Abstract
Xylose utilization is of commercial interest for efficient conversion of abundant plant material to ethanol. Perhaps the most important ethanol-producing organism, Saccharomyces cerevisiae, however, is incapable of xylose utilization. While S. cerevisiae strains have been metabolically engineered to utilize xylose, none of the recombinant strains or any other naturally occurring yeast has been able to grow anaerobically on xylose. Starting with the recombinant S. cerevisiae strain TMB3001 that overexpresses the xylose utilization pathway from Pichia stipitis, in this study we developed a selection procedure for the evolution of strains that are capable of anaerobic growth on xylose alone. Selection was successful only when organisms were first selected for efficient aerobic growth on xylose alone and then slowly adapted to microaerobic conditions and finally anaerobic conditions, which indicated that multiple mutations were necessary. After a total of 460 generations or 266 days of selection, the culture reproduced stably under anaerobic conditions on xylose and consisted primarily of two subpopulations with distinct phenotypes. Clones in the larger subpopulation grew anaerobically on xylose and utilized both xylose and glucose simultaneously in batch culture, but they exhibited impaired growth on glucose. Surprisingly, clones in the smaller subpopulation were incapable of anaerobic growth on xylose. However, as a consequence of their improved xylose catabolism, these clones produced up to 19% more ethanol than the parental TMB3001 strain produced under process-like conditions from a mixture of glucose and xylose.
Over the last decade, metabolic engineering has become a standard procedure for strain improvement and has been very successful when simple cellular traits have been targeted (19, 26). Although the genomics age and the associated genome-wide analytical technologies provide further impetus for rational approaches, metabolic engineering of more complex cellular systems or cellular systems that are not fully understood remains a challenge (4). Along with random, combinatorial approaches, such as directed evolution in contemporary protein engineering (3), evolutionary approaches are becoming increasingly important for augmenting metabolic engineering of complex phenotypes (21, 22, 24, 36). In certain cases, however, even seemingly simple metabolic systems resist straightforward rational engineering.
One example of a seemingly simple trait is expanding the substrate range of Saccharomyces cerevisiae for utilization of pentoses for ethanol formation. The commercial interest in utilization of pentoses, particularly xylose, is related to the prevalence of pentoses in abundant plant material; for instance, they are the major structural units in hemicelluloses. While metabolic engineering has successfully endowed S. cerevisiae with the ability to utilize the pentose xylose (7, 12, 17, 33) and recently also arabinose (J. Becker and E. Boles, submitted for publication), it has not yet succeeded in developing strains that convert pentoses to ethanol with a high yield and at a high specific rate (10, 13).
An additional puzzling fact is the fact that xylose is not able to support anaerobic growth of both natural and recombinant xylose-utilizing yeasts (16). Since many bacteria can grow anaerobically on xylose (14), the reason for this inability is not really understood at present, but it has been ascribed to a general restriction of eukaryotic xylose metabolism to respirative conditions (16). This conclusion was based on the fundamental difference between eukaryotic xylose catabolism and prokaryotic xylose catabolism because bacteria convert xylose directly to xylulose by using xylose isomerase, whereas eukaryotes rely on two consecutive redox reactions that are catalyzed by the NADPH-dependent xylose reductase (XR) and the NADH-dependent xylitol dehydrogenase (XDH), with xylitol as the pathway intermediate. By providing NADPH through the oxidative pentose phosphate pathway, which operates actively in S. cerevisiae and Pichia stipitis (8, 9) and by respiring NADH, eukaryotes can efficiently drive these coupled redox reactions under aerobic conditions but possibly not under anaerobic conditions. While this could potentially explain the inability of many yeasts to grow anaerobically on xylose, it does not suffice as an explanation for the xylose-utilizing S. cerevisiae strains that functionally overexpress xylose isomerase (32). Hence, it appears that at least one additional component is missing in our understanding of xylose metabolism.
Such understanding cannot be obtained from the available databases and previously published information; hence, in this study we attempted to evolve strains that are capable of anaerobic growth on xylose by performing long-term selection experiments. Since anaerobic xylose-utilizing eukaryotes apparently have not evolved naturally, we decided that selection should be initiated with the best xylose-utilizing S. cerevisiae strains available. At present, the best xylose-utilizing strains overexpress the XR and XDH genes from P. stipitis (17) in combination with the endogenous xylulokinase (XK) gene (7, 12, 28). In this study we used S. cerevisiae strain TMB3001, which overexpresses the three genes of the xylose utilization pathway from a chromosomal integration, as the initial strain in various long-term evolution experiments (7).
MATERIALS AND METHODS
Strains and media.
In all evolution experiments the cultures were inoculated with the recombinant S. cerevisiae strain TMB3001 [CEN.PK 113-7A (MATa his3-Δ1 MAL2-8c SUC2) his3::YIpXR/XDH/XK], which contains the entire xylose utilization pathway (7). Overexpression of XR is controlled by the alcohol dehydrogenase promoter and terminator, whereas XDH and XK are both under control of phosphoglycerate kinase promoters and terminators. The strain suffixes C1 to C15 refer to clones that were isolated after 460 generations of selection. TMB3001C1 and TMB3001C5 are representatives of phenotypic classes II and I, respectively.
For physiological analysis and evolution experiments, yeast cultures were grown at 30°C in minimal medium containing (per liter) 5 g of (NH4)2SO4, 3 g of KH2PO4, 0.5 g of MgSO4 · 7H2O, 15 mg of EDTA, 4.5 mg of ZnSO4 · 7H2O, 0.3 mg of CoCl2 · 6H2O, 1 mg of MnCl2 · 4H2O, 0.3 mg of CuSO4 · 4H2O, 4.5 mg of CaCl2 · 2H2O, 3 mg of FeSO4 · 7H2O, 0.4 mg of Na2MoO4 · 2H2O, 1 mg of H3BO3, 0.1 mg of KI, 0.05 mg of biotin, 1 mg of calcium pantothenate, 1 mg of nicotinic acid, 25 mg of inositol, 1 mg of thiamine HCl, 1 mg of pyridoxine HCl, and 0.2 mg of para-aminobenzoic acid (pH 5.0) (30). In chemostat cultures, 0.1 g of polypropylene glycol P2000 per liter was added to prevent foam formation. The medium was supplemented with ergosterol (Fluka) and Tween 80 (Sigma) for anaerobic cultivation; these two components were dissolved in boiling 99.8% (vol/vol) ethanol and were added to the medium at final concentrations of 0.01 and 0.42 g liter−1, respectively. Solid media were prepared by adding 1.5% (wt/vol) technical agar (Becton Dickinson). For anaerobic growth on xylose plates, aliquots were washed twice with phosphate-buffered saline (PBS) (8 g of NaCl per liter, 0.2 g of KCl per liter, 1.44 g of Na2HPO4 per liter, 0.24 g of KH2PO4 per liter; pH 7.0) and plated on anaerobic minimal medium containing 20 g of xylose per liter as the sole carbon source. The plates were incubated at 30°C in sealed jars by using the GasPack Plus system (Becton Dickinson) to provide an anaerobic atmosphere, which was verified by indicator strips (Becton Dickinson).
Long-term selection cultures.
Chemostat selection was performed in a Sixfors 6 minireactor system (Infors, Botmingen, Switzerland) at a dilution rate (D) of 0.05 h−1 with mixing at 300 rpm. A constant working volume of 300 ml was maintained by continuously removing excess culture broth through a needle that was fixed at a predetermined height. The culture pH was maintained at 5.0 ± 0.3 by supplementing the minimal medium with 50 mM potassium hydrogen phthalate (Fluka) (31). Aerobic conditions were established by aerating cultures at a rate of 0.3 liter min−1. Microaerobic conditions were established by stepwise reduction of the aeration rate until no measurable flow was seen in the reactor effluent gas. Anaerobic conditions were established by slight sparging (<1 ml min−1) with technical N2 (<200 ppm of O2; independently quantified with a Prima 600 mass spectrometer [Fisons Instruments, Uxbridge, England]). It should be noted that due to the contaminating O2, these conditions were not strictly anaerobic. To ensure robust long-term operation for up to 4 months, marprene tubing (Ismatech, Glattbrugg, Switzerland) was used with external peristaltic pumps for feeding and harvesting. Contamination controls were analyzed at 2-week intervals by plating culture aliquots on YPD medium (10 g of yeast extract per liter, 20 g of peptone per liter, 20 g of glucose per liter) plates and by microscopic examination.
Selection in serial, strictly anaerobic batch cultures was done in Hungate tubes, which were 17-ml Pyrex glass tubes that were sealed with butyl rubber septa and plastic screw caps (Bellco Glass Inc., Vineland, N.J.). Cultures were grown in minimal medium containing 10 g of xylose per liter as the sole carbon source. New cultures were inoculated when the growth rate declined, which typically occurred after about 1 week.
Growth conditions.
Aerobic cultures were grown at 30°C in 500-ml baffled shake flasks with 50 ml of minimal medium with shaking at 300 rpm on a rotary shaker. To adapt TMB3001 to aerobic growth on xylose as the sole carbon source, it was grown first on YPX medium (10 g of yeast extract per liter, 20 g of peptone per liter, 20 g of d-xylose per liter), then on YNB xylose medium (6.7 g of yeast nitrogen base per liter, 20 g of d-xylose per liter), and finally in minimal medium containing only xylose prior to inoculation.
Fermentation performance was evaluated in anaerobic batch cultures containing 50 g of glucose per liter and 50 g of xylose per liter. The concentrations of all other minimal medium components except KH2PO4 were doubled. To avoid major decreases in the pH, 100 mM citric acid buffer (pH 5.5) was added, which maintained the pH at values above 4.7 in all cases. Cultures were grown at 30°C in 175-ml serum bottles filled with 150 ml medium and stirred magnetically at 100 rpm. Anaerobic (but not strictly anaerobic) conditions were maintained by slight continuous sparging (1 to 2 bubbles s−1) with technical N2 (O2 concentration, <200 ppm; PanGas, Dagmersellen, Switzerland). Inocula were prepared by growing frozen stock cultures first on YPD medium and then in minimal medium with 20 g of glucose per liter.
Strictly anaerobic growth experiments with xylose as the sole carbon source were performed in Hungate tubes or serum bottles sealed with butyl rubber septa by sparging the basic salt solution of the minimal medium with pure N2 (O2 concentration, <5 ppm; PanGas) for 15 min. After autoclaving, the remaining filter-sterilized, N2-sparged medium components and 10 g of xylose per liter were added. To ensure that the conditions were strictly anaerobic, 0.25 g of Na2S per liter or 0.5 g of l-cysteine per liter was added in selected cases as a reducing agent after incubation at 60°C for 5 min. Culture aliquots were withdrawn during purging with pure N2. To verify that the conditions were strictly anaerobic, the redox indicator resazurin was added to the medium at a final concentration of 0.0001% (wt/vol) before sparging with pure N2. The preparations for strictly anaerobic growth experiments with xylose as the sole carbon source were inoculated with cultures grown on minimal medium containing 20 g of glucose per liter. The inocula were washed twice with PBS prior to inoculation to avoid glucose contamination.
Stocks for strain maintenance were generated from overnight cultures grown in YPD medium by adding glycerol to a final concentration of 15% (wt/vol) and were then stored at −80°C. To preserve the original clonal compositions of selection chemostats, aliquots of the populations from the selection cultures were frozen directly without intermediate batch growth.
EMS mutagenesis.
To increase genetic variability, cultures were randomly mutagenized with ethyl methane sulfonate (EMS) (Sigma) after aliquots of the populations were harvested by centrifugation at 1,500 rpm and 4°C for 3 min from minimal medium batch cultures in the early stationary phase. The pellets were washed once with PBS and resuspended in 10 ml of PBS. Then 300 μl of EMS was added to each suspension, and the suspension was incubated on a rotary shaker at 300 rpm and 30°C. After 40 min, 20 ml of 5% (wt/vol) Na2S2O3 was added to inactivate the mutagen. After centrifugation, the pellet was washed twice with 5% (wt/vol) Na2S2O3 to remove the residual EMS, resuspended in 20 ml of minimal medium, and stored at −80°C after addition of 15% (wt/vol) glycerol. Survival rates of 5 to 30% were verified by counting CFU.
Analytical methods.
Cell growth was monitored by determining the optical density at 600 nm (OD600) or by determining the Klett value with a Klett meter (Bel-Art Products, Pequonock, N.J.). Cellular dry weight was determined by using 10-ml culture aliquots that were centrifuged at 5,000 rpm for 20 min in preweighed glass tubes, washed once with water, and dried at 110°C for 24 h to constant weight. Commercially available kits were used for enzymatic determination of the concentrations of glucose (Beckman), xylose (Medichem, Steinenbronn, Germany), xylitol (R-Biopharm, Darmstadt, Germany), acetate (R-Biopharm), and glycerol (Sigma). Ethanol concentrations were determined by gas chromatography (5890E chromatograph; Hewlett-Packard) with a Permabond-CW20 M-0.25 column (Macherey-Nagel); butyrate was used as the internal standard.
Determination of physiological parameters.
In batch cultures, exponential growth rates were determined by log-linear regression of OD600 versus time with growth rate as the regression coefficient. The specific biomass yield (YX/S) was determined from a plot of the coefficient of linear regression of the biomass concentration (X) versus substrate concentration (S) during the exponential growth phase. The biomass concentration was estimated from predetermined correlations between OD600 and cellular dry weight during the mid-exponential growth phase of aerobic cultures grown on glucose for strains TMB3001, TMB3001C5, and TMB3001C1 (0.530, 0.581, and 0.479 g OD600 unit−1, respectively). During the exponential growth phase, specific glucose and xylose uptake rates were calculated by determining the ratio of the growth rate to YX/S. Ethanol, xylitol, acetate, and glycerol yields were calculated by linear regression of by-product concentration versus S.
In the mixed-substrate fermentation analysis, the specific xylose uptake rate was determined by determining the ratio of the linear regression coefficient of xylose concentration versus time to the average biomass concentration between the onset of xylose consumption and about 100 h after inoculation. In these cases, the correlation between OD600 and cellular dry weight was determined at the end of each fermentation.
RESULTS
Chemostat selection.
Evolution of a yeast strain capable of anaerobic growth on xylose as the sole carbon source was started with the metabolically engineered S. cerevisiae strain TMB3001, which overexpresses XR and XDH from P. stipitis and native XK from a stable chromosomal insertion (7). To increase genetic variability, an EMS-mutagenized population of TMB3001 was used to inoculate all selection preparations. Direct gain-of-function selection for growth on xylose as the sole carbon source in batch cultures or in petri dishes proved to be unsuccessful under anaerobic conditions (data not shown). Similarly, extended selection in serial anaerobic batch cultures with xylose in combination with glucose for more than 30 generations did not yield a population capable of anaerobic growth on xylose as the sole carbon source (data not shown). Hence, we prepared two long-term anaerobic chemostats with a D of 0.05 h−1 that contained a limiting concentration (1 g liter−1) of the growth-promoting sugar glucose or galactose and 5 g of xylose per liter. Galactose was chosen to avoid catabolite repression and competitive inhibition of xylose transport by glucose (10). Within about 170 generations (100 days), the steady-state biomass concentrations remained the same, and only 5 to 10% of the supplied xylose was consumed in both cultures (data not shown), indicating that there was no evolutionary progress toward anaerobic growth on xylose.
To facilitate sequential evolution of multigene changes that may be required for efficient anaerobic xylose catabolism, an aerobic chemostat culture with 5 g of xylose per liter and 1 g of glucose per liter was prepared (Fig. 1A). After about 30 generations, the steady-state xylose concentration declined and the OD600 increased. Within 90 generations a new steady state was attained, during which 80% of the supplied xylose was consumed. At this stage, a culture aliquot was withdrawn, EMS mutagenized, and used to inoculate two new aerobic chemostat cultures. The settings for the first chemostat were identical to those used for the previous chemostat, and a comparable steady state was attained immediately. This chemostat was then switched to anaerobic conditions, and the OD600 decreased to 0.4 while the residual xylose concentration increased to 4.5 g liter−1 (data not shown). This steady-state physiology was similar to the steady-state physiology observed after the previous direct anaerobic selection with 5 g of xylose per liter and 1 g of glucose per liter. Since no significant improvements were observed during the following 30 generations, this culture was not monitored further. The second aerobic chemostat contained 5 g of xylose per liter as the sole carbon source (Fig. 1B). In contrast to the initial EMS-mutagenized TMB3001 population, we obtained a growing population which consumed increasingly more xylose and thus decreased the residual xylose concentration from 1.5 to 0.3 g liter−1 after 60 generations. To establish microaerobic conditions, the aeration rate was drastically reduced from 0.3 liter min−1 to less than 1 ml min−1 at generation 140. Within the following 20 generations, the residual xylose concentration increased and the OD600 decreased rapidly. When the OD600 appeared to be stable, aeration was turned off at generation 170. After an immediate increase, the residual xylose concentration decreased, and the OD600 increased steadily for 100 generations (Fig. 1B). At a residual xylose concentration of 0.4 g liter−1 and an OD600 of 3.1 at generation 270, anaerobic conditions were established by continuous sparging with technical N2. Soon after the onset of anaerobiosis, a stable steady state was attained, although the OD600 was low (Fig. 1B). To determine whether anaerobic growth on xylose could be improved further, we mutagenized an aliquot withdrawn at generation 310 with EMS. After anaerobic batch growth on xylose as the sole carbon source, an anaerobic chemostat culture was grown for another 150 generations on xylose, during which a gradual increase in biomass formation was observed (Fig. 1C). It should be noted that due to a level of O2 contamination of less than 200 ppm in the N2, the conditions were not strictly anaerobic in this selection culture, as discussed below.
FIG. 1.
Evolution of S. cerevisiae TMB3001 in carbon-limited chemostat cultures at a D of 0.05 h−1 under aerobic conditions with 5 g of xylose per liter and 1 g of glucose per liter (A); under aerobic, microaerobic (light gray background), and anaerobic (dark gray background) conditions with 5 g of xylose per liter (B); and under anaerobic conditions with 5 g of xylose per liter (C). Arrow 1 indicates the time when the airflow was reduced from 0.3 liters min−1 to <1 ml min−1; arrow 2 indicates the time when the airflow was shut off; and arrow 3 indicates the time when anaerobiosis was initiated by sparging with technical N2. The evolving population was subjected to EMS mutagenesis prior to inoculation of the chemostats.
To identify the time at which the ability to grow anaerobically on xylose as the sole carbon source emerged first in the 460 generations (266 days) of evolution, frozen aliquots of different generations were plated on xylose minimal medium and incubated in a strictly anaerobic atmosphere. Colonies were detected in aliquots of the populations only after 270 generations. To exclude the possibility that anaerobic growth of the evolved population was due to trace amounts of O2 in the technical N2, the final evolved population was grown in batch cultures with increasing strengths of anaerobiosis. When anaerobiosis was established in serum bottles by continuous sparging with technical N2 (O2 concentration, <200 ppm), the maximum biomass concentration was 0.50 ± 0.08 g (dry weight) of cells per liter in the presence of 5 g of xylose per liter. When anaerobiosis was established in tightly sealed, anaerobic Hungate tubes (without continuous N2 sparging), however, the maximum biomass concentration was only 0.24 ± 0.03 g (dry weight) of cells liter− in the presence of 10 g of xylose per liter. This lower biomass yield under more stringent anaerobic conditions clearly demonstrated the physiologically relevant role of contaminating O2, not only in these batch cultures but probably also during anaerobic selection. Finally, anaerobic growth on xylose as the sole carbon source in Hungate tubes containing Na2S or cysteine as a reducing agent and resazurin as a redox indicator confirmed the gain-of-function phenotype (data not shown). In all cases xylose was completely consumed at the end of growth.
Clonal analysis.
Since chemostat-evolved, asexual populations are typically heterogeneous (18, 23, 34), an aliquot of the population after 460 generations was plated on xylose minimal medium. Under anaerobic conditions the number of CFU was 54% ± 4% of the number of CFU found on aerobic YPD medium plates, thus providing the first evidence that there was population heterogeneity. The fermentation performance of parental strain TMB3001, the fermentation performance of the evolved population after 460 generations, and the fermentation performance of 15 clones, which were isolated from anaerobic xylose plates, were then compared in anaerobic batch cultures with 50 g of glucose per liter and 50 g of xylose per liter. During the initial phase of exponential growth on glucose, almost no xylose was consumed, but when the glucose was depleted, growth ceased and xylose was consumed in a second phase (Fig. 2).
FIG. 2.
Fermentation profiles for TMB3001 (A), the 460-generation population (B), clone TMB3001C5 representing the first phenotypic class (C), and clone TMB3001C1 representing the second phenotypic class (D) during anaerobic growth on 50 g of glucose per liter and 50 g of xylose per liter. The glucose and xylose consumption phases are indicated by I and II, respectively. Gray shading indicates the time when there was simultaneous consumption of glucose and xylose.
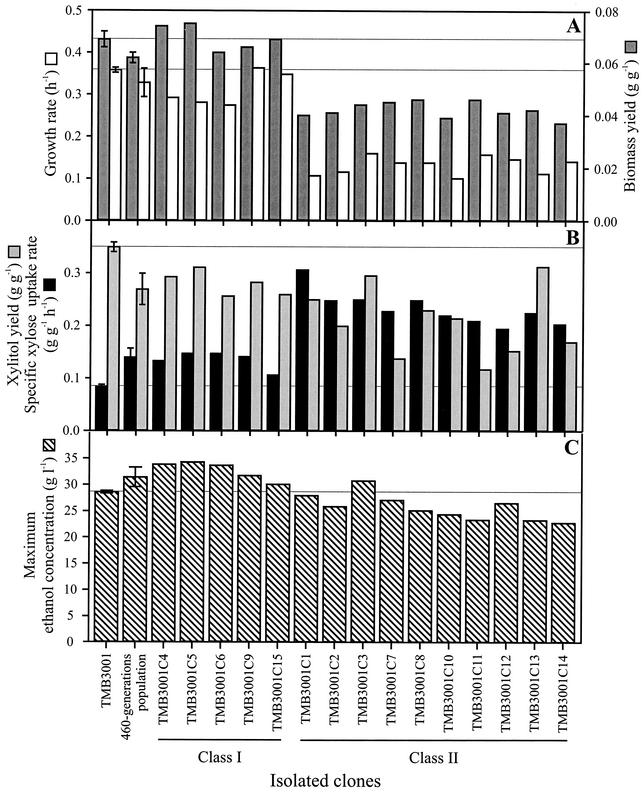
To quantitatively compare clonal fermentation performance, physiological parameters were determined during the glucose and xylose consumption phases (Fig. 3). Since the specific xylose uptake decreased after about 100 h of fermentation (Fig. 2), parameters for the second phase were calculated for the period between the time when glucose was depleted and 100 h. Generally, two major phenotypic classes could be discerned. The first class of clones (class I) and the evolved population were similar to TMB3001 during growth on glucose but exhibited on average an approximately 60% higher specific xylose uptake rate and reduced formation of the by-product xylitol during the second phase (Fig. 2A, B, and C and 3). As a consequence, these clones accumulated up to 19% higher final ethanol concentrations (Fig. 3C). The more abundant second class of clones (class II) exhibited a radically different mode of growth on glucose (Fig. 2D and 3). On average, the maximum growth rate and biomass yield on glucose were reduced by 60 and 40%, respectively. Unlike TMB3001 and the class I clones, the class II clones began to consume xylose prior to the time when the glucose was depleted (Fig. 2D). The specific xylose uptake rates were at least double in the class II clones (range, 0.19 g g−1 h−1 for TMB3001C12 to 0.31 g g−1 h−1 for TMB3001C1) compared to the value for TMB3001 (0.08 g g−1 h−1). Also, the xylitol yields (Fig. 3B) and the final ethanol concentrations (Fig. 3C) were reduced in these clones compared to the values for TMB3001.
FIG. 3.
Physiological parameters during anaerobic growth on 50 g of glucose per liter and 50 g of xylose per liter for TMB3001, the 460-generation population, and 15 clones isolated from this population. (A) Maximum growth rate and biomass yield during exponential growth on glucose. (B) Specific xylose uptake rate and xylitol yield on xylose between the time that glucose was depleted and 100 h of fermentation. (C) Final ethanol concentration at 180 h. Values for TMB3001 and the populations are averages from duplicate experiments. The horizontal lines indicate the reference values for TMB3001.
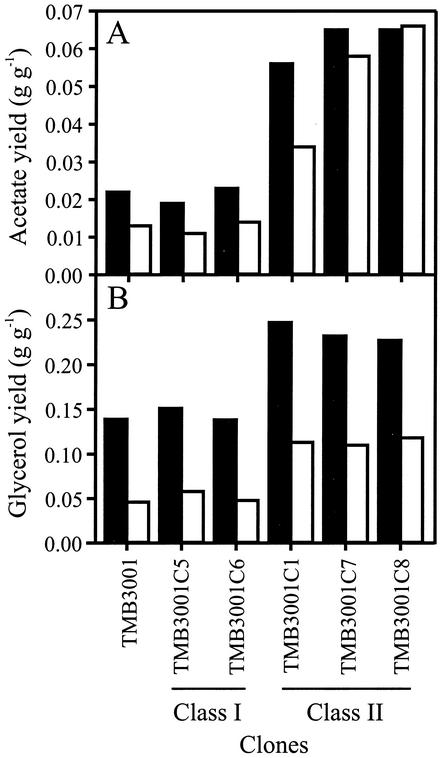
Determination of the time courses of the acetate and glycerol contents for selected clones belonging to both classes confirmed the drastic physiological changes in the class II phenotype (Fig. 4). Compared to TMB3001 and the class I clones, the class II clones produced significantly more acetate and glycerol on both glucose and xylose.
FIG. 4.
Yields of acetate (A) and glycerol (B) on glucose (solid bars) and xylose (open bars) during anaerobic growth on 50 g of glucose per liter and 50 g of xylose per liter for TMB3001 and selected clones from both phenotypic classes. Yields on glucose were determined between the time of inoculation and the beginning of the xylose uptake phase. Yields on xylose were determined between the time that glucose was depleted and 130 h. Values were determined in single experiments.
Physiological characterization.
To further elucidate the phenotypic differences between the two coevolved subpopulations, the best representatives of each phenotypic class were grown in single-substrate batch cultures. The class II representative TMB3001C1 exhibited significantly slower aerobic glucose catabolism, including a growth rate that was reduced 36% and a specific rate of glucose uptake that was reduced 48%, than TMB3001 (Table 1). Although the efficiency of exponential growth on glucose was not affected, as judged from the maximum YX/S, the maximum biomass concentration attained by TMB3001C1 was significantly lower.
TABLE 1.
Physiological parameters for TMB3001, TMB3001C5 (class I), and TMB3001C1 (class II) in aerobic batch cultures with 5 g of glucose per liter
| Strain | Maximum growth rate (h−1) | Specific glucose uptake rate (g g−1 h−1) | YX/S (g g−1) | Maximum biomass concn (g liter−1) |
|---|---|---|---|---|
| TMB3001 | 0.44a | 3.14 ± 0.05 | 0.14b | 2.1c |
| TMB3001C5 | 0.41 | 2.61 ± 0.09 | 0.16 | 2.2 |
| TMB3001C1 | 0.28 | 1.62 ± 0.02 | 0.17 | 1.4 |
The standard deviation for duplicate experiments was ±0.01.
The standard deviation was ±0.01.
The standard deviation was ±0.02.
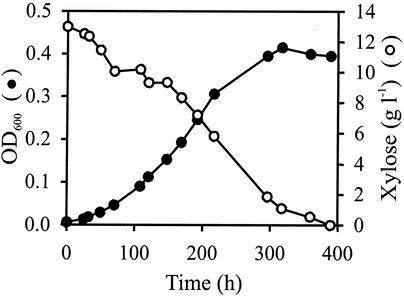
During aerobic growth on xylose as the sole carbon source, both clones grew significantly faster than their parent, but TMB3001C1 grew most rapidly by far (Table 2). Surprisingly, TMB3001C1 but not TMB3001C5 was capable of strictly anaerobic growth on xylose as the sole carbon source in Hungate tubes (Fig. 5). Increasing the strength of anaerobiosis further by addition of cysteine (Table 3) or Na2S (data not shown) had no significant effect on the growth of TMB3001C1. TMB3001C1 grew significantly faster on xylose under anaerobic conditions than the evolved population, which also included this clone.
TABLE 2.
Physiological parameters for TMB3001, TMB3001C5 (class I), and TMB3001C1 (class II) in aerobic batch cultures with 5 g of xylose per liter
| Strain | Maximum growth rate (h−1) | Specific xylose uptake rate (g g−1 h−1) | YX/S (g g−1) | Maximum biomass concn (g liter−1) |
|---|---|---|---|---|
| TMB3001 | 0.016a | NDb | ND | 2.1c |
| TMB3001C5 | 0.064 | 0.13 ± 0.00 | 0.50 ± 0.02 | 1.9 |
| TMB3001C1 | 0.119 | 0.27 ± 0.02 | 0.45 ± 0.04 | 2.0 |
Aerobic growth on xylose as the sole carbon source was observed only after serial growth in YPX medium and YNB medium with 20 g of xylose per liter. The standard deviation for duplicate experiments was ±0.001.
ND, not determined.
The standard deviation was ±0.02.
FIG. 5.
OD600 and xylose concentration during strictly anaerobic growth of TMB3001C1 in minimal medium with xylose as the sole carbon source.
TABLE 3.
Physiological parameters for TMB3001C1 and the 460-generation population in strictly anaerobic batch cultures with 10 g of xylose per liter
| Strain(s) | Maximum growth rate (h−1) | Specific xylose uptake rate (g g−1 h−1) | Yields (g g−1)a
|
|||
|---|---|---|---|---|---|---|
| Biomass | Ethanol | Xylitol | Glycerol | |||
| TMB3001C1 | 0.012b | 0.56c | 0.021 ± 0.004 | 0.24d | 0.32 ± 0.00 | 0.044 ± 0.005 |
| TMB3001C1 + Cyse | 0.010 | 0.52 | 0.022 ± 0.008 | 0.21 | 0.37 ± 0.09 | 0.047 ± 0.008 |
| 460-generation population | 0.004 | 0.23 | 0.018 ± 0.006 | 0.25 | 0.33 ± 0.01 | 0.036 ± 0.001 |
The acetate yields were less than 0.006 g g−1 in all cases.
The standard deviation for duplicate experiments was ±0.001.
The standard deviation was ±0.15.
The standard deviation was ±0.01.
Anaerobic conditions were established by addition of cysteine.
To determine the reason for the persistence of the class I clones in the evolved population despite their inability to grow anaerobically on xylose as the sole carbon source, we established anaerobic conditions like those in the final selection chemostat by continuous sparging (<1 ml min−1) with technical N2 (<200 ppm of O2). Surprisingly, all three strains, TMB3001, TMB3001C1, and TMB3001C5, grew to an OD600 of about 0.2 on minimal medium without carbon source supplementation, presumably on the 0.5 g of ethanol per liter which was added with the ergosterol and Tween 80 stock solution and which provided sufficient carbon for the growth observed. Since neither TMB3001 nor TMB3001C5 grew on xylose under these conditions, which were not strictly anaerobic, it appears that the class I clones survived during the selection process by scavenging contaminating O2 for oxidation of the ethanol produced or possibly other metabolic by-products of the class II clones. The growth rate of TMB3001C1 on xylose was 0.07 h−1 under these conditions, compared to 0.012 h−1 under strictly anaerobic conditions (Table 3). This indicates that TMB3001C1 also benefited from O2 contamination and explains why the class II clones were not washed out in the anaerobic chemostat at a D of 0.05 h−1.
Batch culture selection.
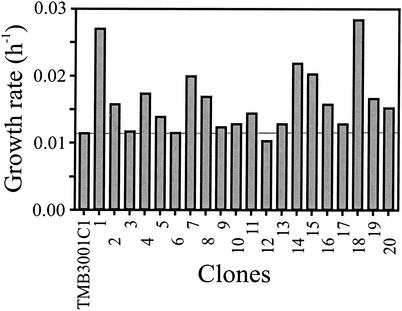
Since strictly anaerobic growth on xylose of the best isolated clone was still relatively slow, an EMS-mutagenized population of TMB3001C1 was grown sequentially in seven serial batch cultures, which corresponded to 40 generations. Twenty isolated clones were then grown on xylose as the sole carbon source in strictly anaerobic Hungate tubes (Fig. 6). Relative to the maximum growth rate of TMB3001C1, seven clones grew at about the same rate, 10 clones grew 1.2- to 2-fold faster, and three clones grew 2- to 2.5-fold faster. The highest growth rate observed was 0.028 h−1 for clones 1 and 18. The three best clones were then characterized more accurately in anaerobic xylose batch cultures. While TMB3001C1 grew at the previously determined rate of 0.012 h−1, clones 1, 14, and 18 grew at rates of 0.027 ± 0.002, 0.021 ± 0.002, and 0.018 ± 0.002 h−1, respectively.
FIG. 6.
Strictly anaerobic growth rates on xylose minimal medium for 20 clones that were isolated after seven serial anaerobic batch cultures on xylose. The horizontal line indicates the growth rate of parental strain TMB3001C1 before selection.
DISCUSSION
To our knowledge, we describe here the first yeast strain that grows on xylose as the sole carbon source under strictly anaerobic conditions. Such strains were isolated in a long-term, multistep chemostat evolution experiment, which was initiated with the metabolically engineered S. cerevisiae strain TMB3001, which overexpresses the xylose utilization pathway of P. stipitis (7). The selection procedure was based on the well-known evolution of mutants with increased substrate affinity and utilization in chemostat cultures (1, 6, 24, 25, 34). However, the key to successful evolution was the fact that selection for aerobic xylose utilization and selection for anaerobic xylose utilization were decoupled (Fig. 1). Thus, the selective pressure was adjusted to the capabilities of the evolving culture, allowing advantageous mutations to accumulate under permissive growth conditions.
Although the clones described here were isolated after 460 generations or 266 days of selection, the ability to grow anaerobically on xylose as the sole carbon source was first detected after 270 generations, immediately after the culture conditions were changed to anaerobic conditions (<200 ppm of O2). The phenotype of the best xylose-utilizing clone TMB3001C1, which had a maximum specific growth rate of 0.012 h−1 and a biomass yield of 0.021 g g−1 during strictly anaerobic growth on xylose, by no means represents the final stage of evolution. For instance, the anaerobic growth rate of TMB3001C1 could be more than doubled by 40 generations of batch culture selection (Fig. 6). Although the rate of anaerobic xylose metabolism is still relatively low, isolation of the improved clones argues against the view that eukaryotic xylose metabolism is necessarily tied to respiration (16). Our results are more consistent with the view that anaerobic growth on xylose does not occur naturally in yeasts because the rate of xylose metabolism is too low, so that the rate of ATP production is insufficient (10, 17). Since the strains evolved here consumed xylose at severalfold-higher specific rates than, for example, control strain TMB3001, one would expect that the accumulated beneficial mutations affect, at least in part, the rate of catabolism and thus ATP formation. The nature of the underlying genetic changes that cause the observed phenotypic changes remains unclear at present and is the subject of further investigation in our lab. Multiple mutations were probably necessary to endow TMB3001 with the ability to grow under strictly anaerobic conditions on xylose, since direct selection on plates, in batch cultures, or in chemostat cultures was not successful. This may also explain to some extent why intense rational metabolic engineering efforts have not yielded such strains (2, 10, 13).
After 460 generations, the population consisted of at least two subpopulations with distinct phenotypes and thus exhibited the heterogeneity (or polymorphism) that is often observed during evolution experiments (11, 18, 23, 29, 34). The phenotype of the smaller class I subpopulation, representing one-third of the clones isolated, was rather similar to that of parental strain TMB3001 on glucose but was significantly improved on xylose. The best representative of these clones, TMB3001C5, exhibited a 60% higher specific xylose uptake rate and a fourfold-higher aerobic growth rate on xylose as the sole carbon source than TMB3001 exhibited (Table 2). Consequently, this strain accumulated up to 19% more ethanol when it was grown anaerobically under process-like conditions in a mixture containing 50 g of glucose per liter and 50 g of xylose per liter (Fig. 3C). None of the class I clones, however, grew anaerobically on xylose as the sole carbon source, either under strictly anaerobic conditions or in the presence of contaminating O2. The phenotype of the more abundant class II subpopulation was characterized by even more improved xylose metabolism, and the clones were also able to grow on xylose under strictly anaerobic conditions. The best representative of this subpopulation, TMB3001C1, exhibited a more-than-threefold-higher specific xylose uptake rate and an eightfold-higher aerobic growth rate on xylose than TMB3001 exhibited (Table 2). All of the class II clones grew more slowly and less efficiently on glucose than TMB3001 grew and exhibited significantly increased overflow metabolism to acetate and glycerol (Fig. 4), indicating that there was a drastic reorganization of the central metabolism.
The inability of the class I subpopulation to grow anaerobically on xylose as the sole carbon source is surprising because it stably reproduced in the anaerobic selection chemostat. Moreover, the maximum anaerobic growth rate of all of the clones isolated on xylose was significantly lower than the D in the anaerobic selection chemostat; thus, these clones would be expected to wash out. The most likely explanation for this obvious discrepancy is the O2 contamination (<200 ppm) in the technical N2 that was used to establish anaerobic conditions in the bioreactor. This contamination was independently verified by mass spectroscopy (data not shown). Although the class I clone TMB3001C5 was incapable of anaerobic growth on xylose even in the presence of contaminating O2, we showed that this clone could grow on ethanol and possibly other metabolic by-products of the class II clones with the contaminating O2 as an external electron acceptor. Likewise, the growth rate of the class II clone TMB3001C1 was significantly higher than the D of the anaerobic selection chemostat when the clone was cultivated with contaminating O2. This finding is also consistent with the obvious absence of strong selection pressure for a high anaerobic growth rate on xylose during chemostat selection, since faster-growing clones were readily selected within comparatively few generations in strictly anaerobic batch cultures.
The strategy which we used was a fruitful combination of rational metabolic engineering to render a strain amenable to selection and evolutionary techniques. Recently, two industrial ethanol-producing strains were metabolically engineered with the same xylose utilization pathway that was used here (35). Compared to these industrial strains, the evolved strains described here accumulated less xylitol, and some of the clones had higher xylose consumption rates (e.g., TMB3001C1). Moreover, the engineered industrial strains produced only about 8% more ethanol than TMB3001 produced from a mixture of glucose and xylose (35), while our best clone, TMB3001C5, produced about 19% more ethanol than TMB3001 produced (Fig. 3). Evolutionary engineering that enables or improves substrate utilization is not confined to the recombinant strain used here but can in principle be applied to other substrates or organisms (e.g., the industrial strains described above). As is the case for many pentoses in yeasts (5, 15), the organism subjected to selection should have the genetic potential to utilize the new substrate. Evolution may then be used to improve substrate utilization or to improve substrate utilization under novel conditions. While simpler traits may be directly selected for (20, 27), more complex, multigene modifications require an evolutionary approach for stepwise improvement (24).
Acknowledgments
We thank Bärbel Hahn-Hägerdal for sharing S. cerevisiae TMB3001 with us.
This work was supported by the Swiss Bundesamt für Bildung und Wissenschaft within European Commission Framework V.
REFERENCES
- 1.Arensdorf, J. J., A. K. Loomis, P. M. DiGrazia, D. J. Monticello, and P. T. Pienkos. 2002. Chemostat approach for the directed evolution of biodesulfurization gain-of-function mutants. Appl. Environ. Microbiol. 68:691-698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aristidou, A., and M. Penttilä. 2000. Metabolic engineering applications to renewable resource utilization. Curr. Opin. Biotechnol. 11:187-198. [DOI] [PubMed] [Google Scholar]
- 3.Arnold, F. H., and A. Volkov. 1999. Directed evolution of biocatalysts. Curr. Opin. Chem. Biol. 3:54-59. [DOI] [PubMed] [Google Scholar]
- 4.Bailey, J. E. 1999. Lessons from metabolic engineering for functional genomics and drug discovery. Nat. Biotechnol. 17:616-618. [DOI] [PubMed] [Google Scholar]
- 5.Dien, B. S., C. P. Kurtzman, B. C. Saha, and R. J. Bothast. 1996. Screnning for l-arabinose fermenting yeasts. Appl. Biochem. Biotechnol. 57/58:233-242. [PubMed] [Google Scholar]
- 6.Dykhuizen, D. E., and D. L. Hartl. 1983. Selection in chemostats. Microbiol. Rev. 47:150-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eliasson, A., C. Christensson, C. F. Wahlbom, and B. Hahn-Hägerdal. 2000. Anaerobic xylose fermentation by recombinant Saccharomyces cerevisiae carrying XYL1, XYL2, and XKS1 in mineral medium chemostat cultures. Appl. Environ. Microbiol. 66:3381-3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fiaux, J., Z. P. Çakar, M. Sonderegger, K. Wüthrich, T. Szyperski, and U. Sauer. 2003. Metabolic flux profiling of the yeasts Saccharomyces cerevisiae and Pichia stipitis. Eukaryot. Cell 2:170-180. [DOI] [PMC free article] [PubMed]
- 9.Gombert, A. K., M. M. dos Santos, B. Christensen, and J. Nielsen. 2001. Network identification and flux quantification in the central metabolism of Saccharomyces cerevisiae under different conditions of glucose repression. J. Bacteriol. 183:1441-1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hahn-Hägerdal, B., C. F. Wahlbom, M. Gardonyi, W. H. van Zyl, R. R. Cordero Otero, and L. J. Jönsson. 2001. Metabolic engineering of Saccharomyces cerevisiae for xylose utilization. Adv. Biochem. Eng. Biotechnol. 73:53-84. [DOI] [PubMed] [Google Scholar]
- 11.Helling, R. B., C. N. Vargas, and J. Adams. 1987. Evolution of Escherichia coli during growth in a constant environment. Genetics 116:349-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ho, N. W. Y., Z. Chen, and A. P. Brainard. 1998. Genetically engineered Saccharomyces yeast capable of effective cofermentation of glucose and xylose. Appl. Environ. Microbiol. 64:1852-1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ho, N. W. Y., Z. Chen, A. P. Brainard, and M. Sedlak. 1999. Successful design and development of genetically engineered Saccharomyces yeasts for effective cofermentation of glucose and xylose from cellulosic biomass to fuel ethanol. Adv. Biochem. Eng. Biotechnol. 65:163-192. [DOI] [PubMed] [Google Scholar]
- 14.Jeffries, T. W. 1983. Utilization of xylose by bacteria, yeasts, and fungi. Adv. Biochem. Eng. Biotechnol. 27:1-32. [DOI] [PubMed] [Google Scholar]
- 15.Jeffries, T. W., and C. P. Kurtzman. 1994. Strain selection, taxonomy, and genetics of xylose-fermenting yeasts. Enzyme Microb. Technol. 16:922-932. [Google Scholar]
- 16.Jeffries, T. W., and N.-Q. Shi. 1999. Genetic engineering for improved xylose fermentation by yeasts. Adv. Biochem. Eng. Biotechnol. 65:117-161. [DOI] [PubMed] [Google Scholar]
- 17.Kötter, P., and M. Ciriacy. 1993. Xylose fermentation by Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 38:776-783. [Google Scholar]
- 18.O'Kennedy, R. D., and J. W. Patching. 1999. The isolation of strains of Saccharomyces cerevisiae showing altered plasmid stability characteristics by means of selective continuous culture. J. Biotechnol. 69:203-214. [DOI] [PubMed] [Google Scholar]
- 19.Ostergaard, S., L. Olsson, and J. Nielsen. 2000. Metabolic engineering of Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 64:34-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parekh, S., V. A. Vinci, and R. J. Strobel. 2000. Improvement of microbial strains and fermentation processes. Appl. Microbiol. Biotechnol. 54:287-301. [DOI] [PubMed] [Google Scholar]
- 21.Patnaik, R., S. Louie, V. Gavrilovic, K. Perry, W. P. C. Stemmer, C. M. Ryan, and S. del Cardayre. 2002. Genome shuffling of Lactobacillus for improved acid tolerance. Nat. Biotechnol. 20:707-712. [DOI] [PubMed] [Google Scholar]
- 22.Rohlin, L., M.-K. Oh, and J. C. Liao. 2001. Microbial pathway engineering for industrial processes: evolution, combinatorial biosynthesis and rational design. Curr. Opin. Microbiol. 4:330-335. [DOI] [PubMed] [Google Scholar]
- 23.Rosenzweig, R. F., R. R. Sharp, D. S. Treves, and J. Adams. 1994. Microbial evolution in a simple unstructured environment: genetic differentiation in Escherichia coli. Genetics 137:1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sauer, U. 2001. Evolutionary engineering of industrially important microbial phenotypes. Adv. Biochem. Eng. Biotechnol. 73:129-170. [DOI] [PubMed] [Google Scholar]
- 25.Schneider, K.-H., G. Jäkel, R. Hoffmann, and F. Giffhorn. 1995. Enzyme evolution in Rhodobacter sphaeroides: selection of a mutant expressing a new galactitol dehydrogenase and biochemical characterization of the enzyme. Microbiology 141:1865-1873. [DOI] [PubMed] [Google Scholar]
- 26.Stafford, D. E., and G. Stephanopoulos. 2001. Metabolic engineering as an integrating platform for strain development. Curr. Opin. Microbiol. 4:336-340. [DOI] [PubMed] [Google Scholar]
- 27.Teunissen, A., F. Dumortier, M. F. Gorwa, J. Bauer, A. Tanghe, A. Loïez, P. Smet, P. Van Dijck, and J. M. Thevelein. 2002. Isolation and characterization of a freeze-tolerant diploid derivative of an industrial baker's yeast strain and its use in frozen doughs. Appl. Environ. Microbiol. 68:4780-4787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Toivari, M. H., A. Aristidou, L. Ruohonen, and M. Penttilä. 2001. Conversion of xylose to ethanol by recombinant Saccharomyces cerevisiae: importance of xylulokinase (XKS1) and oxygen availability. Metab. Eng. 3:236-249. [DOI] [PubMed] [Google Scholar]
- 29.Treves, D. S., S. Manning, and J. Adams. 1998. Repeated evolution of an acetate-crossfeeding polymorphism in long-term populations of Escherichia coli. Mol. Biol. Evol. 15:789-797. [DOI] [PubMed] [Google Scholar]
- 30.Verduyn, C., E. Postma, W. A. Scheffers, and J. P. van Dijken. 1992. Effect of benzoic acid on metabolic fluxes in yeasts: a continuous-culture study on the regulation of respiration and alcoholic fermentation. Yeast 8:501-517. [DOI] [PubMed] [Google Scholar]
- 31.Votruba, J., and J. Paca. 1992. Phenomenological theory of substrate-induced acidification with application to Candida utilis dissimilating ethanol. Folia Microbiol. 37:133-139. [DOI] [PubMed] [Google Scholar]
- 32.Walfridsson, M., X. Bao, M. Anderlund, G. Lilius, L. Bülow, and B. Hahn-Hägerdal. 1996. Ethanolic fermentation of xylose with Saccharomyces cerevisiae harboring the Thermus thermophilus xylA gene, which expresses an active xylose (glucose) isomerase. Appl. Environ. Microbiol. 62:4648-4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walfridsson, M., J. Hallborn, M. Penttilä, S. KerDenen, and B. Hahn-Hägerdal. 1995. Xylose-metabolizing Saccharomyces cerevisiae strains overexpressing the TKL1 and TAL1 genes encoding the pentose phosphate pathway enzymes transketolase and transaldolase. Appl. Environ. Microbiol. 61:4184-4190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weikert, C., U. Sauer, and J. E. Bailey. 1997. Use of a glycerol-limited, long-term chemostat for isolation of Escherichia coli mutants with improved physiological properties. Microbiology 143:1567-1574. [DOI] [PubMed] [Google Scholar]
- 35.Zaldivar, J., A. Borges, B. Johansson, H. P. Smits, S. G. Villas-Boas, J. Nielsen, and L. Olsson. 2002. Fermentation performance and intracellular metabolite patterns in laboratory and industrial xylose-fermenting Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 59:436-442. [DOI] [PubMed] [Google Scholar]
- 36.Zelder, O., and B. Hauer. 2000. Environmentally directed mutations and their impact on industrial biotransformation and fermentation processes. Curr. Opin. Microbiol. 3:248-251. [DOI] [PubMed] [Google Scholar]