Abstract
The inhibitor of apoptosis (IAP) proteins form a highly conserved gene family that prevents cell death in response to a variety of stimuli. Herein we describe a newly defined murine IAP, designated Tiap, that proved to be a murine homologue of human survivin based on sequence comparison. TIAP has one baculovirus IAP repeat and lacks a C-terminal RING finger motif. TIAP interacted with the processed form of caspase 3 and inhibited caspase-induced cell death. Histological examinations revealed that TIAP is expressed in growing tissues such as thymus, testis, and intestine of adult mice and many tissues of embryos. In in vitro studies, TIAP was induced in splenic T cells activated with anti-CD3 antibody or Con A, and the expression of TIAP was up-regulated in synchronized NIH 3T3 cells at S to G2/M phase of the cell cycle. We propose that during cell proliferation, cellular protective activity may be augmented with inducible IAPs such as TIAP.
Apoptosis, or programmed cell death, plays a major role in development, tissue homeostasis, and defense against infectious agents (1). When the machinery of apoptosis is improperly regulated, unwanted cells survive or required cells die, resulting in numerous diseases such as cancer, autoimmune disease, and degenerative neuronal disease (2). The cysteine proteinases termed caspases can be activated by diverse stimuli and play a central role during apoptosis as executors of cell death (3). The caspases are present as proenzymes in viable cells and are proteolytically processed to generate active forms in apoptotic cells. In the case of caspase 3, the most intensely studied cell death protease, the inactive form (32 kDa) is processed to produce active large (17 kDa) and small (12 kDa) domains by cleavage at an aspartic residue between the two subunits and by the autocatalytic removal of the N-terminal prodomain (4, 5). The activities of caspases and their counteraction by apoptosis-inhibitory proteins, such as the Bcl-2 family and inhibitor of apoptosis (IAP) protein family, are crucial regulators in the molecular mechanism of apoptosis.
IAP was first identified in baculovirus genes that can complement the loss of the caspase inhibitor, p35, in mutant viruses (6, 7). Cellular homologues of IAPs have also been noted in mammals and Drosophila (8). The neuronal apoptosis-inhibitory protein, the first human IAP gene to be identified, was isolated as a candidate gene for the neuromuscular disease, spinal muscular atrophy (9). Two mammalian IAPs, c-IAP1 (HIAP-2/hMIHB) and c-IAP2 (HIAP-1/hMIHC), were isolated as molecules associated with tumor necrosis factor (TNF) receptor 2-associated factor 2 (10) and in a homology search (11–13). It has been shown that IAPs can counteract apoptosis induced by a variety of stimuli such as anti-Fas antibody (14), TNF-α (14, 15), virus infection (11), chemotherapeutic agents (14), exposure to UV radiation (14), serum withdrawal (12), x-irradiation (8), and overexpression of caspase family proteins (11, 13). Recent studies showed that three mammalian IAPs, XIAP (hILP/MIHA), c-IAP1, and c-IAP2, bind to specific cell death proteases, caspase 3 and caspase 7, and inhibit their proteolytic activity in vitro (16, 17).
All of the iap genes isolated from different species have the common structure termed the baculovirus IAP repeat (BIR) that is present in either two or three copies. Another common feature among IAP proteins is a RING finger domain at the C terminus, the function of which still remains unclear. Although the RING finger domain of baculoviral IAP is required for suppression of apoptosis in insect cells (7) and the RING finger domain of c-IAP2 is required for NF-κB induction by TNF in vitro (15), overexpression of the Drosophila IAP or human XIAP in the absence of the RING finger domain still suppresses cell death (8, 14, 17). Furthermore, two human IAP proteins, the neuronal apoptosis inhibitory protein and survivin, lacking the RING finger domain, can suppress apoptosis in vitro (12, 18), suggesting that the BIR domains are sufficient to inhibit apoptosis in some cases.
The present work was done to determine whether there are other IAP family proteins regulating apoptosis of mammalian cells. We have now identified a murine IAP homologue, designated TIAP (because of its high expression in thymus and testis), exhibiting 84% sequence identity with human survivin. TIAP can bind to caspase 3 in vitro and inhibits caspase-induced cell death of transfected Rat-1 cells. High expression of TIAP was detected specifically in proliferating cells in vitro and in vivo. Tiap is, therefore, a newly identified member of the growing IAP gene family coding for caspase inhibitors and may be involved in molecular mechanisms of apoptosis during cell proliferation.
MATERIALS AND METHODS
Molecular Cloning.
cDNA clones for Tiap were isolated from a cDNA library made from a 16-day murine embryo (Stratagene) by using a digoxigenin-labeled probe specific to Tiap, designated probe-351bp (586–936) and generated from an expressed sequence tag (EST) clone, W34764. DNA sequencing revealed an ORF of 140 amino acids.
A murine XIAP-encoding cDNA was obtained by reverse transcription-coupled PCR using a first-strand cDNA derived from mRNA of a day 14.5 murine embryo as the template and specific primers based on GenBank accession no. U36842 (forward, 5′-CGGAATTCATGACTTTTAACAGTTTTGAAGGAAC; reverse, 5′-CCGCTCGAGAGACATAAAAATTTTTTGCTTGAACG).
Full-length (amino acids 1–277), p20 (amino acids 1–159), and p17 (amino acids 29–159) murine caspase 3 were obtained by PCR methods and a first-strand cDNA made from mRNA purified from a day 14.5 mouse embryo as a template and primers (full-length forward, 5′-CGCGGATCCATGGAGAACAACAAAACCTCAGTGG; full-length reverse, 5′-CGCGGATCCCTAGTGATAAAAGTACAGTTCTTTCG; p20 forward, 5′-CGCGGATCCATGGAGAACAACAAAACCTCAGTGG; p20 reverse, 5′-CGCGGATCCCTAGTCTGTCTCAATGCCACAGTCCAG; p17 forward, 5′-CGCGGATCCTCTGGGATCTATCTGGACAGTAGTTAC; p17 reverse, 5′-CGCGGATCCCTAGTCTGTCTCAATGCCACAGTCCAG). All cDNA clones were verified by sequence analysis.
Expression Vectors.
To construct the Tiap and Xiap expression constructs, EcoRI–XhoI fragments encoding murine TIAP or XIAP were subcloned into the pHAKIT mammalian expression vectors (19) in frame. Flag-tagged Tiap and Xiap were constructed by subcloning EcoRI–XhoI fragments encoding murine TIAP or XIAP into pCR2FL (20) vectors. A BamHI fragment encoding the ORF of the Tiap gene was subcloned into pGEX-4T-2, and a NotI fragment encoding homeodomain of homeobox protein NCX (21) was subcloned into pGEX-5X-3 in frame with the glutathione S-transferase (GST) reading frame to generate pGEX-Tiap and pGEX-Ncx, respectively. Human caspase 3 expression vector, pCI-human caspase 3 was a gift from T. Momoi (National Institute of Neuroscience, Tokyo).
Cells and Transfections.
Rat-1 (rat fibroblast cell line) cells and NIH 3T3 (mouse fibroblast) cells were maintained in DMEM (GIBCO/BRL) with 10% fetal calf serum (Bioserum, Victoria, Australia), penicillin (100 units/ml), and streptomycin (100 μg/ml) at 37°C in 5% CO2/95% air.
Nearly confluent Rat-1 cells were transfected with 0.2 μg of pSV-β-galactosidase (Promega), 1 μg of pCI-human caspase 3, and 1 μg of test plasmids by using 6 μl of LipofectAmine (GIBCO/BRL) per well in 12-well tissue culture dishes. In the control transfection, 0.2 μg of pSV-β-galactosidase (Promega) and 1 μg of pSV2-neo were used. After 20 h of incubation, the cells were fixed with 0.25% glutaraldehyde in PBS for 15 min at room temperature and stained with 5-bromo-4-chloro-3-indolyl β-d-galactoside solution [0.2% 5-bromo-4-chloro-3-indolyl β-d-galactoside/2 mM MgCl2/5 mM K4Fe(CN)6⋅3H2O/5 mM K3Fe(CN)6] for 3 h at 37°C. Blue cells were visually scored as viable or not based on their appearance by microscopy, as described by Miura et al. (22). The experiment was repeated three times.
For cell cycle analysis, NIH 3T3 cells were cultured in 0.1% fetal calf serum for 48 h and released to reenter the cell cycle by addition of serum.
Jurkat cells were maintained in RPMI 1640 medium (GIBCO/BRL) with 10% fetal calf serum (Bioserum), penicillin (100 units/ml), and streptomycin (100 μg/ml) at 37°C in 5% CO2/95% air. Ten micrograms of Flag-tagged TIAP, XIAP, or control vector (pCR2FL) was transfected into Jurkat cells by electroporation with a Gene pulser (Bio-Rad) at 0.25 kV and 960 μF, and stable clones were obtained after selection with G418 (4 mg/ml).
Antibody Production.
Polyclonal antibodies against TIAP were raised in rabbits against GST-TIAP, by using a standard immunization protocol. The immune sera were precleared by passage through GST-Sepharose columns followed by affinity purification on antigen columns on which GST-TIAP protein was coupled to activated-CH Sepharose (Pharmacia Biotech).
In Vitro Binding Assay.
Full length, p20, and p17 fragments of murine caspase 3 were subcloned into the BamHI site of pCITE4a and translated in the TNT T7-coupled reticulocyte system (Promega) according to the manufacturer’s instructions. Fifteen microliters of each [35S]methionine-labeled in vitro-translated caspase 3 was incubated with 40 μg of GST-TIAP or GST-NCX for 3 h and washed three times with 500 μl of IP buffer (23), followed by heating to 95°C for 3 min, resolution by SDS/PAGE, and analysis using an image analyzer (BAS2000, Fuji).
Coimmunoprecipitation and Immunoblotting.
Jurkat cells stably transfected with Flag-TIAP, Flag-XIAP, or Flag control vector (each at 2 × 107 cells) were stimulated with anti-Fas monoclonal antibody (clone CH-11, Medical and Biological Laboratories, Nagoya, Japan) at 100 ng/ml for 4 h. Cells were collected by centrifugation, washed twice in ice-cold PBS, and lysed in IP buffer. Aliquots of cell lysate were reacted with 1 μg of anti-FLAG antibody (Kodak) for 1 h at 4°C. The immune complexes were precipitated with protein G-Sepharose beads (Zymed) for 2 h at 4°C. Immunoprecipitates were washed three times with IP buffer and analyzed by SDS/PAGE and immunoblotting using rabbit anti-caspase 3 polyclonal antibody (PharMingen).
Northern Blot Analysis.
Total RNAs were extracted from cultured splenocytes, NIH 3T3 cells or adult female mouse tissues using the Trizol reagent (Life Technologies). Northern blot analysis was done as described (23). For the preparation of a Tiap-specific probe, 351 bp, the fragment was subcloned into the pGEM-T vector (Promega) and labeled with digoxigenin (Boehringer Mannheim) by PCR with T7 and SP6 primers.
Western Blot Analysis.
Splenocytes and NIH 3T3 cells (1 × 106 cells) were disrupted by sonication in 1 ml of IP buffer at 4°C and the clarified lysate was obtained by centrifugation. The amount of protein in cell lysates was determined by using the Bio-Rad protein assay. Fifteen micrograms of cell lysates were resolved by SDS/PAGE and transferred to a poly(vinylidene difluoride) membrane (Immobilon-P, Millipore). Blots were blocked in Blockace (Yukijirushi, Sapporo, Japan) for 1 h, washed four times with Tris-buffered saline containing 0.2% Tween 20 (TBST), and incubated with the anti-TIAP antibody in TBST for 1 h at room temperature. The blots were then washed four times with TBST, incubated with affinity-purified donkey anti-rabbit antibody conjugated with horseradish peroxidase (Amersham International) for 1 h at room temperature, washed four times with TBST, and developed with enhanced chemiluminescence reagents (Amersham International).
Immunohistochemistry.
C57BL/6 mice (Japan SLC, Hamamatsu, Japan) were perfused with a solution of 4% paraformaldehyde in 0.1 M sodium phosphate (pH 7.4). Organs were dissected from mice and postfixed with 4% paraformaldehyde for 12 h. The tissues were equilibrated with 20% sucrose and sectioned at 10 μm on a cryostat. After quenching the activity of endogenous peroxidase with 3% H2O2 in methanol, sections were stained with rabbit antibodies against TIAP. A biotinylated goat anti-rabbit antibody (Nichirei, Tokyo) and streptABComplex/horseradish peroxidase (Dako) were used as the second- and third-phase reagents, respectively. Bound horseradish peroxidase activity was visualized with the diaminobenzidine kit (Nichirei). Cells were counterstained with hematoxylin.
In Situ Hybridization.
The RNA probe specific for the Tiap was synthesized on Tiap cDNA by Sp6 RNA polymerase, using the digoxigenin RNA labeling kit (Boehringer Mannheim). Frozen sections fixed with 4% paraformaldehyde were incubated with a proteinase K solution (proteinase K at 10 μg/ml in 10 mM Tris⋅HCl, pH 8.0/1 mM EDTA) at 37°C for 10 min. The sections were hybridized overnight with the RNA probe at 55°C, followed by RNase A treatment. Positive signals were detected by using the nucleic acid detection kit (Boehringer Mannheim).
Cell Cycle Analysis.
One million NIH 3T3 cells were incubated in 1 ml of Krishan’s reagent [propidium iodide(0.05 mg/ml)/0.1% sodium citrate/RNase A (0.02 mg/ml)/0.3% Nonidet P-40, pH 8.3] on ice for 30 min. Fluorescence from propidium iodide-nuclear DNA complexes was analyzed with a FACSCalibar (Becton Dickinson). Proportions of cells in G1, S, and G2/M phase of the cell cycle were analyzed by using the modfit lt software (Verity Software House, Topsham, ME).
Preparation and Stimulation of T Cells.
Isolation of splenocytes and their stimulation with anti-CD3 antibody (2C11) or Con A (Sigma) was done as described (24).
RESULTS
Molecular Cloning of Tiap.
To identify novel genes related to IAP, we adopted three approaches. The first strategy involved the use of degenerate oligonucleotide PCR primers encoding the BIR domains and/or the RING finger motif. The second strategy was to screen a mouse embryo cDNA library at low stringency by using probes encoding the BIR domain of mouse IAPs. The third strategy was to search the GenBank database of expressed sequenced tags (ESTs). We identified an EST (W34764) that generated an ORF containing the BIR domain. Using the cDNA corresponding to this EST as a probe, we isolated the longest cDNA clone from the mouse embryonic day 16 library. The cDNA encodes an ORF of 140 amino acids, named Tiap, with predicted molecular mass of 17 kDa (Fig. 1).
Figure 1.
Nucleotide and deduced amino acid sequences of the Tiap cDNA. The clone contains a 140-amino acid ORF beginning with an initiation methionine preceded by a well conserved Kozak consensus sequence and ends with a stop codon (indicated by an asterisk). The putative polyadenylation signal is underlined. Conserved amino acids of the BIR domain are shaded.
TIAP protein has no RING finger motif and one BIR domain which is similar to that of other IAP family members (41% identity and 57% similarity to the third BIR domain of murine XIAP in amino acid sequence), with a relatively small molecular size compared with other IAP family proteins. Sequence analysis revealed that Tiap has a high homology (84% identity and 92% similarity) to the recently identified novel human IAP family gene survivin (18), suggesting that Tiap is likely to be the murine counterpart of human survivin.
Inhibition of Caspase-Induced Cell Death and Interaction with Caspase 3.
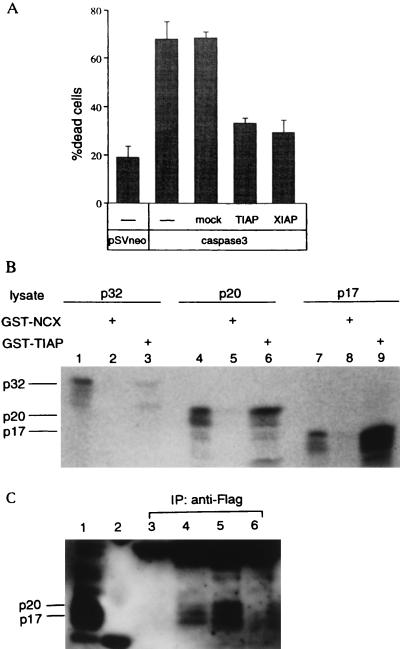
Although TIAP is a small IAP protein without a RING finger motif, a single conserved BIR domain may be able to function and contribute to the inhibition of apoptosis. To address this issue, we tested its potential to suppress caspase-induced cell death. An expression vector encoding Tiap or Xiap was transfected to Rat-1 cells, together with a lacZ expression vector and a caspase 3 expression vector. Eighteen h later the cells were studied for β-galactosidase expression, and the viability of β-galactosidase-positive cells was determined by morphological examination. As shown in Fig. 2A, transient expression of caspase 3 led to cell death of about 70% of the cells staining with 5-bromo-4-chloro-3-indolyl β-d-galactoside, whereas when pSV2neo was transfected alone only 20% of the staining cells were nonviable. Coexpression of Tiap with caspase 3, but not empty vector control (mock; pHAKIT) with caspase 3, reduced the proportion of dead cells to almost the same level seen by Xiap with caspase 3.
Figure 2.
Inhibition of apoptosis induced by overexpression of caspase and association with processed form of caspase 3. (A) Rat-1 cells were cotransfected with plasmids expressing caspase 3 and a plasmid expressing Tiap or Xiap and cell death was measured. (B) [35S]Methionine-labeled in vitro-translated p17, p20, or p32 of caspase 3 was mixed with GST-TIAP or GST-NCX, as indicated, followed by precipitation with glutathione-agarose beads and analysis by SDS/PAGE. In vitro-translated proteins were directly applied to lane 1, 4, or 7. (C) Jurkat cells stably transfected with Flag-tagged TIAP (lane 4), XIAP (lane 5), or control vector (pCR2FL) (lane 6) were stimulated with anti-Fas antibody and immunoprecipitated with anti-Flag antibody. Immunoprecipitates were analyzed by immunoblot with anti-caspase 3 antibody. Lanes: 1, lysate from Jurkat control transfectant stimulated with anti-Fas antibody; 2, marker; 3, Jurkat parent cells.
Since complex formation with caspase family proteins is a characteristic property of caspase inhibitors, including p35 (25), crmA (26), XIAP (16), and c-IAP1 and c-IAP2 (17), we considered whether TIAP also formed a complex with caspases. The potential of TIAP to bind caspase 3 was examined by pull-down experiments and immunoprecipitation studies. (i) GST fusion protein encoding TIAP was immobilized on glutathione-Sepharose and incubated with in vitro-translated [35S]methionine-labeled procaspase 3 (p32), pro- and large domain (p20), or large domain (p17). GST fusion protein with NCX was used as a control. p17 large domain of caspase 3 exhibited specific binding to GST-TIAP but not to GST-NCX (see Fig. 2B) and GST protein only (data not shown). In contrast to p17, p20 bound to GST-TIAP less efficiently, and p32 showed much less binding to GST-TIAP. (ii) Jurkat cells transfected with expression vectors encoding Flag-tagged TIAP or XIAP were stimulated with anti-Fas antibody. Whole cell lysates were prepared and immunoprecipitated by anti-Flag antibody. Endogenous caspase 3 in Jurkat cells was detected by anti-caspase3 antibody. Molecules of approximately 17 and 20 kDa were detected in the cell lysates transfected with Flag-TIAP or Flag-XIAP but not in the control (Fig. 2C). These data suggest that TIAP can bind efficiently to processed active caspase 3.
Relationship Between Cell Proliferation and Expression of TIAP.
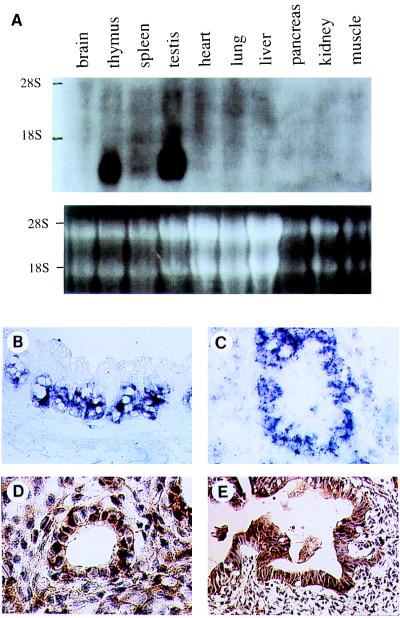
We examined expression of Tiap in various murine tissues by Northern blot analysis, in situ hybridization using the probe specific to Tiap, and immunohistochemistry. Northern blot analysis revealed ≈1.4-kb and much fainter ≈3.4-kb transcripts in thymus and testis, and a weak ≈1.4-kb transcript in spleen of adult mice (Fig. 3A). Tiap mRNA was also detected in total mouse embryos at day 11.5 and 15.5 and all cell lines used including T cell lines, B cell lines, and fibroblast cell lines (data not shown).
Figure 3.
Expression of TIAP in various tissues. The tissue distribution of TIAP was determined by Northern blot analysis (A), in situ hybridization (B and C), and immunohistochemistry (D and E). Histological examination of colon (B) and testis (C) of adult mouse and lung (D) and intestine (E) of mouse day 14.5 embryo was performed.
In situ hybridization studies revealed that Tiap was prominently expressed in crypts of ileum and colon (Fig. 3B), late spermatocytes (Fig. 3C), thymus, and the granulosa cell layer of the ovary during oogenesis (data not shown). In day 15.5 embryonic tissues, Tiap was detected prominently in liver and modestly in cerebrum, epithelium of nasal cavity, submandibular gland, thymus, lung, intestinal epithelium, skin, hair follicle, kidney, and muscles of the back (data not shown).
Immunohistochemically, the anti-TIAP antibody reacted with several adult tissues and many fetal tissues. Consistent with the in situ hybridization result, thymus and germ cells in testis were stained (data not shown). In tissues of day 14.5 embryo, bronchial epithelium and mesenchymal cells of lung (Fig. 3D), intestinal epithelium (Fig. 3E), cerebrum, hair follicle, muscles of the back, submandibular gland, hepatocytes, and hematopoietic cells in liver, epidermis of skin, and dorsal root ganglia (data not shown) reacted with the anti-TIAP antibody.
Most TIAP-positive cells in embryos at these stages are proliferating rapidly to form mature organs. In adult mouse tissues, TIAP is also expressed in proliferating tissues, most of which have a large population of cells undergoing mitosis, except for meiosis of spermatocytes. These data suggest a possible relationship between cell proliferation and TIAP expression.
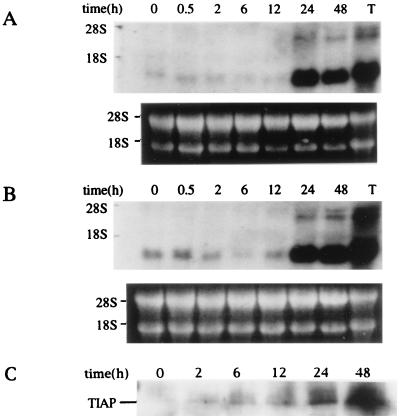
To address this issue, we examined TIAP expression in vitro in primary lymphocytes and NIH 3T3 cells. Splenic T cells are at G0/G1 phase of the cell cycle without stimulation but can enter S phase of the cycle after activation by mitogens. Expression of Tiap in splenocytes stimulated with anti-CD3 antibody (Fig. 4A) or Con A (Fig. 4B) was examined by Northern blot analysis. Tiap was detected in splenocytes at the resting state (0 h) and was strongly induced 24 h after activation. Western blot analysis using anti-TIAP antibody showed that TIAP was consistently induced 24 h after activation by Con A (Fig. 4C).
Figure 4.
Expression of Tiap mRNA in activated T cells. Splenocytes were stimulated with T cell-specific mitogen, anti-CD3 antibody (10 μg/ml) (A) or Con A (10 μg/ml) (B) and expression of Tiap mRNA as indicated was analyzed by Northern blot. RNA sample from thymus is indicated as T. (C) Western blot analysis of TIAP protein in splenocytes stimulated with Con A.
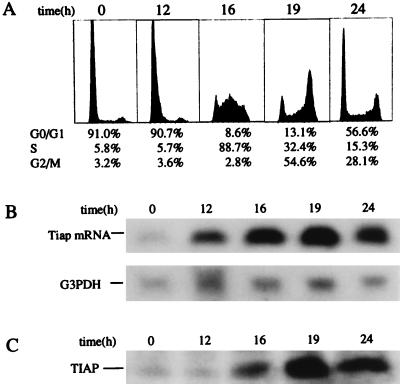
Fig. 5A shows cell cycle analysis of synchronized NIH 3T3 cells by flow cytometry. The cells were starved of serum for 48 h and released to enter the cell cycle by restimulation with serum. The percentage of cells in S phase of the cell cycle reached a peak (approximately 90%) at 16 h after restimulation, and most cells had reentered the G1 phase 24 h after restimulation. Expression of Tiap mRNA and TIAP protein in those cells was examined by Northern and Western blot analysis, respectively. Northern blot analysis revealed that the level of Tiap mRNA was up-regulated at 12 h, reached a peak at 19 h, and was slightly reduced at 24 h after restimulation (Fig. 5B). Consistent with the results of Northern blot analysis, Western blot analysis showed that TIAP protein was up-regulated at 16 h after restimulation (Fig. 5C).
Figure 5.
Expression of TIAP in synchronized NIH 3T3 cells. (A) Cell cycle analysis of synchronized NIH 3T3 cells. Expression of Tiap mRNA (B) and TIAP protein (C) was analyzed by Northern blotting and Western blotting, respectively.
DISCUSSION
The emergence of a variety of mammalian IAP family proteins and their common function in inhibiting apoptosis suggests redundancy among IAPs. For example, XIAP, c-IAP1, and c-IAP2 are widely expressed in human tissues and have the same specificity for inhibition of caspase 3 and caspase 7 (16, 17). Association of TIAP with processed caspase 3 (Fig. 2 B and C) and inhibition of caspase-induced cell death (Fig. 2A) suggests that TIAP also has specificity similar to that of other IAP family proteins, but binding of TIAP to other caspases needs to be elucidated. However, it is conceivable that particular IAPs function in certain tissues and under certain situations. Indeed, the expression pattern of Tiap is very different from that of the other IAP family members. We found a close correlation between expression of TIAP and cell proliferation. In histological examinations, TIAP was expressed in tissues in which cells have high proliferating activity (Fig. 3 B–E). TIAP was induced in splenic T cells after activation (Fig. 4) and in synchronized NIH 3T3 at S to G2/M phase of the cell cycle (Fig. 5). The specific regulation of TIAP expression can discriminate TIAP from other IAP family proteins and suggests that TIAP contributes to regulation of apoptosis during cell proliferation. Several lines of reasoning indicate a relationship between cell proliferation and sensitivity to apoptosis. Within the organism, apoptosis is almost exclusively found in proliferating tissues. Many oncogenes, including c-fos and c-myc that promote cell cycle progression also induce apoptosis (27, 28). Targeted disruption of Rb protein, a key cell cycle regulator, results in abnormal mitotic figures and neuronal cell death in embryos (29, 30). Activated T cells but not resting T cells undergo apoptosis mediated by signaling via the T cell receptor (31). Therefore, proper cell proliferation for development and cellular homeostasis may require the specific molecular regulation of apoptosis, and TIAP may be one of the antiapoptotic regulators that counteract the increasing sensitivity to cell death during cell proliferation.
The expression of TIAP related to cell proliferation is compatible with the data of Ambrosini et al. (18) that human survivin is prominently expressed in most common human cancers and found frequently in high-grade non-Hodgkin lymphomas but not in low-grade lymphomas. The characteristic expression pattern of TIAP in proliferating cells suggests that high expression of TIAP in tumors may be a causative factor for the generation of neoplasms, or at least a deteriorating factor, but may also be the result of rapid growth after neoplastic transformation.
Another inducible mammalian IAP, c-IAP2, is induced via NF-κB signaling after TNF-α stimulation and exerts positive feedback control on NF-κB via an IκB-targeting mechanisms (15). Because c-IAP2 is induced soon after stimulation, the precise role of c-IAP2 is distinct from that of TIAP in stimulated cells. Other IAP family proteins such as c-IAP1 are constitutively expressed (15), implying that constitutively expressed IAPs may form a “threshold” or act as a “buffer” for the death signal by interacting with death inducing molecules such as caspases and that inducible IAPs like c-IAP2 and TIAP may increase the protective activity by IAP family proteins during special events such as TNF signaling or cell proliferation.
Thus, our findings suggest that the newly cloned Tiap functions as an apoptosis inhibitor and that expression is related to cell proliferation in most tissues. Regulatory mechanisms of expression and functional roles of TIAP in cell proliferation and tissue development remain to be elucidated.
Acknowledgments
We are indebted to T. Momoi for the human caspase 3 expression vector; to M. Hayano, H. Shimizu, A. A. Jusuf, and H. Satake for technical assistance; S. Okada for advice and discussion; and N. Fujita for secretarial assistance. K.K. was supported by a grant from the Naito Foundation of Tokyo.
ABBREVIATIONS
- IAP, inhibitor of apoptosis
BIR, baculovirus IAP repeat
- GST
glutathione S-transferase
- TNF
tumor necrosis factor
- EST
expressed sequence tag
Footnotes
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. AB013819 for Tiap).
References
- 1.Steller H. Science. 1995;267:1445–1449. doi: 10.1126/science.7878463. [DOI] [PubMed] [Google Scholar]
- 2.Thompson C B. Science. 1995;267:1456–1462. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- 3.Salvesen G S, Dixit V M. Cell. 1997;91:443–446. doi: 10.1016/s0092-8674(00)80430-4. [DOI] [PubMed] [Google Scholar]
- 4.Nicholson D W, Ali A, Thornberry N A, Vaillancourt J P, Ding C K, Gallant M, Gareau Y, Griffin P R, Labelle M, Lazebnik Y A, et al. Nature (London) 1995;376:37–43. doi: 10.1038/376037a0. [DOI] [PubMed] [Google Scholar]
- 5.Wang X, Pai J T, Wiedenfeld E A, Medina J C, Slaughter C A, Goldstein J L, Brown M S. J Biol Chem. 1995;270:18044–18055. doi: 10.1074/jbc.270.30.18044. [DOI] [PubMed] [Google Scholar]
- 6.Crook N E, Clem R J, Miller L K. J Virol. 1993;67:2168–2174. doi: 10.1128/jvi.67.4.2168-2174.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clem R J, Robson M, Miller L K. J Virol. 1994;68:6759–6762. doi: 10.1128/jvi.68.10.6759-6762.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hay B A, Wassarman D A, Rubin G M. Cell. 1995;83:1253–1262. doi: 10.1016/0092-8674(95)90150-7. [DOI] [PubMed] [Google Scholar]
- 9.Roy N, Mahadevan M S, McLean M, Shutler G, Yaraghi Z, Farahani R, Baird S, Besner-Johnston A, Lefebvre C, Kang X, et al. Cell. 1995;80:167–178. doi: 10.1016/0092-8674(95)90461-1. [DOI] [PubMed] [Google Scholar]
- 10.Rothe M, Pan M G, Henzel W J, Ayres T M, Goeddel D V. Cell. 1995;83:1243–1252. doi: 10.1016/0092-8674(95)90149-3. [DOI] [PubMed] [Google Scholar]
- 11.Duckett C S, Nava V E, Gedrich R W, Clem R J, Van Dongen J L, Gilfillan M C, Shiels H, Hardwick J M, Thompson C B. EMBO J. 1996;15:2685–2694. [PMC free article] [PubMed] [Google Scholar]
- 12.Liston P, Roy N, Tamai K, Lefebvre C, Baird S, Cherton-Horvat G, Farahani R, McLean M, Ikeda J E, MacKenzie A, Korneluk R G. Nature (London) 1996;379:349–353. doi: 10.1038/379349a0. [DOI] [PubMed] [Google Scholar]
- 13.Uren A G, Pakusch M, Hawkins C J, Puls K L, Vaux D L. Proc Natl Acad Sci USA. 1996;93:4974–4978. doi: 10.1073/pnas.93.10.4974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duckett C S, Li F, Wang Y, Tomaselli K J, Thompson C B, Armstrong R C. Mol Cell Biol. 1998;18:608–615. doi: 10.1128/mcb.18.1.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chu Z L, McKinsey T A, Liu L, Gentry J J, Malim M H, Ballard D W. Proc Natl Acad Sci USA. 1997;94:10057–10062. doi: 10.1073/pnas.94.19.10057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deveraux Q L, Takahashi R, Salvesen G S, Reed J C. Nature (London) 1997;388:300–304. doi: 10.1038/40901. [DOI] [PubMed] [Google Scholar]
- 17.Roy N, Deveraux Q L, Takahashi R, Salvesen G S, Reed J C. EMBO J. 1997;16:6914–6925. doi: 10.1093/emboj/16.23.6914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ambrosini G, Adida C, Altieri D C. Nat Med. 1997;3:917–921. doi: 10.1038/nm0897-917. [DOI] [PubMed] [Google Scholar]
- 19.Nakano H, Oshima H, Chung W, Williams-Abbott L, Ware C F, Yagita H, Okumura K. J Biol Chem. 1996;271:14661–14664. doi: 10.1074/jbc.271.25.14661. [DOI] [PubMed] [Google Scholar]
- 20.Akiba H, Nakano H, Nishinaka S, Shindo M, Kobata T, Atsuta M, Morimoto C, Ware C F, Malinin N L, Wallach D, et al. J Biol Chem. 1998;273:13353–13358. doi: 10.1074/jbc.273.21.13353. [DOI] [PubMed] [Google Scholar]
- 21.Hatano M, Iitsuka Y, Yamamoto H, Dezawa M, Yusa S, Kohno Y, Tokuhisa T. Anat Embryol. 1997;195:419–425. doi: 10.1007/s004290050061. [DOI] [PubMed] [Google Scholar]
- 22.Miura M, Zhu H, Rotello R, Hartweig E A, Yuan J. Cell. 1993;75:653–660. doi: 10.1016/0092-8674(93)90486-a. [DOI] [PubMed] [Google Scholar]
- 23.Kobayashi K, Phuchareon J, Inada K, Tomita Y, Koizumi T, Hatano M, Miyatake S, Tokuhisa T. J Immunol. 1997;158:2050–2056. [PubMed] [Google Scholar]
- 24.Ochi Y, Koizumi T, Kobayashi S, Phuchareon J, Hatano M, Takada M, Tomita Y, Tokuhisa T. J Immunol. 1994;153:3485–3490. [PubMed] [Google Scholar]
- 25.Bump N J, Hackett M, Hugunin M, Seshagiri S, Brady K, Chen P, Ferenz C, Franklin S, Ghayur T, Li P, et al. Science. 1995;269:1885–1888. doi: 10.1126/science.7569933. [DOI] [PubMed] [Google Scholar]
- 26.Zhou Q, Snipas S, Orth K, Muzio M, Dixit V M, Salvesen G S. J Biol Chem. 1997;272:7797–7800. doi: 10.1074/jbc.272.12.7797. [DOI] [PubMed] [Google Scholar]
- 27.Smeyne R J, Vendrell M, Hayward M, Baker S J, Miao G G, Schilling K, Robertson L M, Curran T, Morgan J I. Nature (London) 1993;363:166–169. doi: 10.1038/363166a0. [DOI] [PubMed] [Google Scholar]
- 28.Evan G I, Wyllie A H, Gilbert C S, Littlewood T D, Land H, Brooks M, Waters C M, Penn L Z, Hancock D C. Cell. 1992;69:119–128. doi: 10.1016/0092-8674(92)90123-t. [DOI] [PubMed] [Google Scholar]
- 29.Lee E Y, Chang C Y, Hu N, Wang Y C, Lai C C, Herrup K, Lee W H, Bradley A. Nature (London) 1992;359:288–294. doi: 10.1038/359288a0. [DOI] [PubMed] [Google Scholar]
- 30.Jacks T, Fazeli A, Schmitt E M, Bronson R T, Goodell M A, Weinberg R A. Nature (London) 1992;359:295–300. doi: 10.1038/359295a0. [DOI] [PubMed] [Google Scholar]
- 31.Kabelitz D, Pohl T, Pechhold K. Immunol Today. 1993;14:338–339. doi: 10.1016/0167-5699(93)90231-9. [DOI] [PubMed] [Google Scholar]