Abstract
Xenorhabdus nematophilus secretes a large number of proteins into the culture supernatant as soluble proteins and also as large molecular complexes associated with the outer membrane. Transmission electron micrographs of X. nematophilus cells showed that there was blebbing of the outer membrane from the surface of the bacterium. The naturally secreted outer membrane vesicles (OMVs) were purified from the culture supernatant of X. nematophilus and analyzed. Electron microscopy revealed a vesicular organization of the large molecular complexes, whose diameters varied from 20 to 100 nm. A sodium dodecyl sulfate-polyacrylamide gel electrophoresis profile of the vesicles showed that in addition to outer membrane proteins, several other polypeptides were also present. The membrane vesicles contained lipopolysaccharide, which appeared to be of the smooth type. Live cells of X. nematophilus and the OMV proteins derived from them exhibited oral insecticidal activity against neonatal larvae of Helicoverpa armigera. The proteins present in the OMVs are apparently responsible for the biological activity of the OMVs. The soluble proteins left after removal of the OMVs and the outer membrane proteins also showed low levels of oral toxicity to H. armigera neonatal larvae. The OMV protein preparations were cytotoxic to Sf-21 cells in an in vitro assay. The OMV proteins showed chitinase activity. This is the first report showing toxicity of outer membrane blebs secreted by the insect pathogen X. nematophilus into the extracellular medium.
Xenorhabdus nematophilus is a gram-negative bacterium belonging to family Enterobacteriaceae (7, 12). The bacteria reside as endosymbionts in the foreguts of soil nematodes belonging to the genus Steinernema (1). Bacteria are released from the gut upon invasion of the insect hemocoel by the nematode. Bacterial multiplication and secretion of toxic proteins are the primary causes of death of the insect host. The bacteria alone are known to be sufficient to cause larval mortality following injection into the hemocoel (4, 13) or following oral administration when they are mixed in the diet (27).
Xenorhabdus and Photorhabdus are two closely related genera of bacteria associated with soil nematodes. Members of both of these genera produce insecticidal proteins that are toxic to a wide variety of lepidopteran insects. The genes encoding larvicidal proteins in members of both of these bacterial genera have been identified and have been found to exhibit significant degrees of homology (10, 21). Large protein complexes containing several polypeptide species with larvicidal activity have been isolated from the culture supernatant of Photorhabdus luminescens (9, 15). However, there is no information on the activity profiles of the secreted proteins in Xenorhabdus culture media.
All gram-negative bacteria are known to produce spherical two-layer outer membrane (OM) blebs in culture media (5). The general importance of release of these blebs has only recently been recognized. OM vesicles (OMV) have been found emanating from bacteria growing under very different conditions, including in biofilms (6), on solid media or in liquid media (17), and in natural environments (6). Encapsulation of toxic proteins in the membrane vesicles protects the proteins from the degradative enzymes of the host (18) and also helps deliver the enclosed substances by facilitating fusion with lipid-rich host cell membranes (17). Several gram-negative pathogens have been shown to excrete their virulence factors enclosed in OMVs (5). Pseudomonas aeruginosa, Proteus mirabilis, and Serratia marcescens package phospholipase C, proteases, proelastases, and hemolysins in OMVs (5). P. aeruginosa is known to exhibit predatory activity towards other bacteria by secreting OMVs which fuse with the bacterial OM and subsequently release degradative enzymes (20).
The mutualistic mode of living of the entomopathogenic bacterium X. nematophilus in the nematode gut turns into a pathogenic mode when the bacterium enters the larval hemocoel. X. nematophilus is known to secrete highly potent protein toxins into the hemocoel, which rapidly kill the larval host. The insect carcass provides a rich nutrient source on which both the bacteria and the nematode feed, grow, and replicate. The OM of gram-negative bacteria performs many specialized functions under adverse environmental conditions (5), and protein secretion is one such mechanism used by bacterial pathogens when they are inside their hosts (23). Likewise, X. nematophilus also secretes many effector proteins to inactivate the host immune defense and establish itself in the host (12).
In this study we demonstrated the oral larvicidal activity of proteins secreted in association with OMVs into the extracellular medium by X. nematophilus 19061. We found that when the OMVs were incorporated into the diet, they were active against the common lepidopteran pest Helicoverpa armigera.
MATERIALS AND METHODS
Bacteria and growth conditions.
X. nematophilus strain 19061 was obtained from the American Type Culture Collection (Rockville Md.), and the culture was stored as stock preparations in Luria broth (LB) containing glycerol (1:1) at −80°C. The bacteria were plated on nutrient agar supplemented with 0.004% (wt/vol) triphenyl tetrazolium chloride and 0.025% (wt/vol) bromothymol blue (7). Broth cultures were grown from a single blue colony obtained from the plate described above in LB at 29°C with shaking. Escherichia coli K-12 was used as a reference strain.
Isolation of OMVs and soluble proteins from the culture medium.
The OMVs naturally secreted into the medium were collected from 20- to 24-h-old culture supernatant as described by Kadurugamuva and Beveridge (17). Briefly, the cells were pelleted by centrifugation at 10,000 × g for 20 min, and the supernatant was filtered through a 0.45-μm-pore-size filter to remove the remaining bacterial cells. The OMVs were obtained by centrifugation at 150,000 × g for 2 h in a Ti 45 rotor with a Beckman ultracentrifuge, washed and resuspended in 50 mM Tris-HCl buffer (pH 7.5), and stored at −20°C. The supernatant obtained after the high-speed centrifugation was concentrated with a Centricon 3000 and used as a source of extracellular soluble proteins.
Preparation of OMs from X. nematophilus cells.
OMs were prepared as described by Leisman et al. (19). A bacterial pellet was washed once in LB and sonicated in 20 mM sodium phosphate buffer (pH 7.0) on ice by using 10 1-min pulses at 140 W. The cell lysate was centrifuged at 10,000 × g for 15 min, and the clear supernatant was subjected to ultracentrifugation at 150,000 × g for 15 min in a Ti 50 rotor. The crude membrane pellet was suspended in 0.5% Sarkosyl (N-lauroyl sarcosine; Sigma) in 20 mM sodium phosphate buffer for 1 h to solubilize the cytoplasmic membrane components. The suspension was centrifuged at 300,000 × g for 15 min. The pellet was washed once in sodium phosphate buffer and resuspended in the same buffer.
Isolation of LPS from Xenorhabdus cells.
Lipopolysaccharide (LPS) was isolated from the Xenorhabdus cells by phenol-water extraction as described by Apicella et al. (2). To determine the LPS profile of the OMVs, a known amount of protein was denatured by boiling with sodium dodecyl sulfate (SDS)-containing sample buffer for 5 min, followed by treatment with proteinase K for 1 h at 56°C. The protease-treated samples were electrophoresed on SDS-polyacrylamide gel electrophoresis (PAGE) gels and visualized by silver staining.
Protease and chitinase activities.
Protease activity was determined by using Hide Powder Azure (Sigma) as described by Schmidt et al. (26). Chitinase activity was determined as described by Hollis et al. (16) by using 4-methylumbelliferyl-β-N,N′,N"-triacetylchitotrioside as the substrate. Release of free methyl umbelliferone was measured with a fluorescence spectrophotometer by using excitation at 360 nm and emission at 450 nm.
Electron microscopy.
Xenorhabdus cells that were grown overnight were washed once and fixed in 0.1% glutaraldehyde-2% formaldehyde in phosphate-buffered saline (PBS) for 20 min at room temperature. The cells were collected by centrifugation at 12,000 × g for 5 min at 4°C, washed twice in PBS, sequentially dehydrated in N,N-dimethylformamide, and embedded in CY 212 araldite blocks. Thin sections were cut with an ultramicrotome, and the sections were stained with alcoholic uranyl acetate and lead acetate (10 min each). The grids were examined with an electron microscope. The OMVs collected from the culture supernatant were visualized by negative staining with a 2% uranyl acetate solution on a Formvar-coated copper grid, rinsed, and examined with a Philips CM10 transmission electron microscope under standard conditions at 60 to 80 kV.
Insect bioassay.
X. nematophilus was grown in LB overnight, washed once with sterile PBS, diluted in the same buffer, and added to the diet. The test protein preparations were diluted in 20 to 100 μl of water and mixed with the artificial diet. Each group contained 10 to 20 neonatal larvae placed on the surface of the diet, and the plate was sealed and placed in a humidified growth chamber at 28°C. Mortality was scored for 1 to 4 days. Each dose was used in triplicate to establish the toxin potency. The experiments were repeated three times. The 50% lethal dose (LD50) was defined as the protein concentration that resulted in 50% mortality, as determined by Probit analysis (11).
Cytotoxicity assay with cultured Sf-21 cells.
The cytotoxicity assay used was based on the procedure described by Armstrong et al. (3). The insect cells (Sf-21 cell line) were cultured at pH 6.2 in Grace's insect medium (Invitrogen) supplemented with yeast extract and lactalbumin and containing 10% fetal bovine serum and 10 μg of gentamicin per ml. Each experiment was performed in a 48-well tissue culture plate. Approximately 107 cells in serum-free medium were added to each well and allowed to attach for 1 to 2 h at 28°C. The medium was removed, different concentrations of sterile OMV preparations in 0.25 ml of serum-free medium were added to the wells, and the plate was incubated at 28°C. Heat-inactivated OMVs from X. nematophilus, OMVs from E. coli K-12, and serum-free medium were used as controls. Each plate was monitored at regular intervals, and the experiment was terminated after 6 to 7 h. The supernatant was removed, and 200 μl of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide dissolved in serum-free medium was added to each well to a final concentration of 0.5 mg ml−1. The plate was incubated at 28°C for 2 h. The supernatant was decanted, and the plate was air dried. The resulting precipitate was solubilized in a solution of 25 mM HCl in 90% isopropyl alcohol containing 0.5% SDS, and the optical density at 620 nm (OD620) was measured. The percentage of viability was calculated as follows: (OD620 of treated cells/OD620 of control cells) × 100. The assay was performed three times in triplicate, and the results shown below are representative of one experiment.
RESULTS AND DISCUSSION
The cell walls of gram-negative bacteria are dynamic in that OMVs are constantly sloughed off from the surfaces of the cells during growth. During this discharge process a number of periplasmic components are entrapped and are exported out of the cell enclosed in the OM covering. Several pathogenic bacterial species have been shown to release OMVs containing toxic proteins (5) and infectious DNA (18, 29) into the medium during in vitro growth. Greiner and Maryland (14) demonstrated that Bacteroides gingivalis vesicles adhered to the epithelial cells of a host during toxicity tests under in vitro conditions.
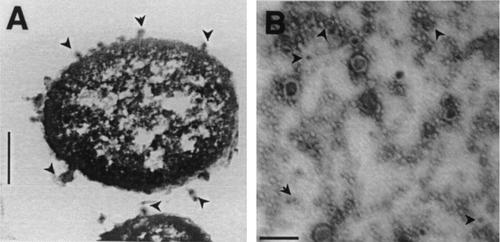
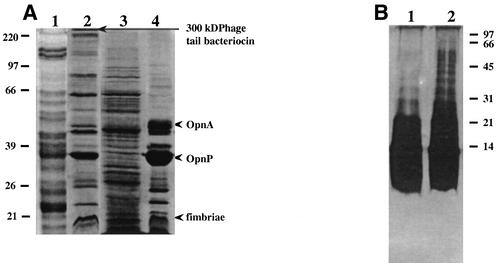
Like other gram-negative bacteria, X. nematophilus also secreted OM blebs into the growth medium. Electron micrographs of Xenorhabdus cells clearly showed that there was blebbing of the OM at several places (Fig. 1A). The diameters of the membrane vesicles varied from 20 to 100 nm (Fig. 1B), although the possibility that there were some modifications in size during vesicle isolation cannot be ruled out. Many of vesicles contained electron-dense contents, which stained dark. The OMVs contained a number of proteins in addition to the OM proteins, as revealed by a comparison of the SDS-PAGE profiles of the OM of X. nematophilus and proteins isolated from the medium (Fig. 2A). The porins OpnP (as determined by the N-terminal sequence) and OpnA (52 kDa; with the N-terminus sequence AEIFNKDGNKLDRYG), as described by Leisman et al. (19), were the predominant proteins in the OMVs, as well as in OM preparations (Fig. 2A, lanes 2 and 4). Another major OM protein identified in the OMV preparation was a 17-kDa protein whose N-terminal sequence (APTQGDGTVK) was very similar to the N-terminal sequence of the P pilin protein of a uropathogenic strain of E. coli (25) (Fig. 2A). Moureaux et al. (22) described a fimbrial protein having a similar molecular weight in X. nematophilus. The N-terminal sequence of a high-molecular-mass band at about 300 kDa (ALPRKLKYLN) exhibited 90% homology with the sequences of phage tail-like proteins. Xenorhabdus species have been reported to produce phage tail-like bacteriocins in the culture medium in a highly oligomeric form (8, 28). The OMVs produced additional protein bands at 22 to 27 and 60 to 75 kDa and several bands at molecular masses greater than 100 kDa, while the OM preparation contained almost no proteins having molecular masses greater than 100 kDa. The soluble protein fraction obtained after separation of the OMVs from the culture supernatant contained a large number of polypeptide species, as revealed by SDS-PAGE (Fig. 2A, lane 3), and many of them appeared to have electrophoretic mobilities similar to those of the OMV-associated proteins. The presence of LPS in the OMV preparation confirmed its OM origin (Fig. 2B, lanes 1 and 2). The LPS of Xenorhabdus was visualized by silver staining of the SDS-PAGE gels (Fig. 2B). The presence of repeating saccharide units forming O side chains of different lengths produced the typical ladder-like structure of the smooth phenotype.
FIG. 1.
(A) Thin section of an X. nematophilus 19061 cell, showing OM blebs on the surface of the cell and also in the surroundings. (B) Negatively stained OMV preparation isolated from the culture supernatant of X. nematophilus 19061 cells, showing electron-dense substances enclosed in vesicles.
FIG. 2.
(A) SDS-PAGE profiles of different X. nematophilus 19061 fractions. Lane 1, OMVs obtained from the medium of a Photorhabdus strain; lane 2, OMVs from X. nematophilus culture supernatant; lane 3, soluble proteins remaining after removal of OMVs from the culture supernatant of X. nematophilus; lane 4, OM proteins from X. nematophilus. (B) LPS profile of the OMVs obtained from the X. nematophilus culture supernatant. Lanes 1 and 2 contained the LPS present in 5 and 10 μg of OMV protein.
When X. nematophilus 19061 was grown in LB to saturation and tested for larvicidal activity, it exhibited oral toxicity against H. armigera neonatal larvae. A dose-dependent toxic effect was observed. Incorporation of 102 cells/g of diet resulted in the death of 10% ± 2% (mean ± standard deviation) of the larvae of H. armigera after 48 h of exposure, and the value increased to 82% ± 2% at a concentration of 106 cells/g. The LD50 was calculated to be 4 × 104 cells for H. armigera larvae (Table 1). At cell densities greater than the LD50 the larvae that remained alive were small and moribund. These results indicated that Xenorhabdus cells were insecticidal and that growth in the nematode host was not necessary to produce the toxin proteins.
TABLE 1.
Toxicity of X. nematophilus 19061 cells for neonatal larvae of H. armigera
| No. of live bacterial cells per g of diet | % Larval deatha |
|---|---|
| 0 | 6 ± 3b |
| 102 | 10 ± 2 |
| 103 | 18 ± 3 |
| 104 | 33 ± 2 |
| 105 | 56 ± 4 |
| 106 | 82 ± 2 |
Each group contained 20 neonatal larvae, and mortality was determined after 48 h.
Mean ± standard deviation.
The OMVs also showed larvicidal activity when they were incorporated into the diet of neonatal larvae of H. armigera (Table 2). At a concentration of 10 μg of OMV protein/g of diet, 12% ± 5% larval death was recorded, and the death rate increased to 40% ± 0% when the concentration of the proteins was increased to 50 μg/g. The maximum mortality (98% ± 2%) was observed when the protein concentration was 100 μg/g, and this occurred within 48 h. The LD50 was calculated to be 37 μg/g of diet. Protein levels greater than 25 μg/g were feeding deterrents, and the larvae that did not die within 48 h remained thin and small. The major part of the insecticidal activity of the excreted proteins was present in the particulate fraction obtained after high-speed centrifugation. The soluble proteins remaining in the supernatant were also tested with the bioassay. The proteins in the latter fraction were found to be less active, causing 10% ± 7% and 30% ± 11% mortality when the protein concentrations were 25 and 100 μg/g of diet, respectively (Table 2). The identity of the insecticidal protein(s) in the soluble fraction is not known; there could be a distinct set of proteins, or proteins could be produced by disintegration of OMVs. Purified LPS, the endotoxin of gram-negative bacteria, which is typically active at very low concentrations, showed no oral toxicity to the insect larvae even at levels of 10 to 20 μg/g (Table 2). The OM preparations also showed low-level oral toxicity to the Helicoverpa larvae, killing 20% ± 4% of the larvae at a concentration of 50 μg/g. Heating OMVs at 80°C for 15 min completely inactivated the insecticidal activity (Table 2). Furthermore, OMVs isolated from E. coli culture supernatant had negligible toxicity. Since the OM preparations alone showed low-level toxicity in the bioassay, the more potent insecticidal factors may be enclosed in the lumina of OMVs or associated with the surfaces of the OMVs. The results described above demonstrate the insecticidal potential of Xenorhabdus cells, as well as the OMVs. When the OMVs were treated with proteinase K, 80 to 90% of the proteins were degraded, as determined by SDS-PAGE (data not shown), and the digested OMVs were found to be inactive in the insecticidal assay. The active factors in the OMVs are proteinaceous, as suggested by their heat and protease sensitivities. The requirement for larger doses of OM and OMV protein preparations for toxicity in these experiments compared to the previously reported values for purified proteins (9, 15) could be due to the presence of a number of protein species in the OMV preparations, and the major OM proteins present in large excess may not have any role in toxicity. In addition, larval age and insect species are also important factors determining susceptibility to external toxins. It is also possible that other proteins are needed for synergistic action of the OMV preparations, as has been suggested previously for a cloned toxin of X. nematophilus (21). Our efforts to determine the toxicity of individual proteins present in the OMV mixture proved to be unsuccessful. Purification of the proteins was attempted after detergent solubilization. Different detergents, including Triton X-100, octyl glucoside, 3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate (CHAPS), and SDS, were used to solubilize the OMV proteins, and only SDS was able to solubilize the protein complex efficiently. However, treatment with SDS resulted in total loss of the biological activity of the proteins.
TABLE 2.
Toxicity of secreted proteins of X. nematophilus for neonatal larvae of H. armigera
| Sample | Protein concn (μg/g of diet) | % Mortalitya |
|---|---|---|
| Control | 0 | |
| OMV protein | 10 | 12 ± 5b |
| 25 | 27 ± 11 | |
| 50 | 40 ± 0 | |
| 75 | 80 ± 5 | |
| 100 | 98 ± 2 | |
| Heat-treated OMVs | 50 | 0 |
| Protease-treated OMVs | 50 | 6 ± 5 |
| Soluble proteins in culture supernatant | 25 | 7 ± 5 |
| 50 | 10 ± 7 | |
| 100 | 30 ± 11 | |
| OM | 50 | 21 ± 4 |
| LPS | 20 | 0 |
| OMVs from E. coli | 30 | 5 ± 2 |
Each group contained 10 neonatal larvae, and mortality was determined after 48 h.
Mean ± standard deviation.
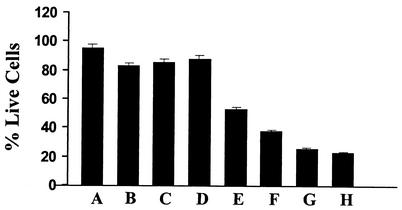
The cytotoxicity of the OMV proteins for the Sf-21 cells of insect origin also reflects the insecticidal potential of the secreted toxins of X. nematophilus. Figure 3 shows that in the presence of 1 μg of the OMV proteins (per well), the number of viable cells was reduced to 43% of the original number after 6 to 7 h and that the toxicity increased in a dose-dependent manner. The number of live cells was reduced to 20% of the original number in the presence of 10 μg of protein per well. Heating the proteins at 80°C for 15 min substantially reduced the toxicity, and OMV preparations from E. coli had no effect on the Sf-21 cells (Fig. 3). Similar to their low oral toxicity for H. armigera neonates, the toxicity of the OM proteins for the Sf-21 cells was also marginal. At a protein concentration of 25 μg per well, 88% of the cells remained viable after 6 to 7 h (Fig. 3).
FIG. 3.
Cytotoxicity assay performed with Sf-21 cells and OMV preparations from X. nematophilus. Bar A, Sf-21 cells in serum-free medium; bar B, 35 μg of OMVs from E. coli K-12 culture supernatant; bar C, 25 μg of heat-inactivated OMVs of X. nematophilus; bar D, 25 μg of OMs from X. nematophilus; bars E to H, 1, 5, 10, and 25 μg of OMV proteins of X. nematophilus, respectively.
OMVs were also prepared from a Photorhabdus strain (24) and tested in the oral toxicity assays. These OMVs were found to be biologically active against neonatal H. armigera larvae (data not shown), although the specific activity of the OMVs isolated from the Photorhabdus strain was lower than the specific activity of the OMVs isolated from the Xenorhabdus strain used in this study. SDS-PAGE analysis of the OMV proteins (Fig. 2A, lane 1) showed that there were a number of proteins in the higher-molecular-weight range, as determined by electrophoretic mobility, similar to the proteins found in the larvicidal, large polypeptide complexes isolated from the culture supernatant of P. luminescens (9, 15). In this context it is tempting to speculate that the large protein complexes isolated from the culture supernatants in the previous study (15) could be organized like the Xenorhabdus OMV proteins.
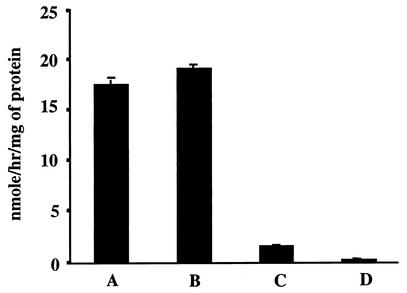
The characteristics of the individual active factors are currently being investigated. A low level of protease activity (data not shown) was detected in the OMV preparations; however, it does not appear to be enough to account for the level of activity observed in the larvicidal assay, suggesting that there is more than one active moiety in the OMV preparation. Association of strong chitinase activity with the OM and OMV fractions (Fig. 4) indicates that the chitinase is probably an OM protein. We do not know the insecticidal efficacy of this chitinase at this stage; however, its presence in the OMVs further strengthens the pathogenic potential of the secreted protein complex. Recently, Morgan et al. (21) described a DNA region of X. nematophilus encoding an insecticidal protein together with other attributes of pathogenicity, including a chitinase gene, organized as a pathogenicity island, suggesting the multicomponent nature of the Xenorhabdus toxins. Similarly, the toxin complex secreted by P. luminescens into the culture medium has also been shown to contain multiple polypeptides with larvicidal activity (9, 15).
FIG. 4.
Chitinase activity in the secreted OMVs of X. nematophilus. Enzyme activity is expressed as nanomoles of methyl umbelliferone released per hour per milligram of protein. Bar A, OMVs; bar B, OMs; bar C, heat-denatured OMVs; bar D, OMVs prepared from E. coli.
In conclusion, this study demonstrated for the first time the insecticidal potential of the OM-associated proteins that are secreted as OMVs by X. nematophilus into its growth medium. The presence of chitinase activity together with bacteriocin, adhesin protein, and pore-forming proteins in the insecticidal multiprotein complex supports the role of this complex in pathogenicity, as these proteins are known to mediate host-pathogen interactions in other pathogenic bacteria. The membrane vesicles provide an efficient mechanism to transport effector molecules to the larval host. Work is in progress to characterize the individual members of the protein complex, which should throw more light on the virulence mechanisms of the bacteria which have symbiotic relationships with insects.
REFERENCES
- 1.Akhurst, R. J., and G. B. Dunphy. 1988. Symbiotically associated entomopathogenic bacteria, nematodes and their insect hosts, p. 1-23. In N. Beckage, S. Thompson, and B. Federici (ed.), Parasites and pathogens of insects, vol. 2. Academic Press, Inc., New York, N.Y.
- 2.Apicella, M. A., J. M. Griffiss, and H. Schneider. 1994. Isolation and characterization of lipopolysaccharides, lipooligosaccharides and lipid A. Methods Enzymol. 235:242-252. [DOI] [PubMed] [Google Scholar]
- 3.Armstrong, J. L., G. F. Rohrmann, and G. S. Beaudreau. 1985. Delta endotoxin of Bacillus thuringiensis subsp. israelensis. J. Bacteriol. 161:39-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bedding, R. A., and R. J. Akhurst. 1975. Nematodes and their biological control of insect pests. Nematologia 21:109-110. [Google Scholar]
- 5.Beveridge, T. J. 1999. Structures of gram-negative cell walls and their derived membrane vesicles. J. Bacteriol. 181:4725-4733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beveridge, T. J., S. A. Makin, J. L. Kadurugamuwa, and Z. Li. 1997. Interaction between biofilm and the environment. FEMS Microbiol. Rev. 20:291-303. [DOI] [PubMed] [Google Scholar]
- 7.Boemare, N. E., and R. J. Akhrust. 1988. Biochemical and physiological characterization of colony variants in Xenorhabdus ssp. (Enteriobacteriaciae). J. Gen. Microbiol. 134:751-761. [Google Scholar]
- 8.Boemare, N. E., M.-H. Boyer-Giglio, J.-O. Thaler, R. J. Akhurst, and M. Brehelin. 1992. Lysogeny and bacteriocinogeny in Xenorhabdus nematophilus and other Xenorhabdus spp. Appl. Environ. Microbiol. 58:3032-3037. [DOI] [PMC free article] [PubMed]
- 9.Bowen, D., T. A. Rocheleau, M. Blackburn, O. Andreev, E. Golubeva, R. Bhartia, and R. H. Ffrench-Constant. 1998. Insecticidal toxins from the bacterium Photorhabdus luminescens. Science 280:2129-2132. [DOI] [PubMed] [Google Scholar]
- 10.FFrench-Constant, R., and D. Bowen. 1999. Photorhabdus toxins: novel biological insecticides. Curr. Opin. Microbiol. 2:284-288. [DOI] [PubMed] [Google Scholar]
- 11.Finney, D. J. 1971. Probit analysis. Cambridge University Press, Cambridge, United Kingdom.
- 12.Forst, S., B. Dowds, N. Boemare, and E. Stackebrandt. 1997. Xenorhabdus and Photorhabdus spp.: bugs that kill bugs. Annu. Rev. Microbiol. 51:47-72. [DOI] [PubMed] [Google Scholar]
- 13.Götz, P., A. Boman, and H. G. Boman. 1981. Interactions between insect immunity and an insect pathogenic nematode with symbiotic bacteria. Proc. R. Soc. Lond. Ser. B Biol. Sci. 212:333-350. [Google Scholar]
- 14.Grenier, D., and D. Maryland. 1987. Functional characterization of extracellular vesicles produced by Bacteroides gingivalis. Infect. Immun. 55:111-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guo, L., R. O. Fatig III, G. L. Orr, B. W. Schafer, J. A. Strickland, K. Sukhapinda, A. T. Woodsworth, and J. K. Petell. 1999. Photorhabdus luminescens W-14 insecticidal activity consists of at least two similar but distinct proteins. J. Biol. Chem. 274:9836-9842. [DOI] [PubMed] [Google Scholar]
- 16.Hollis, T., Y. Honda, T. Fukamizo, E. Marcotte, P. J. Day, and J. D. Robertus. 1997. Kinetic analysis of barley chitinase. Arch. Biochem. Biophys. 344:335-342. [DOI] [PubMed] [Google Scholar]
- 17.Kaduragamuwa, J. L., and T. J. Beveridge. 1995. Virulence factors are released from Pseudomonas aeruginosa in association with membrane vesicles during normal growth and exposure to gentamicin: a novel mechanism of enzyme secretion. J. Bacteriol. 177:3998-4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kolling, G. L., and K. R. Matthews. 1999. Export of virulence genes and Shiga toxin by membrane vesicles of Escherichia coli O157:H7. Appl. Environ. Microbiol. 65:1843-1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leisman, G. B., J. Waukau, and S. Forst. 1995. Characterization and environmental regulation of outer membrane proteins in Xenorhabdus nematophilus. Appl. Environ. Microbiol. 61:200-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li, Z., A. J. Clarke, and T. J. Beveridge. 1996. A major autolysin of Pseudomonas aeruginosa: subcellular distribution, potential role in cell growth, division, and secretion of surface membrane vesicles. J. Bacteriol. 178:2479-2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morgan, J. A. W., M. Sargeant, D. Ellis, M. Ousley, and P. Jarrett. 2001. Sequence analysis of insecticidal genes from Xenorhabdus nematophilus PMF1296. Appl. Environ. Microbiol. 67:2062-2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moureaux, N., T. Karjalainen, A. Givaudan, P. Bourlioux, and N. Boemare. 1995. Biochemical characterization and agglutinating properties of Xenorhabdus nematophilus F1 fimbriae. Appl. Environ. Microbiol. 61:2707-2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pugsley, A. P. 1993. The complete general secretary pathway in gram-negative bacteria. Microbiol. Rev. 57:50-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raman, R., and R. K. Bhatnagar. 2002. Insecticidal toxic proteins produced by Photorhabdus luminescens akhurstii, a symbiont of Heterorhabditis indica. J. Nematol. 34:23-27. [PMC free article] [PubMed] [Google Scholar]
- 25.Sauer, F. G., K. Fütterer, J. S. Pinkner, K. W. Dodson, S. J. Hultgren, and G. Waksman. 1999. Structural basis of chaperone function and pilus biogenesis. Science 285:1058-1061. [DOI] [PubMed] [Google Scholar]
- 26.Schmidt, T. M., B. Bleakley, and K. H. Nealson. 1988. Characterization of an extracellular protease from the insect pathogen Xenorhabdus luminescens. Appl. Environ. Microbiol. 54:2793-2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tachibana, M., H. Hori, N. Suzuki, T. Uechi, D. Kobayashi, H. Iwahana, and H. K. Kaya. 1996. Larvicidal activity of the symbiotic bacterium Xenorhabdus japonicum from the entomopathogenic nematode Steinernema kushidia against Anomala cuprae. J. Invertebr. Pathol. 68:152-159. [DOI] [PubMed] [Google Scholar]
- 28.Thaler, J. O., S. Baghdiguian, and N. Boemare. 1995. Purification and characterization of xenorhabdicin, a phage tail-like bacteriocin, from the lysogenic strain F1 of Xenorhabdus nematophilus. Appl. Environ. Microbiol. 61:2049-2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yaron, S., G. L. Kolling, L. Simon, and K. R. Matthews. 2000. Vesicle-mediated transfer of virulence genes from Escherichia coli O157:H7 to other enteric bacteria. Appl. Environ. Microbiol. 66:4414-4420. [DOI] [PMC free article] [PubMed] [Google Scholar]