohousing if this was a role of sIg. The animals used in the present study were housed conventionally, and so a certain degree of variation in the microbiota was both expected and observed. More contained housing conditions or introduction of a defined microbiota would have reduced this variation, but at the cost of limiting the potential for increased diversity of the GI microbiota in the absence of sIg. Under the experimental conditions used in the present study, if sIg plays a major role in shaping the commensal microbiota, a more heterogeneous microbial community would be observed in pIgR−/− mice, since transient organisms from the environment would be able to colonize at the ileal wall, whereas the presence of sIg in C57BL/6 mice would prevent this.
Choice of method.
The effect of sIg on the GI microbiota was measured by using T-RFLP, which is a useful tool for measuring complexity within and differences between microbial communities (11, 27, 33). The technique is based on sequence heterogeneity within the 16S rRNA gene, allowing the entire bacterial community to be surveyed in one assay. Amplification of 16S rRNA genes can be affected by PCR bias, and extrapolating back to the original community is made difficult by differential bacterial cell lysis and various rrn copy numbers. However, all PCR-based analytical techniques are hampered by these problems (51). Amplification of 16S rRNA genes with a 6-FAM-labeled forward oligonucleotide primer, followed by restriction enzyme digestion of the amplified products, yields 5′-6-FAM-labeled-T-RFs of different sizes from different 16S rRNA genes. Some species with different 16S rRNA genes may yield the same T-RFs but, overall, a fingerprint of the 16S rRNA gene population is produced. Resolution of the T-RFs on sequencing gels yields electropherograms in which each individual peak represents a different-sized T-RF (33). Using this technique, altered fecal profiles of mice fed a probiotic containing Lactobacillus rhamnosus DR20 have been observed, with the ingested strain detected as a unique T-RF (21). Changes have also been detected in complex microbial communities exposed to different environmental stimuli (8, 41).
Many factors have been reported to influence T-RFLP profiles (12, 36). The impact of one of the more significant of these, the effect of the amount of labeled, digested DNA analyzed in any one profile on the data obtained from that T-RFLP profile (12, 36), was minimized by the adoption of a 5% relative peak area threshold. By correcting T-RFLP profiles to focus the analysis on the important members of the microbial community and limit the influence of the amount of labeled product loaded on the gel, possible experimental bias introduced by minor “background” T-RFs is removed. After applying a 5% threshold, T-RFLP profiles generated from replicate PCRs and digests resulted in highly similar electropherograms, while nonreplicate samples still retain differences conferred by differences in the major populations. T-RFLP analysis of replicate T-RFLP profiles showed that, despite variation being apparent between replicate T-RFLP profiles, the mean distance variations (Es and Ew) between replicates were much smaller than the mean distance variations detected in T-RFLP profiles from different samples. Therefore, differences introduced by PCR and restriction endonuclease digestion were much smaller than the differences resulting from different ileal bacterial community compositions. Whereas it is impossible to remove all biases in the PCR, or in the DNA extraction, we are confident that since each sample was analyzed consistently any biases affecting samples from C57BL/6 mice should be seen also in samples from pIgR−/− mice.
Effect of sIg.
Analysis of T-RFLP profiles by both the weighted and Sorensen's similarity coefficients showed that the ileal bacterial communities of any one mouse phenotype were not more similar to each other than they were to those from mice of the other phenotype. This is illustrated in both the overlapping distribution of the T-RFLP profiles from the ilea of animals of both phenotypes in the dendrograms generated from matrices of the Sorensen's and weighted distances and the lack of statistical support for any groupings by pIgR−/− and C57BL/6 mice. In addition, the richness of the bacterial communities in C57BL/6 and pIgR−/− mice compared by using Simpson's and Shannon's diversity indices were not significantly different. The methods used to analyze the T-RFLP profiles were sensitive enough to detect any change in the GI microbiota, since we could observe a clear shift in the ileal bacterial community composition between the two independent replicate experiments, carried out 3 months apart, by the Sorensen's index, the weighted index, and Shannon's diversity index.
The majority of dominant T-RFs (>10% of total area) were observed in multiple individuals of both phenotypes, indicating that although the entire GI microbiota is variable between animals, there are common dominant components that vary only in abundance between animals. T-RFs that were not common to both phenotypes were generally present in very few animals and generally constituted smaller parts of the total bacterial community. Clone library and T-RFLP analyses of the bacterial microbiota from the ileum (M. Galic, L. Sait, R. A. Strugnell, and P. H. Janssen., unpublished data) has revealed that the major T-RFs observed in the T-RFLP profiles are consistent with those expected from typical ileal microorganisms. T-RFs of 65, 196, 213, 247, 265, 282, 324, 330, and 372 nucleotides have been identified as originating from 16S rRNA genes of Lactobacillus spp., relatives of “Eubacterium” spp., and Bacteroides spp. These data will be reported as part of another study.
Role of sIg.
Despite the extensive research into the mucosal immune system, the exact function of sIg is still unclear. The interaction of the GI microbiota with sIg and the GI epithelium has been demonstrated (18, 23, 24, 28, 29, 38, 45, 46, 48, 49). Jiang et al. (18) have shown in a monoassociation model that sIg may have a role in controlling the persistence of SFB in mice. We were neither able to confirm nor contradict this result in the presence of a normal microbiota, as SFB were undetectable in mice in our animal facility by microscopy or SFB-specific PCR (unpublished data). We have shown that sIg does not influence the composition (the present study) or size (unpublished data) of the normal epithelial community of the ileum of adult mice. However, enteric viruses and members of the domain Archaea, known to inhabit the GI tract, were not examined in the present study. The interaction between the epithelium, the mucosal immune system, and microbial cells in the GI tract is a complex triptych, requiring further systematic analysis. Our data show that one of the potential interactions, a selection of the commensal microbiota on the epithelial surfaces of the terminal ileum by sIg, may not be significant.
Acknowledgments
This work was supported by a University of Melbourne research development grant.
We thank Kelly Ewen-White, Wayne Ward, Daniel Lucas, and Melinda Ziino at the Melbourne Genotyping Division of the Australian Genome Research Facility for advice and assistance in processing T-RFLP samples.
REFERENCES
- 1.Abreu-Martin, M. T., and S. R. Targan. 1996. Regulation of immune responses of the intestinal mucosa. Crit. Rev. Immunol. 16:277-309. [DOI] [PubMed] [Google Scholar]
- 2.Berg, R. D. 1996. The indigenous gastrointestinal microflora. Trends Microbiol. 4:430-435. [DOI] [PubMed] [Google Scholar]
- 3.Boes, M., A. P. Prodeus, T. Schmidt, M. C. Carroll, and J. Chen. 1998. A critical role of natural immunoglobulin M in immediate defense against systemic bacterial infection. J. Exp. Med. 188:2381-2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bos, N. A., J. C. A. M. Bun, S. H. Popma, E. R. Cebra, G. J. Deenen, M. J. F. van der Cammen, F. G. M. Kroese, and J. J. Cebra. 1996. Monoclonal immunoglobulin A derived from peritoneal B cells is encoded by both germ line and somatically mutated VH genes and is reactive with commensal bacteria. Infect. Immun. 64:616-623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bromander, A. K., L. Ekman, M. Kopf, J. G. Nedrud, and N. Y. Lycke. 1996. IL-6-deficient mice exhibit normal mucosal IgA responses to local immunizations and Helicobacter felis infection. J. Immunol. 156:4290-4297. [PubMed] [Google Scholar]
- 6.Brown, J. F., and E. Balish. 1978. Gastrointestinal microecology of BALB/c nude mice. Appl. Environ. Microbiol. 36:144-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chase, D. G., and S. L. Erlandsen. 1976. Evidence for a complex life cycle and endospore formation in the attached, filamentous, segmented bacterium from murine ileum. J. Bacteriol. 127:572-583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clement, B. G., L. E. Kehl, K. L. DeBord, and C. L. Kitts. 1998. Terminal restriction fragment patterns (TRFPs), a rapid, PCR-based method for the comparison of complex bacterial communities. J. Microbiol. Methods 31:135-142. [Google Scholar]
- 9.Collins, F. M., and P. B. Carter. 1978. Growth of salmonellae in orally infected germfree mice. Infect. Immun. 21:41-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Draser, B. S., and M. J. Hill. 1974. Human intestinal flora. Academic Press, Inc., London, England.
- 11.Dunbar, J., L. O. Ticknor, and C. R. Kuske. 2000. Assessment of microbial diversity in four southwestern United States soils by 16S rRNA gene terminal restriction fragment analysis. Appl. Environ. Microbiol. 66:2943-2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dunbar, J., L. O. Ticknor, and C. R. Kuske. 2001. Phylogenetic specificity and reproducibility and new method for analysis of terminal restriction fragment profiles of 16S rRNA genes from bacterial communities. Appl. Environ. Microbiol. 67:190-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dunstan, S. J., C. P. Simmons, and R. A. Strugnell. 1998. Comparison of the abilities of different attenuated Salmonella typhimurium strains to elicit humoral immune response against a heterologous antigen. Infect. Immun. 66:732-740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fitch, W. M., and E. Margoliash. 1967. Construction of phylogenetic trees. Science 155:279-284. [DOI] [PubMed] [Google Scholar]
- 15.Fubara, E. S., and R. Freter. 1973. Protection against enteric bacterial infection by secretory IgA antibodies. J. Immunol. 111:395-403. [PubMed] [Google Scholar]
- 16.Heczko, U., A. Abe, and B. B. Finlay. 2000. Segmented filamentous bacteria prevent colonization of enteropathogenic Escherichia coli O103 in rabbits. J. Infect. Dis. 181:1027-1033. [DOI] [PubMed] [Google Scholar]
- 17.Hudault, S., J. Guignot, and A. L. Servin. 2001. Escherichia coli strains colonising the gastrointestinal tract protect germfree mice against Salmonella typhimurium infection. Gut 49:47-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang, H. Q., N. A. Bos, and J. J. Cebra. 2001. Timing, localization, and persistence of colonization by segmented filamentous bacteria in the neonatal mouse gut depend on immune status of mothers and pups. Infect. Immun. 69:3611-3617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johansen, F.-E., M. Pekna, I. N. Norderhaug, B. Haneberg, M. A. Hietala, P. Krajci, C. Betsholtz, and P. Brandtzaeg. 1999. Absence of epithelial immunoglobulin A transport, with increased mucosal leakiness, in polymeric immunoglobulin receptor/secretory component-deficient mice. J. Exp. Med. 190:915-922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaetzel, C. S. 2001. Polymeric Ig receptor: defender of the fort or Trojan horse? Curr. Biol. 11:R35-R38. [DOI] [PubMed] [Google Scholar]
- 21.Kaplan, C. W., J. C. Astaire, M. E. Sanders, B. S. Reddy, and C. L. Kitts. 2001. 16S ribosomal DNA terminal restriction fragment pattern analysis of bacterial communities in feces of rats fed Lactobacillus acidophilus NCFM. Appl. Environ. Microbiol. 67:1935-1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kerr, M. A. 1990. The structure and function of human IgA. Biochem. J. 271:285-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klaasen, H. L., P. J. van der Heijden, W. Stok, F. G. Poelma, J. P. Koopman, M. E. van den Brink, M. H. Bakker, W. M. Eling, and A. C. Beynen. 1993. Apathogenic, intestinal, segmented, filamentous bacteria stimulate the mucosal immune system of mice. Infect. Immun. 61:303-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kroese, F. G. M., R. de Waard, and N. A. Bos. 1996. B-1 cells and their reactivity with the murine intestinal microflora. Semin. Immunol. 8:11-18. [DOI] [PubMed] [Google Scholar]
- 25.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 26.Littlejohn, T. G., C. A. Bucholtz, R. M. M. Campbell, B. A. Gaëta, C. Huynh, and S. H. Kim. 1996. Computing for biotechnology: WebANGIS. Australas. Biotechnol. 6:211-217. [Google Scholar]
- 27.Liu, W. T., T. L. Marsh, H. Cheng, and L. J. Forney. 1997. Characterization of microbial diversity by determining terminal restriction fragment length polymorphisms of genes encoding 16S rRNA. Appl. Environ. Microbiol. 63:4516-4522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Macpherson, A. J., D. Gatto, E. Sainsbury, G. R. Harriman, H. Hengartner, and R. M. Zinkernagel. 2000. A primitive T cell-independent mechanism of intestinal mucosal IgA responses to commensal bacteria. Science 288:2222-2226. [DOI] [PubMed] [Google Scholar]
- 29.Macpherson, A. J., U. Y. Khoo, I. Forgacs, J. Philpott-Howard, and I. Bjarnason. 1996. Mucosal antibodies in inflammatory bowel disease are directed against intestinal bacteria. Gut 38:365-375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Macpherson, A. J., A. Lamarre, K. McCoy, G. R. Harriman, B. Odermatt, G. Dougan, H. Hengartner, and R. M. Zinkernagel. 2001. IgA production without mu or delta chain expression in developing B cells. Nature Immunol. 2:288-290. [DOI] [PubMed] [Google Scholar]
- 31.Marcotte, H., and M. C. Lavoie. 1996. No apparent influence of immunoglobulins on indigenous oral and intestinal microbiota of mice. Infect. Immun. 64:4694-4699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marcotte, H., and M. C. Lavoie. 1998. Oral microbial ecology and the role of salivary immunoglobulin A. Microbiol. Mol. Biol. Rev. 62:71-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marsh, T. L. 1999. Terminal restriction fragment length polymorphism (T-RFLP): an emerging method for characterizing diversity among homologous populations of amplification products. Curr. Opin. Microbiol. 2:323-327. [DOI] [PubMed] [Google Scholar]
- 34.Mohammadi, M., S. Czinn, R. Redline, and J. Nedrud. 1996. Helicobacter-specific cell-mediated immune responses display predominant Th1 phenotype and promote a delayed-type hypersensitivity response in the stomachs of mice. J. Immunol. 156:4729-4738. [PubMed] [Google Scholar]
- 35.O'Neal, C. M., G. R. Harriman, and M. E. Conner. 2000. Protection of the villus epithelial cells of the small intestine from rotavirus infection does not require immunoglobulin A. J. Virol. 74:4102-4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Osborn, A. M., E. R. Moore, and K. N. Timmis. 2000. An evaluation of terminal-restriction fragment length polymorphism (T-RFLP) analysis for the study of microbial community structure and dynamics. Environ. Microbiol. 2:39-50. [DOI] [PubMed] [Google Scholar]
- 37.Robinson, J. K., T. G. Blanchard, A. D. Levine, S. N. Emancipator, and M. E. Lamm. 2001. A mucosal IgA-mediated excretory immune system in vivo. J. Immunol. 166:3688-3692. [DOI] [PubMed] [Google Scholar]
- 38.Savage, D. C. 1977. Interactions between the host and its microbes, p. 277-310. In R. T. J. Clarke, and T. Bauchop (ed.), Microbial ecology of the gut. Academic Press, Inc., London, England.
- 39.Savage, D. C., R. Dubos, and R. W. Schaedler. 1968. The gastrointestinal epithelium and its autochthonous bacterial flora. J. Exp. Med. 127:67-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schaedler, R. W., R. Dubos, and R. Costello. 1965. The development of the bacterial flora in the gastrointestinal tract of mice. J. Exp. Med. 122:59-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sessitsch, A., A. Weilharter, M. H. Gerzabek, H. Kirchmann, and E. Kandeler. 2001. Microbial population structures in soil particle size fractions of a long-term fertilizer field experiment. Appl. Environ. Microbiol. 67:4215-4224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shimada, S., M. Kawaguchi-Miyashita, A. Kushiro, T. Sato, M. Nanno, T. Sako, Y. Matsuoka, K. Sudo, Y. Tagawa, Y. Iwakura, and M. Ohwaki. 1999. Generation of polymeric immunoglobulin receptor-deficient mouse with marked reduction of secretory IgA. J. Immunol. 163:5367-5373. [PubMed] [Google Scholar]
- 43.Snel, J., C. C. Hermsen, H. J. Smits, N. A. Bos, W. M. C. Eling, J. J. Cebra, and P. J. Heidt. 1998. Interaction between gut-associated lymphoid tissue and colonization levels of indigenous, segmented filamentous bacteria in the small intestine of mice. Can. J. Microbiol. 44:1177-1182. [DOI] [PubMed] [Google Scholar]
- 44.Thioulouse, J., and F. Chevenet. 1996. NetMul, a world-wide web user interface for multivariate analysis software. Comput. Stat. Data Anal. 21:369-372. [Google Scholar]
- 45.Umesaki, Y., and H. Setoyama. 2000. Structure of the intestinal flora responsible for development of the gut immune system in a rodent model. Microb. Infect. 2:1343-1351. [DOI] [PubMed] [Google Scholar]
- 46.Umesaki, Y., H. Setoyama, S. Matsumoto, A. Imaoka, and K. Itoh. 1999. Differential roles of segmented filamentous bacteria and clostridia in development of the intestinal immune system. Infect. Immun. 67:3504-3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Uren, T. K., F.-E. Johansen, O. L. C. Wijburg, F. Koentgen, P. Brandtzeg, and R. A. Strugnell. 2003.. Role of polymeric Ig receptor in mucosal B cell homeostasis. J. Immunol. 170:2531-2539. [DOI] [PubMed]
- 48.van der Waaij, D., J. M. Berghuis-de Vries, and J. E. C. Lekkerkerk-van der Wees. 1971. Colonization resistance of the digestive tract in conventional and antibiotic-treated mice. J. Hyg. 69:405-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van der Waaij, L. A., P. C. Limburg, G. Mesander, and D. van der Waaij. 1996. In vivo IgA coating of anaerobic bacteria in human faeces. Gut 38:348-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van Egmond, M., C. A. Damen, A. B. van Spriel, G. Vidarsson, E. van Garderen, and J. G. J. van de Winkel. 2001. IgA and the IgA Fc receptor. Trends Immunol. 22:205-211. [DOI] [PubMed] [Google Scholar]
- 51.von Wintzingerode, F., U. B. Göbel, and E. Stackebrandt. 1997. Determination of microbial diversity in environmental samples: pitfalls of PCR-based rRNA analysis. FEMS Microbiol. Rev. 21:213-229. [DOI] [PubMed] [Google Scholar]