Abstract
Lactobacillus brevis is a promising lactic acid bacterium for use as a probiotic dietary adjunct and a vaccine vector. The N-terminal region of the S-layer protein (SlpA) of L. brevis ATCC 8287 was recently shown to mediate adhesion to various human cell lines in vitro. In this study, a surface display cassette was constructed on the basis of this SlpA receptor-binding domain, a proteinase spacer, and an autolysin anchor. The cassette was expressed under control of the nisA promoter in Lactococcus lactis NZ9000. Western blot assay of lactococcal cell wall extracts with anti-SlpA antibodies confirmed that the SlpA adhesion domain of the fusion protein was expressed and located within the cell wall layer. Whole-cell enzyme-linked immunosorbent assay and immunofluorescence microscopy verified that the SlpA adhesion-mediating region was accessible on the lactococcal cell surface. In vitro adhesion assays with the human intestinal epithelial cell line Intestine 407 indicated that the recombinant lactococcal cells had gained an ability to adhere to Intestine 407 cells significantly greater than that of wild-type L. lactis NZ9000. Serum inhibition assay further confirmed that adhesion of recombinant lactococci to Intestine 407 cells was indeed mediated by the N terminus-encoding part of the slpA gene. The ability of the receptor-binding region of SlpA to adhere to fibronectin was also confirmed with this lactococcal surface display system. These results show that, with the aid of the receptor-binding region of the L. brevis SlpA protein, the ability to adhere to gut epithelial cells can indeed be transferred to another, nonadhesive, lactic acid bacterium.
Lactobacilli are commonly found microorganisms in nature and have been widely used in dairy and other food fermentations for centuries. Many Lactobacillus species are also members of the normal microbiota of the human and animal gastrointestinal and genitourinary tracts. Various Lactobacillus species are used commercially as probiotics because of their proposed beneficial effects on the health of the host. Among the requirements commonly suggested for a microorganism to be classified as probiotic are the ability to adhere to gut epithelial tissue and the ability to at least transiently colonize the gastrointestinal tract (10). Compared with the present knowledge of the adhesive mechanisms of many pathogenic bacteria, only limited information is available about the adhesive surface molecules of lactobacilli and their tissue receptors. A recent study suggested fibronectin as one of the eukaryotic receptors that mediate the adhesion of lactobacilli to epithelial cells (16). Lectin-like molecules (25) and lipoteichoic acids (11, 34) have also been shown to function as adhesins in lactobacilli. In several cases, however, the adhesion of lactobacilli has been reported to be protein mediated (1, 7, 8, 22), but thus far, only a few proteinaceous adhesins have been identified, including a protein component of the bacterial ATP-binding cassette (29), an S-layer protein (33, 36), a surface protein that is able to bind to porcine intestinal mucus and gastric mucin (28), and a Mub protein adhering to mucus components (30).
S-layers are regular paracrystalline surface protein arrays commonly found in species belonging to all major phylogenetic groups of Bacteria and are an almost universal feature of Archaea (reviewed in reference 32). Most S-layers are composed of a single protein or glycoprotein species that forms the S-layer lattice by an intrinsic self-assembly process. Diverse functions have been proposed for S-layers, like acting as cell-protective coats, molecular sieves, molecular and ion traps, and cell adhesion mediators (reviewed in reference 37).
S-layers are also found in some species of the genus Lactobacillus (24, 44). To date, only a few S-layer genes of lactobacilli have been described (4, 6, 36, 41). The functions of lactobacillary S-layers are poorly known. In Lactobacillus acidophilus, the S-layer has been proposed to mediate binding to avian intestinal epithelial cells (33), and in Lactobacillus crispatus, the S-protein (CbsA) has been shown to mediate binding to collagen (40). Flagellar display experiments with Escherichia coli have shown that the S-layer protein (SlpA) of Lactobacillus brevis ATCC 8287 mediates adhesion to human intestinal cell lines and fibronectin in vitro via a binding region located within the N-terminal part of the SlpA protein (15).
In this report, we describe the successful transfer and expression of the SlpA receptor-binding region in Lactococcus lactis and demonstrate that nonadhesive lactococci can be endowed with the ability to adhere to a human intestinal epithelial cell line and fibronectin with the aid of the SlpA adhesin. L. lactis was chosen as the model host because of its amenability to genetic engineering, the availability of a wide variety of genetic tools for this bacterium, and its wide experimental use as a producer of bioactive molecules.
MATERIALS AND METHODS
Bacterial strains, plasmids, cell line, and growth media.
The strains and plasmids used in this study are listed in Table 1. Lactobacillus strains were grown in MRS (Difco, Detroit, Mich.) at 37°C. Lactococcus strains were propagated in M17 (Difco) containing 0.5% (wt/vol) glucose at 30°C without shaking or with gentle shaking (60 rpm) for the fibronectin adhesion assay. E. coli strains were grown in Luria-Bertani medium at 37°C under aeration. Antibiotics were used, when appropriate, at the following concentrations: chloramphenicol, 5 μg/ml (for L. lactis); ampicillin, 50 μg/ml (for E. coli).
TABLE 1.
Strains, plasmids, and cell line used in this study
| Strain, plasmid, or cell line | Relevant propertiesa | Reference or source |
|---|---|---|
| Strains | ||
| Lactobacillus brevis ATCC 8287 | ATCC | |
| Lactococcus lactis NZ9000 | pepN::nisR nisK | 9 |
| Lactococcus lactis NCDO712 | Lac+ Prt+ | NCDOb |
| Escherichia coli DH5α | Transformation host | 12 |
| Plasmids | ||
| pUC19 | Apr; E. coli cloning vector | 44 |
| pKTH5033 | pUC19 derivative containing 1.5-kb prtP spacer | This study |
| pKTH5042 | pUC19 derivative containing 0.6-kb prtP spacer | This study |
| pNG101his | Cmr; 4.6 kb; pNG101 derivative carrying His tag and autolysin anchor transcriptionally fused to nisA promoter | K. Leenhouts |
| pKTH5043 | pNG101his derivative carrying 0.6-kb prtP spacer fused to nisA promoter | This study |
| pKTH5046 | pNG101his derivative carrying 1.5-kb prtP spacer fused to nisA promoter | This study |
| pKTH5050 | pKTH5043 derivative carrying mature part of bla gene and 0.6-kb prtP spacer fused to nisA promoter | This study |
| pKTH5051 | pKTH5046 derivative carrying mature part of bla gene and 1.5-kb prtP spacer fused to nisA promoter | This study |
| pKTH5056 | pKTH5046 derivative carrying adhesion region from slpA gene (coding for amino acids 1-247) and 1.5-kb prtP spacer fused to nisA promoter | This study |
| Cell line Intestine 407 | Human intestinal epithelial cell line ATCC CCL-6 | ATCC |
Apr, resistance to ampicillin; Cmr, resistance to chloramphenicol.
NCDO, National Collection of Dairy Organisms.
The Intestine 407 cell line was obtained from the American Type Culture Collection (ATCC). Intestine 407 cells were routinely grown at 37°C in a 95% air-5% CO2 atmosphere in RPMI 1640 medium (Life Technologies, Paisley, Scotland) supplemented with 2 mM l-glutamine, 1% heat-inactivated (30 min 56°C) fetal calf serum, 50 U of penicillin per ml, 50 μg of streptomycin per ml, and a 1% (vol/vol) minimal essential medium nonessential amino acid solution (Life Technologies). For adhesion assays, Intestine 407 cell monolayers were prepared by inoculating eight-well microscope slides (Knittel, Braunschweig, Germany) with 25 μl of a freshly diluted (in growth medium) cell suspension, incubating them for 1 h at 37°C, and then covering the slides with growth medium and further incubating them for 2 days at 37°C. The slides were washed once with phosphate-buffered saline (PBS) before the adhesion assays.
DNA methods and transformation.
Routine molecular biology techniques were used (2). Enzymes were used as recommended by the manufacturers (Promega, Madison, Wis.; New England Biolabs Inc., Beverly, Mass.). Plasmid DNA was isolated from L. lactis and L. brevis by using the QIAfilter Plasmid Midi Kit (Qiagen GmbH, Hilden, Germany) and 8 mg of lysozyme per ml and from E. coli by using the Wizard Minipreps kit (Promega). L. lactis and E. coli cells were transformed as described by Holo and Nes (14) and Sambrook and Russell (31), respectively. Correct PCR amplification was verified by using an ABI Prism 310 genetic analyzer (Applied Biosystems, Foster City, Calif.) in combination with the DNA sequencing kit for BigDye terminator cycle sequencing (Applied Biosystems).
Construction of plasmid vectors.
The proteinase (prtP) gene sequence of L. lactis subsp. cremoris Wg2 (EMBL accessions number M24767) was used to design primers for the amplification of 0.6- and 1.5-kb prtP spacers. For both prtP spacers, the same downstream primer, 5′-CGAAGAATTCGAGTCGTATCATCCGTGC-3′ (EcoRI site underlined), was used for PCR amplification. The upstream primers with an NcoI site (underlined) for the 0.6- and 1.5-kb prtP fragments were 5′-TTACCCATGGGTGCC AATCGAGACC-3′ and 5′-CGAACCATGGTCAAGCACCCAACG-3′, respectively. Plasmid DNA isolated from strain L. lactis NCDO712 was used as the template in PCRs. The resulting PCR products, 0.6- and 1.5-kb prtP fragments, were treated with Klenow polymerase to generate blunt ends, digested with EcoRI, and cloned into EcoRI- and SmaI-digested pUC19 (41), resulting in plasmids pKTH5042 and pKTH5033, respectively (Table 1). The prtP spacers were isolated as NcoI-EcoRI fragments from pKTH5042 and pKTH5033 and cloned into the NcoI-EcoRI sites of pNG101his (Table 1), resulting in plasmids pKTH5043 and pKTH5046, respectively.
For expression of the mature β-lactamase protein (Bla), its gene (bla) was PCR amplified from pUC19 by using primers 5′-TACTTCTAGACCACCCAGAAACGCTGG-3′ and 5′-ATACTCTAGAGCTTACCAATGCTTAATCAG-3′ with an XbaI site (underlined). The approximately 0.8-kb PCR fragment obtained was digested with XbaI and cloned into the XbaI sites of pKTH5043 and pKTH5046, resulting in plasmids pKTH5050 and pKTH5051, respectively.
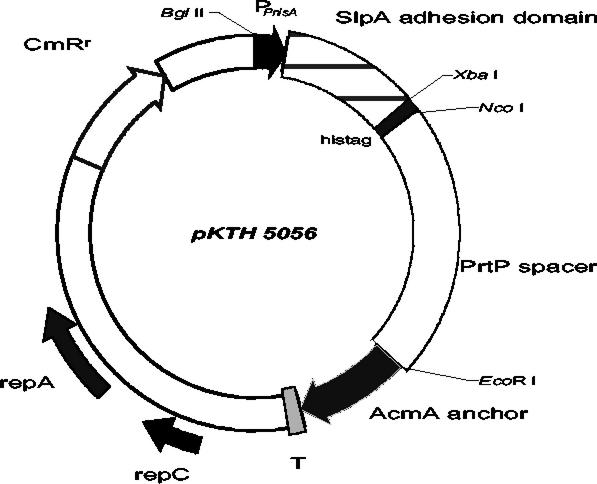
To create an exact joint between the nisA promoter and the signal sequence of the L. brevis slpA gene, the recombinant PCR technique was applied essentially as described earlier (18). Briefly, the nisA promoter fragment was amplified from pNG101his with primers 5′-CCAAGATCTAGTCTTATAACTATACTG (P1; BglII site underlined) and 5′-TAAACTTGATTGCATGGTGAGTGCCTC (P2), resulting in an approximately 0.2-kb fragment. The DNA region encoding the SlpA signal peptide and the SlpA receptor-binding region (amino acids 31 to 247) were synthesized by using primers 5′-GAGGCACTCACCATGCAATCAAGTTTAAAG-3′ (P3) and 5′-CACATCTAGACTGTTATCGTTGGTTGC-3′ (P4; XbaI site underlined), resulting in an approximately 0.7-kb fragment. The 0.2- and 0.7-kb PCR fragments were hybridized, and the hybrid formed was extended by DNA polymerase in a PCR without primers, followed by a further PCR amplification in the presence of the primer pair P1-P4. The resulting 0.9-kb fragment was purified, digested with BglII and XbaI, and ligated with BglII-XbaI-digested pKTH5046, resulting in plasmid pKTH5056 (Fig. 1).
FIG. 1.
Schematic drawing of vector pKTH5056, which was constructed for the controlled expression of the surface-anchored adhesion domain of L. brevis SlpA in L. lactis. PnisA, promoter sequence of the nisA gene of L. lactis; AcmA anchor, anchor peptide from the L. lactis acmA gene; PrtP spacer, 515-amino-acid-encoding region of the L. lactis subsp. cremoris Wg2 prtP gene; T, transcription terminator sequence; CmRr, chloramphenicol resistance gene.
Nisin induction.
For controlled expression, nisin induction of recombinant L. lactis strains was performed as follows. From a culture grown overnight, a 3% (vol/vol) inoculum was transferred to fresh medium and the bacteria were grown at 30°C until the optical density at 600 nm was 0.2 to 0.3. Nisin was added at different concentrations (0.8 to 10 ng/ml), and cells were propagated for 4 to 20 h before harvesting.
Whole-cell ELISA for detection of cell surface-exposed polypeptides.
Recombinant L. lactis cells harboring pKTH5050, pKTH5051, or pKTH5056 were harvested after nisin induction or overnight incubation. Cell surface-displayed polypeptides were assayed with a whole-cell enzyme-linked immunosorbent assay (ELISA) essentially as described earlier (2a). The primary antibodies used were anti-SlpA serum (41) or anti-Bla serum (kindly provided by Matti Sarvas, National Public Health Laboratory).
Protein analysis.
The total protein extracts were prepared from cells harvested after 4 h of nisin induction. The bacteria were resuspended in 500 μl of digestion buffer (50 mM HEPES [pH 7.0], 20% [wt/vol] sucrose, 5 mM MgCl2, 5 mM CaCl2, 10 mg of lysozyme per ml, 42 U of mutanolysin per ml). The digestion reactions were allowed to proceed for 90 min at 37°C in a water bath before the mixtures were chilled and disrupted by sonication for 30 s, resulting in total protein extracts that were mixed with Laemmli buffer (19).
The cell wall-associated polypeptides were extracted as follows. Recombinant L. lactis cells harboring pKTH5056 were harvested after nisin induction (4 h) or from overnight culture. The bacteria were resuspended in 1 ml of 50 mM HEPES (pH 7.0) and centrifuged. Lactococcal cells were disrupted by homogenization with glass beads in a cell mill (Bühler Vibrogen Cell Mill VL4; Edmund Bühler GmbH, Bodelshausen, Germany) for 60 s and resuspended in 500 μl of 50 mM HEPES (pH 7.0). After centrifugation (1,000 × g, 5 min, 4°C), the supernatant containing the cell walls and membranes was recovered and centrifuged again (18,500 × g, 30 min, 4°C) in order to pellet the cell wall-associated material. The pellet was resuspended in digestion buffer and incubated for 90 min at 37°C in a water bath for solubilization of the cell walls, followed by addition of Laemmli buffer.
The protein samples were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and blotted (MiniProtean II; Bio-Rad) onto nitrocellulose membranes. Polyclonal rabbit anti-SlpA antibodies (40) and horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin G (Bio-Rad) were used as primary and secondary antibodies, respectively. Bands were detected by addition of 4-chloro-1-naphthol (horseradish peroxidase color development reagent; Bio-Rad). Semiquantitation of the S-layer and fusion protein (from plasmid pKTH5056) bands was carried out by using a Multi-Analyst (Bio-Rad). Intensity values (numbers of pixels per unit of band area) were adjusted with cell amounts loaded onto the SDS-PAGE gel.
Immunofluorescence assay for detection of peptides on the cell surface of L. lactis.
Detection of SlpA on the cell surface by immunofluorescence assay was performed essentially as described previously (2a). The slides were incubated for 1 h with undiluted or diluted (1:5 to 1:20 in PBS) anti-SlpA antibody (unspecific binding to L. lactis cells was first removed by incubating the serum with L. lactis NZ9000 cells for 4 h at 4°C) in a moist chamber at 37°C, washed twice with PBS, and finally incubated with fluorescein isothiocyanate-conjugated swine anti-rabbit immunoglobulin G serum (dilution, 1:10; DAKO A/S, Glostrup, Denmark) for 1 h in a moist chamber at 37°C.
Bacterial adhesion assays.
Bacteria were harvested from overnight cultures or after nisin induction. For the assay of adhesion to epithelial cells, bacteria were resuspended in RPMI medium and the optical densities at 600 nm of these suspensions were adjusted to approximately 0.8. From these cell suspensions, 10 μl was added to each well of the Intestine 407 monolayer in microscope slides and incubated for 1 h at 37°C in a moist chamber. After treatment, the monolayers were washed five times with PBS at room temperature for 10 min with gentle agitation and fixed for 10 min with methanol. Air-dried slides were stained with Giemsa (Merck Eurolab, Darmstadt, Germany) and examined by light microscopy.
The statistical significance of the adherence capabilities of the bacterial cells was evaluated by one-way analysis of variance, and pairwise differences between the means of groups were determined by the Tukey HSD test for post-analysis-of-variance pairwise comparisons (available at http://faculty.vassar.edu/lowry/VassarStats.html). Differences were considered significant when P values were less than 0.01.
The ability of anti-SlpA serum to prevent the bacterial adherence to Intestine 407 cells was examined by incubating cells with diluted (1:20 in PBS) anti-SlpA serum (or with PBS as a negative control) for 2 h at room temperature. The bacterial cells were washed with PBS after treatment, resuspended in RPMI medium, and applied to Intestine 407 monolayer as described earlier. Bacterial adherence to Intestine 407 cells was scored as the average number of bacteria attached to one Intestine 407 cell (approximately 100 Intestine 407 cells per sample were counted). Student's t test for unpaired values was used to evaluate the statistical significance of the differences in adhesion of lactococcal cells after anti-SlpA antibody treatment compared with the control (PBS treatment). Differences were considered significant when P values were less than 0.01.
Bacterial adhesion to human plasma fibronectin (Becton Dickinson, Bedford, Mass.) was performed as described before (43), with the following modifications. Fibronectin was used at a surface concentration of 46 fmol/mm2, and the slides were blocked with 1% (wt/vol) blocking reagent (Roche) in PBS and washed twice with 0.25% blocking reagent in PBS and once with PBS. The time of incubation with bacteria in PBS was 3 h, and nonadherent bacteria were removed by washing 3 × 10 min in PBS.
The statistical significance of differences between the abilities of bacteria to adhere to fibronectin was analyzed as described for bacterial adherence to Intestine 407 cells.
RESULTS
Construction of expression vectors for surface display.
A derivative of the pNG101his vector (Table 1), allowing expression of surface-located proteins, was constructed. The pNG101his vector carries, under control of the nisA promoter, the signal sequence of the L. lactis acmA gene, a multicloning site, and a gene fragment encoding the cell wall-anchoring regions of the lactococcal AcmA protein (5). To increase the surface accessibility of hybrid proteins to be expressed, we used parts of the L. lactis subsp. cremoris Wg2 prtP gene (17) to construct spacers 0.6 and 1.5 kb in size. The 0.6- and 1.5-kb prtP inserts were cloned into pNG101his in frame with the AcmA cell wall-anchoring gene fragment under the nisA promoter, resulting in plasmids pKTH5043 and pKTH5046, respectively (Table 1).
Characterization of surface accessibility with a β-lactamase reporter.
To establish a reporter system with which to study the utility of the spacers, the β-lactamase gene (bla) was cloned into pKTH5043 and pKTH5046, resulting in plasmids pKTH5050 and pKTH5051, respectively. Thus, pKTH5050 and pKTH5051 encode a fusion protein consisting of the AcmA signal peptide, the mature β-lactamase (Bla), one of the PrtP spacers, and the AcmA cell wall anchor. The surface accessibility of Bla in these chimeric fusion proteins was assessed by whole-cell ELISA with anti-Bla antibodies. The color response obtained with nisin-induced L. lactis strain NZ9000 harboring pKTH5051 was clearly more intense than that of L. lactis NZ9000 carrying pKTH5050 and those of uninduced cells (data not shown). The result indicated that the PrtP1153-1668 spacer encoded by the 1.5-kb prtP fragment present in pKTH5051 gave better surface accessibility of Bla than did the PrtP1453-1668 gene product encoded by the 0.6-kb prtP fragment of pKTH5050.
Surface display vector for expression of the SlpA adhesion domain in lactococci.
On the basis of the results obtained with the whole-cell ELISA of L. lactis cells carrying pKTH5051, the SlpA adhesion-mediating region (amino acids 31 to 247) was cloned into pKTH5046, which carries the same 1.5-kb PrtP spacer as pKTH5051 (Table 1). To maintain a homologous signal sequence and a mature protein structure, the acmA signal sequence was replaced with that of the L. brevis slpA gene, resulting in the vector pKTH5056 (Fig. 1).
Cellular localization of the SlpA adhesion domain fused to PrtP and AcmA.
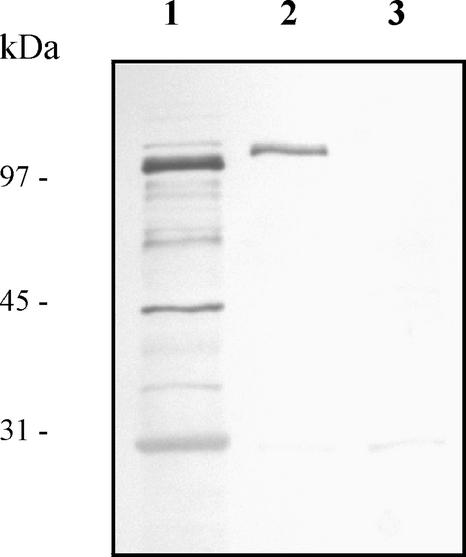
The expression and cellular location of the SlpA-PrtP-AcmA fusion protein were studied with nisin-induced L. lactis NZ9000 cells harboring pKTH5056 by Western blot analysis of total protein extracts and cell wall-associated polypeptides. After separation by SDS-PAGE, blots were probed with anti-SlpA antibodies. As illustrated in Fig. 2 (lane 2), a band corresponding to the expected molecular mass (111 kDa) of the fusion protein was detected among the cell wall-associated polypeptides extracted from L. lactis with pKTH5056. A strong fusion protein band was also detected in the total protein extracts (Fig. 2, lane 1) from induced recombinant L. lactis cells, indicating efficient gene expression from pKTH5056. In contrast, no band corresponding to the fusion protein was detected in the cell wall-associated polypeptide extracts from uninduced L. lactis with pKTH5056 (Fig. 2, lane 3). This assay thus suggested that L. lactis cells transformed with pKTH5056 expressed SlpA adhesion-mediating molecules within its surface structure.
FIG. 2.
Western blot analysis of the SlpA adhesion-mediating region. Total cellular proteins and cell wall-associated polypeptides were extracted from L. lactis NZ9000 cells harboring pKTH5056. The protein samples were prepared from equal amounts of cells as described in Materials and Methods, and samples were separated by SDS-PAGE, electroblotted onto a nitrocellulose membrane, and incubated with polyclonal SlpA antiserum diluted 1:1,000. Lanes: 1, total cellular proteins from nisin-induced recombinant cells; 2, cell wall-associated polypeptides from nisin-induced recombinant cells; 3, cell wall-associated polypeptides from noninduced recombinant cells. Sizes of molecular mass marker proteins are indicated on the left.
Surface accessibility of the SlpA receptor-binding region.
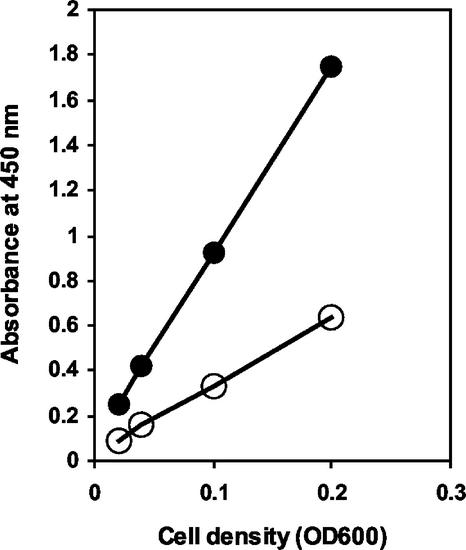
The surface accessibility of the SlpA adhesion-mediating region was studied by whole-cell ELISA and immunofluorescence microscopy. For the whole-cell ELISA, recombinant L. lactis NZ9000 cells harboring pKTH5056 were harvested after nisin induction and from an uninduced culture. The cells were incubated with anti-SlpA antibodies and assayed as described earlier for the Bla construct. The ELISA absorbance values of the induced recombinant lactococcal cells were clearly higher in every cell dilution than those of uninduced cells (Fig. 3). This result demonstrated that the pKTH5056-encoded SlpA receptor-binding region of the fusion protein was successfully displayed on the recombinant L. lactis cell surface.
FIG. 3.
Whole-cell ELISA for detection of the surface-exposed SlpA adhesion-mediating region encoded by pKTH5056. Anti-SlpA antibody was allowed to bind to lactococcal cells harvested from nisin-induced or uninduced cultures, and after the addition of a horseradish peroxidase conjugate, different lactococcal cell densities were incubated with a chromogenic substrate. Symbols: •, nisin-induced NZ9000(pKTH5056); ○, uninduced NZ9000(pKTH5056). OD600, optical density at 600 nm.
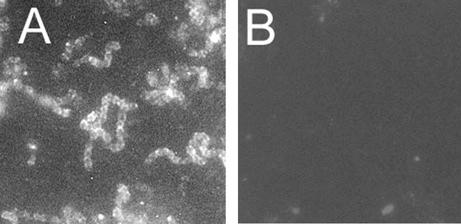
The result obtained with the whole-cell ELISA was further verified with immunofluorescence microscopy by testing the reactivity of nisin-induced and uninduced L. lactis NZ9000 cells carrying pKTH5056 and of wild-type L. lactis NZ9000 cells with anti-SlpA antibodies. The nisin-induced cells carrying pKTH5056 exhibited positive fluorescence when reacted with anti-SlpA antibodies (Fig. 4A), while no fluorescence was obtained with the wild-type L. lactis NZ9000 cells (Fig. 4B). No fluorescence was observed either when uninduced recombinant cells with pKTH5056 were incubated with anti-SlpA antibodies (data not shown). The immunofluorescence assay thus confirmed the surface accessibility of the SlpA adhesion-mediating region of the pKTH5056-encoded fusion protein.
FIG. 4.
Immunofluorescence microscopy of recombinant L. lactis NZ9000 cells harboring pKTH5056 (A) and wild-type L. lactis NZ9000 (B). Recombinant cells were harvested after overnight nisin induction, and wild-type cells were harvested after overnight incubation. Both cell types were treated with anti-SlpA antibodies (diluted 1:20 in PBS) and a fluorescein isothiocyanate-conjugated secondary antibody. Both pictures were taken after 16-s exposures. Magnification, ×4,300.
Adhesion of recombinant lactococci to Intestine 407 cells in vitro.
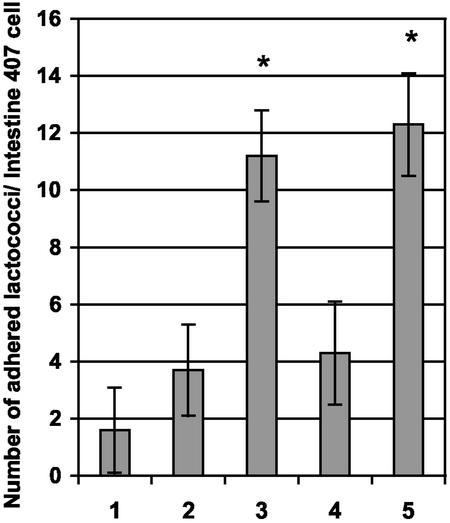
The ability of pKTH5056 carrying L. lactis NZ9000 cells to adhere to human intestinal epithelial cells was assayed by an in vitro adhesion test using Intestine 407 cells. The adhesion assay, testing nisin-induced recombinant L. lactis cells with pKTH5056 or pKTH5046 and wild-type L. lactis NZ9000 cells, indicated that L. lactis NZ9000 cells with pKTH5056 possessed an approximately sevenfold greater ability to adhere to Intestine 407 cells than wild-type NZ9000 cells, whereas an only three- to fourfold increase could be obtained compared to NZ9000 carrying pKTH5046 (Fig. 5). The binding of L. lactis cells with pKTH5056 to Intestine 407 cells was found to be statistically significantly (P < 0.01) greater than that of the negative control cells (wild-type NZ9000 and induced NZ9000 with pKTH5046).
FIG. 5.
Adherence of recombinant and wild-type L. lactis NZ9000 cells to Intestine 407 cells. The mean number of adherent lactococcal cells per Intestine 407 cell was determined from 16 randomized microscopic fields and is illustrated for L. lactis NZ9000 cells harboring no plasmid (bar 1), pKTH5046 (nisin induced) (bar 2), pKTH5056 (nisin induced) (bar 3), pKTH5056 (nisin induced and incubated with anti-SlpA antibodies before the adhesion assay) (bar 4), or pKTH5056 (nisin induced and incubated with PBS before the adhesion assay) (bar 5). The results shown are group means with 95% confidence intervals.*, P < 0.01.
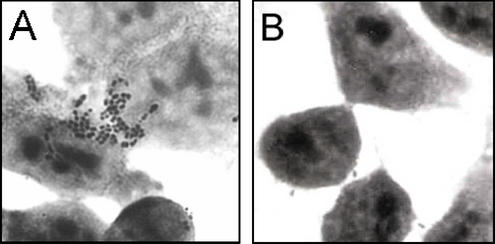
To obtain even more definitive evidence that the adhesion was indeed mediated by the N-terminal part of the L. brevis SlpA protein expressed by pKTH5056, a serum inhibition study was carried out. Nisin-induced recombinant NZ9000 cells with pKTH5056 were incubated with either anti-SlpA antibodies or PBS before use in adhesion assays with Intestine 407 cells. Pretreatment with SlpA antiserum inhibited the binding of induced NZ9000 cells with pKTH5056 to the level of negative controls (wild-type NZ9000 and induced NZ9000 with pKTH5046), and a statistically significant (P < 0.01) difference between the numbers of PBS-treated cells and cells treated with anti-SlpA antibodies bound to Intestine 407 cells was found (Fig. 5 and 6). Thus, the adhesion inhibition tests confirmed that the adherence to Intestine 407 cells of L. lactis NZ9000 harboring pKTH5056 was indeed mediated by the SlpA receptor-binding region encoded by pKTH5056.
FIG. 6.
Adhesion of recombinant L. lactis NZ9000 cells carrying pKTH5056 (A) and wild-type L. lactis NZ9000 (B) to Intestine 407 cells. Recombinant lactococcal cells were incubated with PBS for 2 h before the adhesion assay. Magnification, ×4,500.
Adhesion of recombinant lactococci to human plasma fibronectin in vitro.
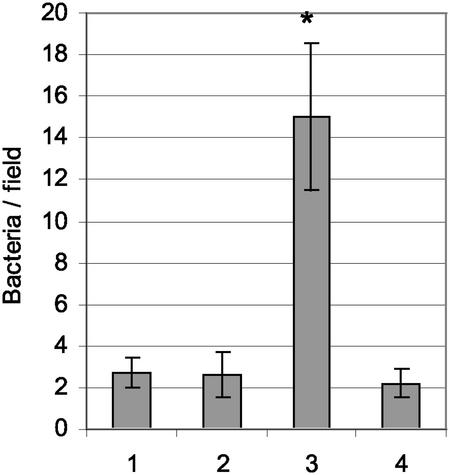
As the adherence of L. brevis ATCC 8287 SlpA to human fibronectin has recently been reported (15), we also tested the binding of induced and uninduced recombinant L. lactis carrying pKTH5056 or pKTH5046 and wild-type L. lactis NZ9000 cells to human fibronectin immobilized on glass slides. The results indicated that recombinant L. lactis with pKTH5056 adhered to fibronectin whereas wild-type L. lactis NZ9000 and recombinant L. lactis with pKTH5046, as well as all of the uninduced counterparts, showed no adherence over the background (Fig. 7). The difference in fibronectin binding between the recombinant L. lactis strain with pKTH5056 and the control strains was approximately sevenfold, further confirming the specificity of the SlpA region-mediated binding of lactococci.
FIG. 7.
Binding of recombinant and wild-type L. lactis NZ9000 to immobilized fibronectin. Means and standard deviations of bacterial numbers in 20 randomly chosen 4 × 102-μm2 microscopic fields are shown for L. lactis NZ9000 harboring no plasmid (bar 1), pKTH5046 (nisin induced) (bar 2), pKTH5056 (nisin induced) (bar 3), or pKTH5056 (uninduced) (bar 4). The concentration of bacteria was 5 × 108 ml−1. The results shown are group means with 95% confidence intervals. *, P < 0.01.
DISCUSSION
The probiotic research field has produced numerous studies in which the adhesion capabilities of lactobacilli have been tested. However, only a few lactobacillary adhesins have been characterized in detail, including the collagen-binding S-layer protein of Lactobacillus crispatus (36), a surface protein of Lactobacillus fermentum that is able to bind to porcine intestinal mucus and gastric mucin (28), a Mub protein adhering to mucus components (29), and the fibronectin-binding L. brevis SlpA protein (15) that was also further studied in this work. It is likely, however, that Lactobacillus strains express several different adhesins (15), and their detailed role in complex adhesion processes may be difficult to reveal, especially if genetic tools and gene-disrupting methods are lacking for the strain in question. A way to study, e.g., the receptor specificity of these binding proteins is to express the putative adhesin in a heterologous host lacking the adhesion property. So far, among lactobacilli, only expression of the L. crispatus collagen-binding S-layer protein (36) has been studied in another Lactobacillus species (23), whereas in lactococci, some heterologous adhesins of non-lactic acid bacterial origin have been tested (13, 27). Another rationale for transferring adhesin molecules in lactic acid bacteria is to provide nonadhesive mucosal delivery vehicles with a specific adhesion capability to test whether more efficient delivery of bioactive molecules of interest can be obtained.
In this work, we have demonstrated that the N-terminal region of the S-layer protein of L. brevis can be used to transform a naturally nonadhesive lactic acid bacterium into an adhesive one. This was achieved by constructing a surface display vector, pKTH5056, encoding the SlpA receptor-binding region as a fusion protein with the spacer and cell wall-anchoring regions of PrtP and AcmA, respectively. We used L. lactis as the host because this organism does not adhere to Intestine 407 cells (A. Palva et al., unpublished results), a wide range of genetic tools with which to study it are available, it has been successfully used for surface display of heterologous proteins (13, 26, 39), and it is being developed as a mucosal delivery vehicle (38, 42).
The cell wall attachment of the receptor-binding region of SlpA was accomplished by using an expression vector that has the cell wall-anchoring sequences of the L. lactis acmA gene (5). The AcmA repeat cell wall anchor has been previously used for surface expression of the Bacillus licheniformis α-amylase and the E. coli β-lactamase (5). The mechanism by which the acmA-encoded attachment domains interact with the cell wall components is unknown, but the interaction has been suggested to be noncovalent in nature (reviewed in reference 21).
A 515-amino-acid PrtP1153-1668-encoding region of the prtP gene of L. lactis subsp. cremoris Wg2, tested to allow efficient surface expression with the Bla reporter protein in pKTH5051, was used as the spacer protein to properly extend the SlpA receptor-binding region out of the cell surface. Surprisingly, with the shorter 215-amino-acid PrtP1453-1668 spacer tested, no surface expression of β-lactamase could be demonstrated. In addition to the length and conformation of a spacer, the nature of the protein to be surface displayed and its interactions with the cell wall molecules also affect the design of the spacer. The 515-amino-acid spacer amplified from the C terminus of the prtP gene spans the putative H domain and part of the W domain determined from PrtP of L. lactis strain SK11 (35). The function of the putative H domain has not been determined, but it has been suggested to act as a spacer directing other PrtP domains farther from the cell wall (35). In putative H domains, alpha-helixes are the dominant secondary structure. Spacers consisting of alpha-helixes of different lengths and origins have recently been used to enhance the expression of M6 protein on the cell surface of Streptococcus gordonii (3).
We chose a 247-amino-acid-long N-terminal region, including the signal peptide of 30 residues, from the L. brevis SlpA protein for surface expression and adhesion studies with L. lactis. This region was previously shown to include the receptor-binding region of SlpA, which reacts with fibronectin and several human epithelial cell types, by using a flagellar display system in E. coli (15). With flagellar display experiments, the binding domain of SlpA could be limited to 81 amino acid residues representing residues 96 through 176 in the unprocessed SlpA protein (15).
In the cell adhesion tests (Fig. 5), some adhesion background with NZ9000 carrying the control vector (pKTH5046) was observed. This is likely due to some aggregation observed both with the control and test vector-carrying strains. However, in the fibronectin assay (Fig. 7), where the lactococci were subjected to gentle agitation during propagation, the aggregation was not significant. Accordingly, the difference between the binding backgrounds with the control strains was almost nonexistent, demonstrating the specificity of the fibronectin binding.
Even though a significant ability to adhere to human epithelial Intestine 407 cells could be provided to L. lactis by the aid of the SlpA adhesion-mediating region, the epithelial cell-binding efficiency of L. lactis harboring pKTH5056 was substantially lower than that of the wild type L. brevis cells synthesizing a surface layer estimated to consist of approximately one-half million SlpA subunits (Palva et al., unpublished; 15). One obvious explanation for this is that the amount of the pKTH5056-encoded region of SlpA in L. lactis is only a few percent of the SplA subunits present in the native S-layer in L. brevis. We cannot, however, rule out the possibility that the receptor-binding domain of SlpA is more effective when displayed at a high density in the S-layer lattice. The assumption that entire S-layers possess more efficient binding capacity than their protein subunits is also supported by the results of studies of the expression of the collagen-binding CbsA protein of L. crispatus in Lactobacillus casei. CbsA was anchored with the cell wall-sorting signal of PrtP to the cell wall and successfully expressed in L. casei, but no S-layer was formed and the amount of collagen bound was smaller than that observed with wild-type L. crispatus (23).
Acknowledgments
We thank Ilkka Palva for valuable discussion and critical reading of the manuscript. We also thank Kees Leenhouts for providing plasmid pNG101his, Matti Sarvas for supplying anti-Bla serum, and Erja Malinen for assistance with the statistical analysis. Esa Pohjolainen and Sinikka Ahonen are acknowledged for technical assistance.
This work was supported by the Academy of Finland (grants 40836 and 44602).
REFERENCES
- 1.Adlerberth, I., S. Ahrné, M-L. Johansson, G. Molin, L. Å. Hanson, and A. E. Wold. 1996. A mannose-specific adherence mechanism in Lactobacillus plantarum conferring binding to the human colonic cell line HT-29. Appl. Environ. Microbiol. 62:2244-2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 1994. Current protocols in molecular biology. Green Publishing Associates, New York, N.Y.
- 2a.Åvall-Jääskeläinen, S., K. Kylä-Nikkilä, M. Kahala, T. Miikkulainen-Lahti, and A. Palva. 2002. Surface display of foreign epitopes on the Lactobacillus brevis S-layer. Appl. Environ. Microbiol. 68:5943-5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bolken, T. C., C. A. Franke, K. F. Jones, R. H. Bell, R. M. Swanson, D. S. King, V. A. Fischetti, and D. E. Hruby. 2002. Analysis of factors affecting surface expression and immunogenicity of recombinant proteins expressed by gram-positive commensal vectors. Infect. Immun. 70:2487-2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boot, H. J., C. P. A. M. Kolen, J. M. van Noort, and P. H. Pouwels. 1993. S-layer protein of Lactobacillus acidophilus ATCC 4356: purification, expression in Escherichia coli, and nucleotide sequence of the corresponding gene. J. Bacteriol. 175:6089-6096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buist, G. 1997. AcmA of Lactococcus lactis, a cell-binding major autolysin. Ph.D. thesis. University of Groningen, Haren, The Netherlands.
- 6.Callegari, M. L., B. Riboli, J. W. Sanders, P. S. Cocconcelli, J. Kok, G. Venema, and L. Morelli. 1998. The S-layer gene of Lactobacillus helveticus CNRZ 892: cloning, sequence and heterologous expression. Microbiology 144:719-726. [DOI] [PubMed] [Google Scholar]
- 7.Coconnier, M.-H., T. R. Klaenhammer, S. Kerneis, M.-F. Bernet, and A. L. Servin. 1992. Protein-mediated adhesion of Lactobacillus acidophilus BG2FO4 on human enterocyte and mucus-secreting cell lines in culture. Appl. Environ. Microbiol. 58:2034-2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Conway, P. L., and S. Kjelleberg. 1989. Protein mediated adhesion of L. fermentum strain 737 to mouse stomach squamous epithelium. J. Gen. Microbiol. 25:245-250. [DOI] [PubMed] [Google Scholar]
- 9.de Ruyter, P. G. G. A., O. P. Kuipers, M. M. Beerthuyzen, I. J. van Alen-Boerrigter, and W. M. de Vos. 1996. Functional analysis of promoters in the nisin gene cluster of Lactococcus lactis. J. Bacteriol. 178:3434-3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dunne, C., L. Murphy, S. Flynn, L. O'Mahony, S. O'Halloran, M. Feeney, D. Morrissey, G. Thornton, G. Fitzgerald, C. Daly, B. Kiely, E. M. M. Quigley, G. C. O'Sullivan, F. Shanahan, and J. K. Collins. 1999. Probiotics: from myth to reality. Demonstration of functionality in animal models of disease and in human clinical trials. Antonie van Leeuwenhoek 76:279-292. [PubMed] [Google Scholar]
- 11.Granato, D., F. Perotti, I. Masserey, M. Rouvet, M. Golliard, A. Servin, and D. Brassart. 1999. Cell surface-associated lipoteichoic acid acts as an adhesion factor for attachment of Lactobacillus johnsonii La1 to human enterocyte-like Caco-2 cells. Appl. Environ. Microbiol. 65:1071-1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hanahan, D. 1983. Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 166:557-580. [DOI] [PubMed] [Google Scholar]
- 13.Holmes, A. R., C. Gilbert, J. M. Wells, and H. F. Jenkinson. 1998. Binding properties of Streptococcus gordonii SspA and SspB (antigen I/II family) polypeptides expressed on the cell surface of Lactococcus lactis MG1363. Infect. Immun. 66:4633-4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holo, H., and I. F. Nes. 1989. High-frequency transformation, by electroporation, of Lactococcus lactis subsp. cremoris grown with glycine in osmotically stabilized media. Appl. Environ. Microbiol. 55:3119-3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hynönen, U., B. Westerlund-Wikström, A. Palva, and T. K. Korhonen. 2002. Fibronectin-binding function in the SlpA surface protein of Lactobacillus brevis. J. Bacteriol. 184:3360-3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kapczynski, D. R., R. J. Meinersmann, and M. D. Lee. 2000. Adherence of Lactobacillus to intestinal 407 cells in culture correlates with fibronectin binding. Curr. Microbiol. 41:136-141. [DOI] [PubMed] [Google Scholar]
- 17.Kok, J., K. J. Leenhouts, A. J. Haandrikman, A. M. Ledeboer, and G. Venema. 1988. Nucleotide sequence of the cell wall proteinase gene of Streptococcus cremoris Wg2. Appl. Environ. Microbiol. 54:231-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kylä-Nikkilä, K., M. Hujanen, M. Leisola, and A. Palva. 2000. Metabolic engineering of Lactobacillus helveticus CNRZ32 for production of pure l-(+)-lactic acid. Appl. Environ. Microbiol. 66:3835-3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 20.Laitinen, R., E. Malinen, and A. Palva. 2002. PCR-ELISA. I. Application to simultaneous analysis of mixed bacterial samples composed of intestinal species. Syst. Appl. Microbiol. 25:241-248. [DOI] [PubMed]
- 21.Leenhouts, K., G. Buist, and J. Kok. 1999. Anchoring of proteins to lactic acid bacteria. Antonie van Leeuwenhoek 76:367-376. [PubMed] [Google Scholar]
- 22.Lorca, G., M. I. Torino, G. F. de Valdez, and Å. Ljungh. 2002. Lactobacilli express cell surface proteins which mediate binding of immobilized collagen and fibronectin. FEMS Microbiol. Lett. 206:31-37. [DOI] [PubMed] [Google Scholar]
- 23.Martínez, B., J. Sillanpää, E. Smit, T. K. Korhonen, and P. H. Pouwels. 2000. Expression of cbsA encoding the collagen-binding S-protein of Lactobacillus crispatus JCM5810 in Lactobacillus casei ATCC 393T. J. Bacteriol. 182:6857-6861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Masuda, K., and T. Kawata. 1983. Distribution and chemical characterization of regular arrays in the cell walls of strains of the genus Lactobacillus. FEMS Microbiol. Lett. 20:145-150. [Google Scholar]
- 25.Mukai, T., K. Arihara, and H. Itoh. 1992. Lectin like activity of Lactobacillus acidophilus strain JCM 1026. FEMS Microbiol. Lett. 77:71-74. [DOI] [PubMed] [Google Scholar]
- 26.Piard, J.-C., I. Hautefort, V. A. Fischetti, S. D. Ehrlich, M. Fons, and A. Gruss. 1997. Cell wall anchoring of the Streptococcus pyogenes M6 protein in various lactic acid bacteria. J. Bacteriol. 179:3068-3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Que, Y.-A., P. François, J.-A. Haefliger, J.-M. Entenza, P. Vaudaux, and P. Moreillon. 2001. Reassessing the role of Staphylococcus aureus clumping factor and fibronectin-binding protein by expression in Lactococcus lactis. Infect. Immun. 69:6296-6302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rojas, M., F. Ascencio, and P. L. Conway. 2002. Lactobacillus fermentum 104R that binds to porcine small intestinal mucus and gastric mucin. Appl. Environ. Microbiol. 68:2330-2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roos, S., P. Aleljung, N. Robert, B. Lee, T. Wadström, M. Lindberg, and H. Jonsson. 1996. A collagen binding protein from Lactobacillus reuteri is part of an ABC transporter system. FEMS Microbiol. Lett. 144:33-38. [DOI] [PubMed] [Google Scholar]
- 30.Roos, S., and H. Jonsson. 2002. A high-molecular-mass cell-surface protein from Lactobacillus reuteri 1063 adheres to mucus components. Microbiology 148:433-442. [DOI] [PubMed] [Google Scholar]
- 31.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 32.Sára, M., and U. B. Sleytr. 2000. S-layer proteins. J. Bacteriol. 182:859-868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schneitz, C., L. Nuotio, and K. Lounatmaa. 1993. Adhesion of Lactobacillus acidophilus to avian intestinal epithelial cells mediated by the crystalline bacterial cell surface layer (S-layer). J. Appl. Bacteriol. 74:290-294. [DOI] [PubMed] [Google Scholar]
- 34.Sherman, L. A., and D. C. Savage. 1986. Lipoteichoic acids in Lactobacillus strains that colonize the mouse gastric epithelium. Appl. Environ. Microbiol. 52:302-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Siezen, R. J. 1999. Multi-domain, cell-envelope proteinases of lactic acid bacteria. Antonie van Leeuwenhoek 76:139-155. [PubMed] [Google Scholar]
- 36.Sillanpää, J., B. Martínez, J. Antikainen, T. Toba, N. Kalkkinen, S. Tankka, K. Lounatmaa, J. Keränen, M. Höök, B. Westerlund-Wikström, P. H. Pouwels, and T. K. Korhonen. 2000. Characterization of the collagen-binding S-layer protein CbsA of Lactobacillus crispatus. J. Bacteriol. 182:6440-6450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sleytr, U. B., and T. J. Beveridge. 1999. Bacterial S-layers. Trends Microbiol. 7:253-260. [DOI] [PubMed] [Google Scholar]
- 38.Steidler, L., K. Robinson, L. Chamberlain, K. M. Schofield, E. Remaut, R. W. F. Le Page, and J. M. Wells. 1998. Mucosal delivery of murine interleukin-2 (IL-2) and IL-6 by recombinant strains of Lactococcus lactis coexpressing antigen and cytokine. Infect. Immun. 66:3183-3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Steidler, L., J. Viaene, W. Fiers, and E. Remaut. 1998. Functional display of a heterologous protein on the surface of Lactococcus lactis by means of the cell wall anchor of Staphylococcus aureus protein A. Appl. Environ. Microbiol. 64:342-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Toba, T., R. Virkola, B. Westerlund, Y. Björkman, J. Sillanpää, T. Vartio, N. Kalkkinen, and T. K. Korhonen. 1995. A collagen binding S-layer protein in Lactobacillus crispatus. Appl. Environ. Microbiol. 61:2467-2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vidgrén, G., I. Palva, R. Pakkanen, K. Lounatmaa, and A. Palva. 1992. S-layer protein gene of Lactobacillus brevis: cloning by polymerase chain reaction and determination of the nucleotide sequence. J. Bacteriol. 174:7419-7427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wells, J. M., K. Robinson, L. M. Chamberlain, K. M. Schofield, and R. W. F. Le Page. 1996. Lactic acid bacteria as vaccine delivery vehicles. Antonie van Leeuwenhoek 70:317-330. [DOI] [PubMed] [Google Scholar]
- 43.Westerlund, B., P. Kuusela, T. Varnio, I. van Die, and T. K. Korhonen. 1989. A novel lectin-independent interaction of P fimbriae of Escherichia coli with immobilized fibronectin. FEBS Lett. 243:199-204. [DOI] [PubMed] [Google Scholar]
- 44.Yanisch-Perron, C., J. Vieira, and J. Messing. 1985. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene 33:103-119. [DOI] [PubMed] [Google Scholar]
- 45.Yasui, T., K. Yoda, and T. Kamiya. 1995. Analysis of S-layer proteins of Lactobacillus brevis. FEMS Microbiol. Lett. 133:181-186. [DOI] [PubMed] [Google Scholar]