Abstract
Candida glabrata, the second most prevalent Candida species colonizing humans, possesses three mating type-like (MTL) loci (MTL1, MTL2, and MTL3). These loci contain pairs of MTL genes with their respective coding regions on complementary Crick and Watson DNA strands. Each pair of genes is separated by a shared intergenic promoter region, the same configuration found at the mating type loci of Saccharomyces cerevisiae. Two of the MTL loci, MTL1 and MTL2, contain either the MTLa1/MTLa2 configuration or the MTLα1/MTLα2 configuration in different strains. All but one of the 38 tested C. glabrata strains were either aaα or aαα. One test strain was ααα. Based on the mating type genotype, the MTL genes at the MTL1 or MTL2 loci, and the size of the XbaI fragment harboring MTL1 or MTL2, four classes of C. glabrata strains (I, II, III, and IV) were distinguished. Northern analysis revealed that strains were either a-expressors or α-expressors and that expression always reflected the genotype of either the MTL1 or MTL2 locus, depending on the class. The expression pattern in each class, therefore, is similar to that observed in S. cerevisiae, which harbors two silent cassette loci, HMR and HML, and the expression locus MAT. High-frequency phenotypic switching between core phenotypes in an α-expressing, but not in an a-expressing, strain modulated the level of MTL expression, suggesting a possible relationship between core phenotypic switching and mating.
Candida glabrata is the second most prevalent Candida species in humans (15, 17, 32, 38, 41). Although it is genetically far more related to Saccharomyces cerevisiae than to Candida albicans (3, 42, 48), it mimics in many respects the pathogenic capabilities of C. albicans, the most prevalent Candida species, residing as a commensal in healthy individuals and causing vaginitis (15, 43, 44) and bloodstream infections (40). Recently, it was demonstrated that in the elderly, C. glabrata has emerged as the major commensal (32). The prominence of C. glabrata as a pathogen is of particular clinical concern because it is naturally resistant to azole drug therapy (4, 16, 20, 36).
Until recently, it was generally assumed that C. glabrata did not undergo the bud-hypha transition, and no reports had been published on phenotypic switching, two developmental programs that contribute to the pathogenic success of C. albicans. However, recent studies have demonstrated that C. glabrata forms pseudohyphae (12, 26, 27, 39), forms noncompartmentalized tubes distinct from true hyphae (27), and undergoes high-frequency phenotypic switching (26, 27). C. glabrata, therefore, possesses developmental programs at least as complex as those of C. albicans. Recently, it was demonstrated that C. albicans, which is diploid, possesses the mating type-like (MTL) loci MTLa and MTLα (21) and that homozygous MTLa and homozygous MTLα strains will mate both in vivo (22) and in vitro (35). It was subsequently demonstrated that while most strains of C. albicans are heterozygous for mating type at the MTL locus and do not undergo white-opaque switching, homozygous strains do undergo the transition (33, 37), and the opaque phenotype dramatically facilitates mating (34, 37). Given the pathogenic similarities of C. albicans and C. glabrata and the developmental correlates between the two species, we have searched for and identified the mating type genes of C. glabrata. We demonstrate that C. glabrata harbors three loci containing mating type-like genes, that two loci, MTL1 and MTL2, can contain either an MTLa1-MTLa2 or an MTLα1-MTLα2 configuration in different strains, and that based on expression patterns, C. glabrata strains can be distinguished as either a mating type or α mating type. Our results further indicate that in any one class, only one of the three MTL loci serves as the mating type expression locus.
MATERIALS AND METHODS
Maintenance and growth of strains.
Thirty-eight different clinical isolates of C. glabrata previously genetically fingerprinted with the C. glabrata DNA fingerprinting probe Cg12 (30) are described in Table 1. All strains were maintained on YPD agar slants (1.5% [weight/vol] agar, 2% [weight/vol] Bacto Peptone, 2% [weight/vol] glucose, 1% [weight/vol] yeast extract). For experimental purposes, cells were streaked on fresh YPD agar plates and incubated for 3 to 4 days at 25°C prior to use. For monitoring phenotypic switching, cells were plated on YPD agar containing 1 mM CuSO4 (26).
TABLE 1.
Strains used in this studya
| Strain | Geographic locale | Body location | |
|---|---|---|---|
| 26B0 | Iowa City, Iowa | Oral | |
| 25T1 | Iowa City, Iowa | Oral | |
| 75P1 | Iowa City, Iowa | Oral | |
| 29P1 | Iowa City, Iowa | Oral | |
| 26B9 | Iowa City, Iowa | Oral | |
| 86B1 | Iowa City, Iowa | Oral | |
| 7549 | Iowa City, Iowa | Oral | |
| 65T1 | Iowa City, Iowa | Oral | |
| 35B11 | Iowa City, Iowa | Oral | |
| LAI89 | Detroit, Mich. | Vaginal | |
| PB921 | Detroit, Mich. | Vaginal | |
| R313 | Detroit, Mich. | Vaginal | |
| LP21 | Detroit, Mich. | Vaginal | |
| PB656 | Detroit, Mich. | Vaginal | |
| NB783 | Detroit, Mich. | Vaginal | |
| PB09 | Detroit, Mich. | Vaginal | |
| CD457 | Detroit, Mich. | Vaginal | |
| 1480.41 | Richmond, Va. | Oral | |
| 1480.42 | Richmond, Va. | Oral | |
| 1480.44 | Richmond, Va. | Oral | |
| 1480.46 | Richmond, Va. | Oral | |
| 1480.50 | Richmond, Va. | Oral | |
| 1480.49 | Richmond, Va. | Oral | |
| 1480.47 | Richmond, Va. | Oral | |
| 1480.43 | Richmond, Va. | Oral | |
| J932405 | Belgium | Vaginal | |
| J942007 | Belgium | Vaginal | |
| J932436 | Belgium | Vaginal | |
| J932597 | Belgium | Vaginal | |
| J932387 | Belgium | Vaginal | |
| J932474 | Belgium | Vaginal | |
| J932285 | Germany | Oral | |
| J932405 | Germany | Oral | |
| 9932258 | Germany | Oral | |
| J931010 | Germany | Oral | |
| J932273 | Germany | Oral | |
| J932283 | Germany | Oral | |
| J941814 | The Netherlands | Blood |
All isolates used in this study were confirmed as C. glabrata by DNA fingerprinting with the probe, Cg12 (22). Each isolate was recovered from a separate human subject.
Isolation and sequence analysis of MTL loci.
Based on homology comparisons with the MATα2p or MTLα2p from the species S. cerevisiae, Kluyveromyces lactis, and C. albicans by using the multiple-alignment editor of Clustal W software developed by Michele Clamp (www.cmbi.kur.nl/bioinf/tools/clustalw.shtml), two highly conserved amino acid sequences containing homeobox domains were identified (1, 2, 21, 29). These sequences, WFAKKNIENPY and WVSNRRRKEK, were used to design the forward primer MP2F2 and the reverse primer MP2R1 (Table 2), respectively. These two degenerate primers were used to amplify similar homeobox-containing regions with Taq polymerase (Life Tech/Invitrogen Inc., Gaithersburg, Md.) by PCR amplification of C. glabrata genomic DNA. PCR products were purified by using the Wizard PCR Clean-up kit (Promega Corp, Madison, Wis.) and cloned into pGEM-T Easy (Promega Corp.). Plasmids pE30.8, pE30.9, and pE30.10, containing 440-, 125-, and 240-bp inserts, respectively, were sequenced in both directions with an ABI model 373A automatic sequencing apparatus and fluorescent Big Dye terminator chemistry (PE-ABI Inc., Foster City, Calif.). Protein-coding regions were determined by the Wu-BLAST/BEAUTY Search algorithm-based program (49).
TABLE 2.
Primers used in this study
| Primer | Sequence |
|---|---|
| MP2F2 | 5′-TGGTTTGCAAAGAAYANNGAGAAYCCNTA-3′ |
| MP2R1 | 5′-TTTTTCTTTTCTTCTTCTATTCGANACCCA-3′ |
| PIRACE1 | 5′-GCCATCAAGGTAGGTCTGAAT-3′ |
| CGPF1 | 5′-TAACCAACTTATATCATTGTGTTCCA-3′ |
| AIRACE1 | 5′-GAACTTGATTGGTGGTGATCCCA-3′ |
| CGAR1 | 5′-CAACACGGTAGGTTTACGATA-3′ |
| CGPIF1 | 5′-ATGAAGTATACTGCCACAAAA-3′ |
| CGPIR2 | 5′-CTGAGAGAATGACGGAGAGTGTA-3′ |
| MLFLR2 | 5′-TATGTCTTGCGCGTCCAATTGCT-3′ |
| MLFLF2 | 5′-ATAAGCAATCAGTATGTGTA-3′ |
| FLR2 | 5′-ACATCACCAACATACGCACCGCT-3′ |
| 12.36F1 | 5′-ATGTCAGTCTGAACTAGTGAATA-3′ |
| MLFLF3 | 5′-AGGGACATCGCTGAGGCCAGA-3′ |
| MLFLR3 | 5′-ATTGACCCAAGAAGTGGTGAGA-3′ |
| MTLOCR1 | 5′-GCTTGCAATCAAAGTGTTCTG-3′ |
| MTLOCF1 | 5′-GTGCTGATCACTATCGAATGC-3′ |
| FuncP2 | 5′-TATTGGATTAAGTAGATGCGTAATACTAATTCTTGATTTCTTTGACAT-3′ |
Sequence analysis and strain comparison.
To obtain the full-length MTL1 locus and its flanking sequences, 105 plaques of a C. glabrata genomic library of strain 7549 (30) were screened with the 125-bp DNA fragment, which spanned the MATα2p homeodomain. Plaque lysates from 55 positive primary clones were used as a template for PCR, using MP2F1 and MP2R1 as primers (Table 2). Five lambda clones, λMP1, λMP2, λMP5, λMP16, and λMP18, which generated 125-bp PCR products, were chosen for secondary screening. λMP1.1, which contained a 3.4-kb insert, was used to characterize the sequence of the MTL1 region and flanking sequences by lambda clone walking, using custom primers and the ABI sequencing apparatus. Similarly, λMP16.1 was used to characterize the MTL2 locus, and λMP5.1 and λMP18.1 were used to characterize the MTL3 locus. Based on the DNA sequence of MTL1, two primers, MLFLF2 and MLFLR2 (Table 2), spanning the flanking region of the MTL1 open reading frames (ORFs) were designed and used in PCRs to generate homologous DNA regions from two additional strains, 1480.47 and PB921. The derived PCR products were cloned into pGEM-T Easy and sequenced. Comparisons and alignments of protein sequences were performed by using the multiple alignment editor of Clustal W/Jalview software (www.embi.kun.nl.bioinf/tools/clustalw.shtml). Promoter analysis was performed with the Matrix method for identifying putative regulatory protein binding sites in the S. cerevisiae Promoter Database (http://ctsigma.cshl.org/jiar/).
Southern and Northern analyses.
Southern and Northern analyses were performed according to procedures previously described (26, 45, 46). In Southern analyses in which more than one probe was used, the originally hybridized blots were stripped at 75°C according to the protocols of Church and Gilbert (7). For Southern analysis, the hybridization probes for MTLa2/α2, MTLa1, and MTLα1 contained ORF regions derived from the primer pairs P1RACE1-CGPF1, A1RACE1-CGAR1, and CGPIF1-CGPIR2, respectively (Table 2). For Southern analysis involving oligonucleotide-based hybridization to distinguish MTLa2 and MTLα2 sequences among three MTL loci, 500 ng of FuncP2 (Table 2) was end labeled with [32P]ATP by using T4 polynucleotide kinase as described by Conner et al. (9). Southern blotting was done with the nylon membrane Hybond N+. The protocols for hybridization and washing of Southern blots were those of Landsman et al. (28). In Northern analyses, the hybridization probes for MTLα1 and MTLa1 contained ORF regions derived from the same primer pairs.
RESULTS
Isolation of a conserved domain of C. glabrata MATa2/MATα2.
Based on homology comparisons between MATα2p and MATa2p of S. cerevisiae, C. albicans, and K. lactis, two highly conserved regions were identified. Assuming that these sequences would also be conserved in C. glabrata based on the high level of genetic relatedness between it and S. cerevisiae (3, 42), degenerate primers that encompassed approximately 125 bp of the MATα2 ORF of S. cerevisiae were designed. Using these primers and C. glabrata DNA, three distinct PCR products of 450, 240, and 125 bp were identified. Sequence analysis of the recombinant plasmids containing the PCR products revealed that the 125- and 450-bp inserts contained uninterrupted ORFs flanked by highly conserved sequences. A BLAST-based sequence similarity search revealed that the 450-bp ORF did not encode a MATα2p but rather encoded a protein homologous to a JUN activation domain binding protein or morphogenetic factor in humans and plants, respectively (6, 8). However, the BLAST search of the 125-bp PCR product revealed that it was derived from a locus homologous to MATα2. The deduced amino acid sequence of the 125-bp PCR product suggested that the ORF encoded 41 amino acids with identities of 69, 57, and 50% and similarities of 82, 77, and 65% to the MATα2p or MTLα2p of S. cerevisiae, K. lactis, and C. albicans, respectively.
Isolation and characterization of the MTL1a and MTL2a loci.
The 125-bp fragment was used in turn as a probe to screen a C. glabrata EMBL3a λ library constructed from genomic DNA of strain 7549 (30) for the gene locus. Fifty-five putative clones were identified and tested for the presence of a 125-bp MATα2-like insert by PCR. One of these clones, λMP1.1, was used to determine the sequence of a 2,587-bp insert by customized primer walking in both directions (Fig. 1A and 2A). A BLAST-type search of the sequence identified a 312-bp S. cerevisiae MATa1p-like protein-coding sequence and a 510-bp S. cerevisiae MATa2p/MATα2p-like protein-coding sequence separated by a 180-bp putative promoter region. The locus containing these two coding sequences and intergenic sequence will be referred to as mating type-like locus 1 (MTL1). The S. cerevisiae MATa1-like coding sequence in MTL1, which we will refer to as MTL1a1, was 126 bp shorter than the S. cerevisiae MATa1 coding region. The deduced 104-amino-acid sequence had 28% overall similarity with S. cerevisiae MATa1p (Fig. 3A). The-carboxy terminal 57 amino acids showed a high level of similarity with the carboxy-terminal regions of C. albicans MTLa1p (21), K. lactis MATa1p (1), and S. cerevisiae MATa1p (2) (Fig. 3A). The S. cerevisiae MATa2/MATα2-like coding sequence in MTL1 was 150 bp longer than the S. cerevisiae MATa2 coding region. The deduced 170-amino-acid sequence had an overall identity of 26% with both S. cerevisiae MATa2p and S. cerevisiae MATα2p. The carboxy-terminal 123 amino acids exhibited 31% identity with the carboxy-terminal 119 amino acids of both S. cerevisiae MATa2p and S. cerevisiae MATα2p (Fig. 3B). The carboxy-terminal two-thirds of MATα2p is identical to the full-length MATa2p in S. cerevisiae (2). Alignment of MTL1a2p with S. cerevisiae MATa2p and MATα2p revealed a region of highest similarity in the carboxy-terminal end, spanning 60 amino acids (Fig. 3B). This region includes a homeodomain signature sequence (WVXNRRR) (Fig. 3B) that is a near match with that of C. glabrata and S. cerevisiae. This region has been implicated in ternary complex formation with DNA and MATa1p in S. cerevisiae (29). The two protein-coding regions in MTL1 of C. glabrata strain 7549 will be referred to as MTL1a1 and MTL1a2, respectively (Fig. 1A).
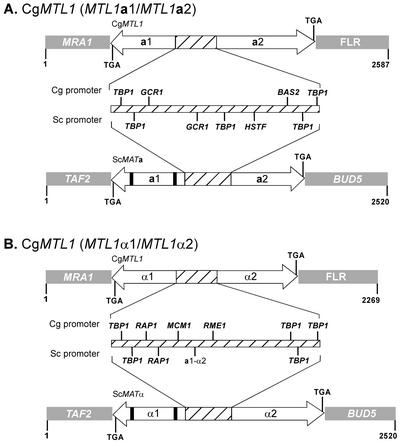
FIG. 1.
Configurations of the C. glabrata MTL1 locus (A) The MTL1 locus containing MTL1a1 and MTL1a2 in strain 7549. (B) The MTL1 locus containing MTL1α1 and MTL1α2 in strains PB921 and 1480.47. In both cases comparisons are made to comparable S. cerevisiae loci MATa and MATα. MRA1, 3′ flanking region of MTL1a1; FLR, 3′ flanking region of MTL1a2; TAF2, 5′ flanking region of the MATa1/α1 locus; BUD5, 3′ flanking region of MATa2/α2. a1 and a2, MTL1a1 and MTL1a2, respectively, or MATa1 or MATa2, respectively; α1 and α2, MTL1α1 and MTL1α2, respectively, or MATα1 or MATα2, respectively. The thick black bars in MATa1 and MATα1 represent introns. Hatched portions of loci represent intergenic promoter regions. Arrow directions reflect orientation of transcription on complementary Crick and Watson DNA strands.
FIG. 2.
Nucleotide sequences of the MTL1a locus derived from strain 7549 and the MTL1α locus derived from strain PB621. (A) The ORFs for MTL1a1 and MTL1a2 are shown in boldface. The primer pairs for MTL1a1 (AIRACE1 and CGAR1) are shown as black boxes with boldface white print, and those for MTL1a2 (CGPF1 and PIRACE1) are shown as grey boxes with boldface white print. The primer pairs which flank the 3′ end of the MTL1a1 ORF and the 3′ end of the MTL1a2 ORF are underlined. (B) The ORFs for MTL1α1 and MTL1α2 are shown in boldface print. The primer pairs for MTL1α1 (CGPIR1 and CGPIF1) are shown as black boxes with white print, and those for MTL1α2 are shown as grey boxes with white print. The primers used to link MTL1α1 with MTL1α2 are underlined. Forty-eight nucleotides at the 5′ end of the MTL1α2 ORF that are common to MTL1α2 and MTL3α2 are show by dashed lines. The rest of MTLα2, excluding the open-box sequence, is common to MTLa2. The nucleotide sequence unique to the 3′ end of the MTL1α2 ORF is shown in an open box.
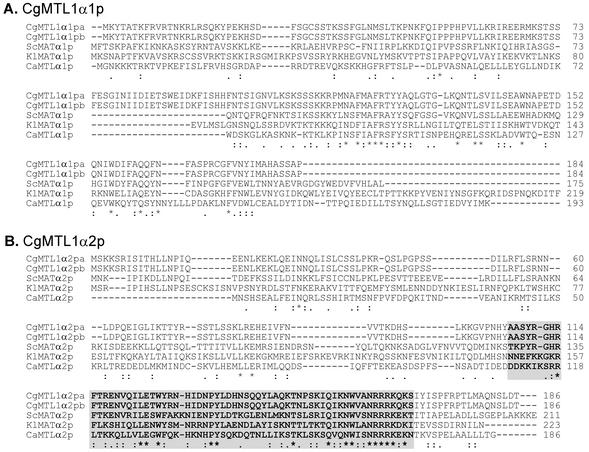
FIG. 3.
Sequence comparison of MTL1a1p and MTL1a2p of C. glabrata strain 7549 with MATa1ps and MATa2ps of other yeast species. (A) Aligned sequences of C. glabrata (Cg) MTL1a1p and MATa1ps or MTLa1ps of S. cerevisiae (Sc), C. albicans (Ca), and K. lactis (Kl). B. Aligned sequences of C. glabrata MTL1a2p and S. cerevisiae MATa2p and MATα2p. The shaded sequences in both panels represent the highly conserved amino acid residues (boldface) involved in the interaction between S. cerevisiae MATa1p and S. cerevisiae MATα2p in the formation of the a1-α2 repressor complex. Note that S. cerevisiae MATa2p and MATα2p share identical amino acid residues in this region, but the physiological role of S. cerevisiae MATa2p is not known. Asterisks indicate identical residues, two stacked dots indicate conservative substitutions based on similar functional groups, and one dot indicates conservative substitutions based on similar effects on secondary structure. The accession numbers for sequences other than those of C. glabrata are as follows: ScMATa1p, PO1366; CaMATa1p, AAD51404.1; KlMATa1p, AAG21094.1; ScMATa2p, CAA246201.1; and ScMATα2p, AAA34762.1. The accession numbers for C. glabrata MTL1a and MTL2a are AY191461 and AY191464, respectively.
No potential methionine initiation amino acid could be identified in frame in either MTL1a1p or MTL1a2p. Sequencing of three independent 5′ rapid amplification of cDNA ends (5′-RACE)-derived PCR products of MTLa1 mRNA revealed an additional 21-bp sequence upstream of the first isoleucine codon (Fig. 2A). 5′-RACE-derived PCR products of MTL1a2 mRNA revealed an additional 15-bp sequence upstream of the first glutamine codon (Fig. 2B). 5′-RACE analysis, therefore, did not identify any typical AUG-type initiation codons or distinguish whether MTL1a1 or MTL1α2 transcripts are translationally functional. The MTL1a1 and MTL1a2 ORFs were positioned on complementary Crick and Watson DNA strands, suggesting divergent transcription from the intervening promoter region, a configuration similar to that in S. cerevisiae (Fig. 1A) (2, 18).
The 3′ end of MTL1a1 was flanked by the gene MRA1 (Fig. 1A), which encodes a multicopy suppressor of RAS1 in S. cerevisiae (http://genome-www.stanford.edu/saccharomyces/). The 3′ end of MTL1a2 was flanked by the undefined sequence FLR (Fig. 1A). In S. cerevisiae, the functional MATa locus is flanked by BUD5 at the 3′ end of MATa1 and by TAF2 at the 3′ end of MATa2 (Fig. 1A), while the silent HMR and HML loci are flanked by YCRWDDta12/YCR097W-a and YCL068C/HCl065W, respectively (http://genome-www.stanford.edu/saccharomyces/). The intergenic promoter region between MTL1a1 and MTL1a2 was similar in size to that for MATa in S. cerevisiae (Fig. 1), but the sequence of the MTL1a1-MTL1a2 intergenic region was dissimilar to that of S. cerevisiae MATa1-MATa2, except for the presence of two putative binding sites for TBP1, one at the 3′ end of each of the presumed overlapping promoters for MTL1a1 and MTL1a2 (Fig. 1A).
To identify a DNA fragment containing a second MTLa locus in strain 7549, a negative PCR selection strategy, involving two primer pairs for the MTL1 flanking regions, was used to screen 55 primary lambda clones. The two primer pairs used, MLFLF3-MLFLR3 and FLR2-MLFLR2, represented the 3′ ends of MTL1a1 and MTL1a2, respectively (Table 2). Lambda clone λ16.1 was selected for sequencing, and a total of 1,960 nucleotides were determined by primer walking. Analysis of the nucleotide sequence revealed that the DNA fragment harbored an MTLa1 ORF and an MTLa2 ORF on Crick and Watson strands and that they were flanked by sequences distinct from those flanking MTL1. We therefore designated this DNA fragment MTL2.
The full-length MTL2a1 ORF, which included 472 nucleotides flanking the 3′ end, was identical to MTL1a1. A stretch of 347 nucleotides at the 3′ end of MTL2a1 represented a unique flanking sequence of MTL2a. The full-length ORFs of both MTL1a2 and MTL2a2 were identical except for the last 11 codons of the former and 20 codons of the latter, which are unique prior to the same TGA stop codon. Thus, MTL2a2p is 9 amino acids longer than MTL1a2p. The MTL2a flanking region, which spans the 3′ end of MTL2a1, contained a short sequence homologous to S. cerevisiae and C. albicans Nep1p (14). The 3′ end of MTL2a2 was flanked by the threonyl-tRNA and a short sequence homologous to the Fadd death effector domain (13).
Isolation and characterization of an MTL1 locus and an MTL3 locus containing MTLα genes.
To test whether the configuration of the MTL1 locus of strain 7549 was common to all C. glabrata strains, we cloned and sequenced an MTL locus in two additional strains of C. glabrata, PB921 and 1480.47. The primers MLFLF2 and MLFLR2 (Table 2), which represented the 3′ ends of the flanking regions of MTL1a1 and MTL1a2, respectively, of strain 7549, were used to generate PCR products from genomic DNA. While these primers generated a PCR product of 2,075 bp for strain 7549, they generated a PCR product of 2,269 bp from strains PB921 and 1480.47 (Fig. 1B). A BLAST-type search identified an S. cerevisiae MATα1-like coding sequence and an S. cerevisiae MATa2/MATα2-like coding sequence (23). Because the MATα1-like and MATα2-like coding sequences were similar to those of S. cerevisiae MATα1 and MATα2, respectively, the paired coding regions were designated MTL1α1 and MTL1α2. The flanking sequences of the MTL1α locus in strains PB921 and 1480.47 were identical to those of MTL1a in reference strain 7549. Both MTL1α2 and MTL1α1 contained uninterrupted ORFs beginning with codons for the initiation amino acid methionine. As in the case of the MTLa2-MTLa1 configuration of the MTL1 and MTL2 loci in strain 7549, MTL1α2 and MTL1α1 were positioned on complementary Crick and Watson DNA strands, with an intergenic promoter (Fig. 1B and 2B). The MTL1α promoter encompassed 333 bp, which was 70 bp longer than the MATα promoter of S. cerevisiae. The MTLα promoter contained three TATA box binding protein sites and a putative binding site for the repressor-activator protein Rap1p, both found in the MATα2 promoter of S. cerevisiae (Fig. 1B). The MTLα promoter of C. glabrata also contained putative Mcm1p and Rme1p binding sites, neither of which was present in the MATα promoter of S. cerevisiae (Fig. 1B). There was no recognizable binding site for the MATa1p-MATα2p heterodimer (a1-α2) (Fig. 1B and 2B) found in the promoter of S. cerevisiae (Fig. 1B).
The MTL1α1 ORF encodes a deduced protein of 184 amino acids with an overall identity of 29% and similarity of 51% with S. cerevisiae MATα1p (Fig. 4A). The carboxy-terminal 88 amino acids showed 50% identity with MATα1p (Fig. 4A). The MTL1α2 ORF encoded a deduced protein of 187 amino acids with an overall identity of 42% and similarity of 70% with S. cerevisiae MATα2p (Fig. 4B) (18). The highly conserved carboxy-terminal end, spanning 62 amino acids, exhibited 60% identity (Fig. 4B). MTL1α2p was identical to MTL1a2p in C. glabrata strain 7549, except that the former was 16 amino acids longer at the amino-terminal end and contained an initial methionine. Since the primers used to clone the MTL1 loci in strains PB921 and 1480.47 represented the flanking regions of MTL1 in strain 7549, these results indicate that the same MTL locus, MTL1, harbors alternative MTL1a or MTL1α genes in different strains.
FIG. 4.
Sequence comparison of MTL1α1p and MTL1α2p of Candida glabrata strains PB921 (CgMTL1α1pa and CgMTL1α2pa) and 1480.47 (CgMTL1α1pb and CgMTL1α2pb) with MATα1ps or MTLα2ps and MATα2ps or MTLα2ps of other yeast species. (A) Aligned sequences of C. glabrata MTLα1pa and MTLα1pb with MATα1p of S. cerevisiae (Sc), MATα1p of K. lactis (Kl), and MTLa1p of C. albicans (Ca). (B) Aligned sequences of C. glabrata MTL1α2pa with MATα2p of S. cerevisiae, MATα2p of K. lactis, and MTL1α2p of C. albicans. The shaded sequences in panel B and the method used to indicate conserved amino acid sequences are described in the legend to Fig. 3. The accession numbers for the sequences other than those of C. glabrata are as follows: ScMATα1p, AAA34763.1; KlMATα1p, AAG21092.1 (note that the carboxy-terminal 42 amino acids of this K. lactis sequence are not shown); CaMATα1p, AAD51411.1; ScMATα2p, AAA34762.1; KlMATα2p, AAG21091.1; and CaMATα2p, AAD51408.1. The accession numbers for C. glabrata MTL1αa, MTL1αb, and MTL3α are AY191462, AY191463, and AY207368, respectively.
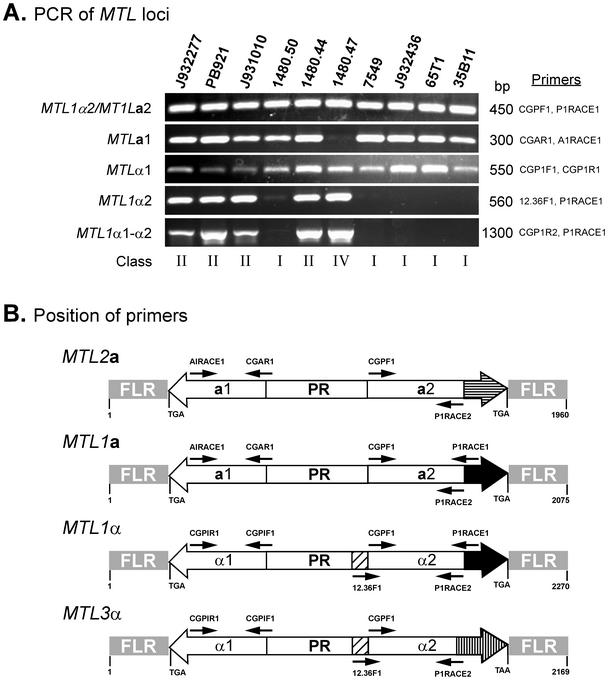
A preliminary Southern analysis of XbaI-digested DNA of strain 7549 hybridized with MTL1a2-α2 revealed three bands, but the same Southern blot probed with MTL1a1 revealed only two of these bands, suggesting a third locus containing an MTLα ORF. To identify this third locus in strain 7549, a positive PCR selection strategy employing the MTL1α1 ORF-specific primer pair CGP1F1-CGP1R2 (Table 2), was used to screen 55 primary lambda clones. Two clones, λMP5.1 and λMP18.1, containing the MTLα1 ORF were selected. The sequence of 2,169 nucleotides was determined by a primer walking strategy. This locus was designated MTL3α. Sequence analysis revealed that this locus harbored divergently transcribed MTL3α1 and MTL3α2 ORFs, both organized similarly to the MTL1α locus (see Fig. 6B). The sequence of the MTL3α1 ORF was identical to that of the MTL1α1 ORF, except that the alanine codon at bp 152 was replaced by a serine codon. Although both the MTL1α2 and MTL3α2 ORFs contained identical stretches of 164 amino acids at their amino-terminal ends, their carboxy termini were dissimilar. MTL3α2p contained a unique stretch of 22 amino acids at the carboxy terminus that was not shared by MTL1α2p (see Fig. 6B). The flanking regions of MTL3 contained unique sequences with no significant homology to any sequence in the database.
FIG. 6.
PCR analysis of the MTL loci of C. glabrata. (A) PCR assays were performed with genomic DNAs of 10 C. glabrata strains. Five separate PCRs were performed with each strain to test the presence or absence of the full-length ORFs. Note that the primers CGPF1 and P1RACE1 do not discriminate between MTLa2 and MTLα2, and the primers CGPIR2 and PIRACE1 generate a fragment containing MTLα1, MTLα2 and the intergenic promoter region. (B) Schematic representation of MTL2a, MTL1a, and MTL3α from strain 7549 and MTL1α from strains PB621 and 1480.47. Positions of key primer pairs used in the PCR analysis are shown.
C. glabrata contains three independent MTL loci.
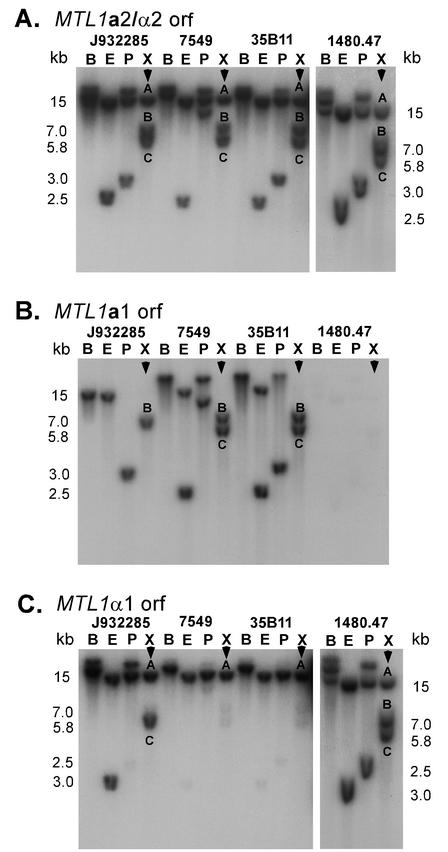
To examine the distribution of the three MTL loci among unrelated strains, Southern blot analyses were initially performed on the genomic DNAs of four independent strains digested with BamHI, EcoRI, PstI, or XbaI and probed with the full-length ORF of MTL1a1, MTL1a2, or MTL1α1. The four test strains were isolated from different geographic locales and proved to be genetically unrelated when DNA fingerprinted with the complex species-specific probe Cg12 (data not shown). The Southern blot hybridization patterns of the four strains probed with a DNA sequence common to MTL1α2 and MTL1a2 (referred to as MTLa2-α2) included two or three bands, depending on the restriction enzyme (Fig. 5A and D). The XbaI patterns of the four test isolates probed with MTLa2-α2 all contained 15-, 7- and 5.8-kb bands, which were designated A, B, and C, respectively (Fig. 5A). The A, B, and C bands represent MTL3, MTL1, and MTL2 loci, respectively. An analysis of 34 additional C. glabrata isolates revealed that all contained three bands either at 15, 7, and 5.8 kb or at 15, 5.8, and 5.2 kb (Table 3). Since MTL1a2 was identical to the 414 bp of the 3′ end of MTL1α2, Southern blot hybridization with MTLa2-α2 could not distinguish between bands containing MTLa2 or MTLα2 sequences.
FIG. 5.
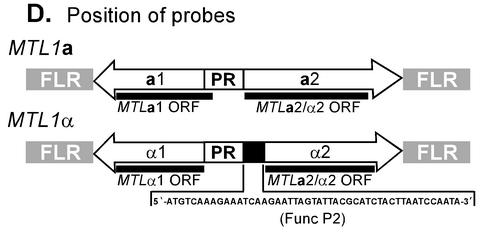
Southern blot analysis of the MTL loci of C. glabrata. Approximately 2.5 μg of total genomic DNA from strains J932285, 7549, 35B11, and 1480.47 were individually digested with the four restriction enzymes BamHI (B), EcoRI (E), PstI (P), and XbaI (X). Digested DNA was resolved in an agarose gel and transferred to a Hybond N nylon membrane. Triplicate Southern blots were individually hybridized with the ORFs for MTL1 genes. (A) Hybridization with the 450-bp PCR fragment (primers CGPF1 and P1RACE1) containing sequences identical to both MTL1a2 and MTL1α2. (B) Hybridization with the 300-bp PCR fragment (primers CGAR1 and A1RACE1) containing the MTL1a1 ORF. (C) Hybridization with the 550-bp PCR fragment (primers CGPIF1 and CGPIR1) containing the MTL1α1 ORF. Three distinct hybridizable fragments in XbaI-digested genomic DNA are designated A (15 kb), B (7 kb) and C (5.8 kb). (D) Schematic representation of MTL1a and MTL1α loci, showing the positions of probes. The primer pairs used to generate the MTL1 ORF probes are described in Fig. 2. The sequence of the MTLα2-specific oligonucleotide FuncP2 is also shown. The molecular sizes of the expected fragments are shown to the right and left of panels A and C and to the left of panel B.
TABLE 3.
Classification of 38 C. glabrata strains based on the distribution of MTLa1, MTLa2 (or MTLα2), and MTLα1 revealed by Southern analysis
| Class | No. of isolates | % of isolates | Band pattern with probe:
|
||||
|---|---|---|---|---|---|---|---|
| MTL1a1 | MTLa2-α2a | FuncP2b | MTL1α1 | MTL1 3′ and 5′ flanking regions | |||
| I | 22 | 58 | A, B, C | A, B, C | A | A | B |
| II | 14 | 36 | C | A, B, C | A, B | A, B | B |
| III | 2 | 5 | B | A, B, C | A, C | A, C | B |
| IV | 1 | A, B, C | A, B, C | A, B, C | B | ||
| V | NAc | NA | A, B, C | A, B, C | B | ||
This probe does not distinguish between MTLa2 and MTLα2.
This probe distinguishes between MTLa2 and MTLα2.
NA, not applicable.
In order to distinguish between MTLa2 and MTLα2 sequences, Southern blots of XbaI-digested genomic DNAs of 38 strains were probed with the end-labeled antisense oligonucleotide primer FuncP2 (Table 2; Fig. 5D), which is unique to the 5′ ends of MTLα2 ORFs. The hybridization identified among the strains three fragments (A, B, and C) containing MTLα2 ORF sequences. The total number of bands in any one strain was either one or two, with the exception of strain 1480.47, which contained three bands (Table 3). Fifty-eight percent of all strains exhibited only one band, and in those cases, it was always the 15-kb A band. Forty-one percent of strains contained a combination of A plus B or A plus C. Hybridization of the exceptional strain 1480.47 with FuncP2 showed that all three DNA fragments, A, B, and C, contained the MTLα2 ORF (Table 3).
The Southern blot hybridization patterns of XbaI-digested DNAs of the four test strains probed with MTLa1 (Fig. 5C) contained no bands, one band (band B), or two bands (bands B and C) (Fig. 5B). Southern blots of the remaining 34 isolates of the test collection probed with MTLa1 revealed patterns of one band (band B or C) or two bands (bands B and C) only (data not shown). In only one unique strain in the entire collection, strain 1480.47, MTLa1 did not hybridize to any band (Fig. 5B), although the MTLα2-specific primer hybridized to three bands (Fig. 5C). MTLa1 did not hybridize to band A in any of the 38 tested strains, in contrast to the MTLα2-specific primer (Table 3).
The Southern blot hybridization patterns of XbaI-digested DNAs of the four test strains probed with MTLα1 (Fig. 5D) contained either one band (band A), two bands (bands A and C), or three bands (bands A, B and C) (Fig. 5C). In the 34 remaining strains in the collection, MTLα1 hybridized to one band (band A) or two bands (bands A and B or bands A and C) (data not shown), the same pattern observed with the FuncP2 probe, suggesting that the MTLα1 ORF always pairs with the MTLα2 ORF. The Southern blot hybridization patterns of all 38 test strains included band A (Fig. 4C; Table 3). In 58% of strains, MTLα1 hybridized only to band A, the same group of strains in which MTLa1 hybridized to bands B and C. We designate strains in this group class I (Table 3). In 18% of strains, MTLα1 hybridized to both A and B; this is the same group of strains in which MTLa1 hybridized only to band C. In this class, the B band exhibited a polymorphism of either 7 or 5.2 kb. Half of the strains in this class contained the 7-kb B band, and the remaining half contained the 5.2-kb B band. We designate strains in this group class II (Table 3). In 5% of strains, MTLα1 hybridized to both A and C; this is the same group of strains in which MTLa1 hybridized only to band B. We designate strains in this group class III (Table 3). The distinguishing feature between class II and class III is the composition of MTL1 and MTL2. In class II isolates, MTL1 includes MTL1α1 and MTL1α2, while in class III isolates, MTL2 contains this configuration. In class IV, there was no hybridization to MTLa1; MTLα1 hybridized to bands A, B, and C (Fig. 5C). If we distinguish loci as a or α by whether they harbor MTLa1 or MTLα1, respectively, then these results suggest that class I strains possess two MTLa loci and one MTLα locus (aaα), class II strains possess two MTLα loci and one MTLa locus (aαα), class III strains possess two MTLα loci and one MTLa locus (aαα), and class IV strains possess three MTLα loci (ααα) (Tables 3 and 4).
TABLE 4.
Models for the six classes based on the distribution of MTLa1, MTLa2 (or MTLα2), and MTLα1 revealed by Southern analysis
| Class | Genes in band:
|
||
|---|---|---|---|
| A | B | C | |
| I (aa α) | MTLα MTL3 | MTLaMTL1 | MTLaMTL2 |
| II (a αα) | MTLα MTL3 | MTLα MTL1 | MTLaMTL2 |
| III (aαα) | MTLα MTL3 | MTLaMTL1 | MTLα MTL1 |
| IV (ααα) (1480.47) | MTLα MTL3 | MTLα MTL1 | MTLα MTL2 |
| V (aaa) | MTLaMTL3 | MTLaMTL1 | MTLaMTL2 |
To test the relationship between the bands containing MTL ORFs and the three MTL loci, XbaI-digested DNAs of the 38 test strains were probed sequentially with the DNA fragments flanking the 3′ ends of MTL1a1 and MTL1a2 of strain 7549. With strain 7549 DNA as template, the primer pair MLFLR2 and FLR2 (Table 2) generated a 395-bp PCR product that spanned the 3′ flanking region of MTLa2 in MTL1, and primer pair MLFLR3 and MLFLF3 (Table 2) generated a 246-bp PCR product that spanned the 3′ flanking region of MTLa1 in MTL1 (Fig. 1A). Southern blots of XbaI-digested DNAs from the 38 test strains probed with either the 395- or 246-bp PCR product revealed that both flanking regions of MTL1 were always associated with the B band, either 7 or 5.2 kb, in all strains (Table 3), indicating that each C. glabrata genome contained only one MTL1 locus in the same relative position. All strains that exhibited bands B and C when probed with MTLa1 and only band A when probed with either MTLα1 or FuncP2 (class I) exhibited only the 7-kb band B when probed with the 3′ flanking regions (Table 3), demonstrating that in these strains only one of the two MTLa1 genes is located in the MTL1 locus. A majority of strains that exhibited bands A and B when probed with MTLα1 exhibited hybridization with only the 5.2- or 7-kb band B (class II) when probed with the 3′ flanking regions (Table 3), demonstrating that in these strains only one of the two MTLα1 genes is located in the MTL1 locus. Surprisingly, two strains that exhibited bands A and C when probed with MTLα1 and FuncP2 (class III) hybridized only to the 7-kb band B when probed with the 3′ flanking regions, demonstrating that in these strains the MTLα1 genes are located at the MTL2 locus. Southern blots of XbaI-digested DNAs from the 38 test strains probed with the 280-bp PCR product flanking the 3′ end of the MTL2a1 ORF, which was generated with the primer pair MTLOCF1-MTLOCR1 (Table 2), revealed multiple hybridization bands, suggesting that the flanking region harbors a repeat element that is dispersed throughout the genome, in addition to its linkage to the C DNA fragments (data not shown). Interestingly, the complex hybridization pattern was identical among strains in each class, suggesting close genetic relatedness of strains within a class.
PCR analysis of MTL size and distribution.
Although Southern analysis revealed three MTL loci (MTL1, MTL2, and MTL3) in each strain and discriminated four classes, it did not demonstrate that the MTL genes were similar in size between strains and between loci. A PCR-based approach was used to investigate this question. To test for any variability in the sequence common to MTL1a2 and MTL1α2 at the MTL1 locus, the primer pair CGPF1 and P1RACE1 (Table 2) was used to generate a single PCR product of 450 bp (Fig. 6B). This 450-bp PCR product was obtained in each of 10 test strains (Fig. 6A), which included five class I strains, four class II strains, and one class IV strain, and in each of the additional 28 isolates in the test collection (data not shown). These results demonstrate that the region common to MTLa2 and MTLα2 is highly conserved in C. glabrata. Since the nucleotide sequences encompassing PIRACE1 is present in MTL1a2and MTL1α2 but not in the MTL3 (band A) or MTL2 (band C) locus, the results clearly suggest that the 450-bp PCR product must have been generated from MTL1 in all classes. To discriminate between (i) MTLa2 and MTLα2 and (ii) MTL1α2 and MTL3α2, the 5′ primer 12.36F1, which is unique to the full-length MTLα2 ORFs of either MTL1α or MTL3α loci, and the 3′ primer P1RACE1, which is common to MTL1a2 and MTL1α2 but not to MTL3α2 (Fig. 6B), were employed. This primer pair generated a 560-bp PCR product for the four class II strains and one class IV strain but produced no product for the five class I strains (Fig. 6A). These results therefore suggest that class II and IV strains contain an MTL1α2 gene at the MTL1 locus, while class I strains contain no MTL1α2 gene. Southern analysis revealed that class I isolates contained MTLα1 and MTLα2 solely at the MTL3 locus in the A fragment (Tables 3 and 4). These PCR results (Fig. 6A) confirm that in class I isolates, MTL3α1 is paired with an MTL3α2-type sequence and not an MTL1α2-type sequence in the A fragment.
To investigate further the linkage between MTL1α1 and MTL1α2, we employed the primer pair CGPIR2, which is common to both MTL3α1 and MTL1α1 (and probably MTL2α1) and PIRACE1, which is unique only to the 3′ end of MTL1α2 (Fig. 6B) but is absent in MTL3α2 and probably in MTL2α2. These primers generated a 1,300-bp PCR fragment which included full-length MTL1α1 and MTL1α2 ORFs for the four class II strains and one class IV strain but not for any of the class I strains (Fig. 6A). These results demonstrate that in class II and IV strains, MTL1α1 paired with MTL1α2 at the MTL1 locus. For two strains, PB921 and 1480.47, sequencing data confirmed that MTL1α1 was paired with MTL1α2 in the B fragment (Fig. 1B and 2B). These results also confirm that MTL3α1 is paired with MTL3α2 and not with an MTL1α2-type ORF in band B in class II strains.
To investigate whether the MTLa1 ORFs present at the MTL1 or MTL2 locus are the same size, the primer pair CGAR1 and A1RACE1 (Table 2), which should generate a single 300-bp PCR product of MTL1a1 (Fig. 6B), was employed. This primer pair generated a 300-bp PCR product in the nine test isolates in classes I and II but not in isolates in class IV (Fig. 6A), which was consistent with the results of the Southern analysis, indicating that all strains but 1480.47 contained at least one copy of MTLa1. These primers also generated a 300-bp PCR product in the 28 additional isolates in the collection. Finally, to test whether MTLα1 ORFs present in all three loci are of the same size, the primer pair CGPIF1 and CGPIR1 (Table 2), which generated a single 550-bp PCR product containing the MTLα1 ORF (Fig. 6B), was employed. This primer pair generated a 550-bp PCR product for all 10 test strains (Fig. 6A), as well as the remaining 28 isolates of the collection; these data are consistent with the results of the Southern analysis demonstrating that all strains contain at least one copy of MTLα1. Since the sequences of MTL1α1 and MTL3α1 were identical, it was not possible to identify the origin of PCR products in class II, III, and IV strains.
Strain-specific transcription of MTL genes.
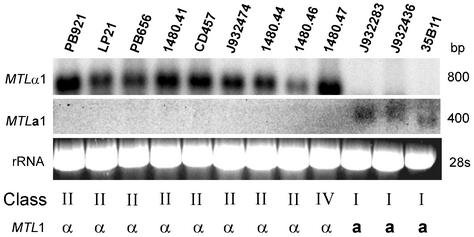
In S. cerevisiae, mating type genes are present at three loci (MAT, HML, and HMR) but are expressed only at one locus (MAT) (18). S. cerevisiae strains can therefore be discriminated phenotypically as MATα or MATa, depending upon the genotype of the MAT locus. To test whether similar distinctions can be made for C. glabrata, and as a strategy for identifying a possible expression locus, 12 isolates were analyzed by Northern blot hybridization for expression of MTLa1 and MTLα1, using the respective ORFs as probes. Three of the test strains were class I (J932283, J932436, and 35B11), eight were class II (PB921, LP21, PB656, CD457, 1480.46, 1480.41, J932474, and 1480.44), and one was class IV (1480.47). While class II and IV strains expressed MTLα1, no class I isolates expressed it (Fig. 7). Conversely, while all tested isolates in class I expressed MTLa1, no class II or IV isolates expressed it (Fig. 7). Therefore, class II and class IV isolates can be classified as α-expressors, while class I isolates can be classified as a-expressors. Predicated on the cassette model in S. cerevisiae, predictions of the MTL expression locus can be made based on the duplication of either MTLα1 or MTLa1 in two of the three MTL loci. While all class II and IV isolates possibly carry identical MTLα1 ORFs at both the MTL1 and MTL3 loci, class III isolates possibly carry identical MTLα1 ORFs at both the MTL2 and MTL3 loci. Therefore, either MTL1 or MTL3 may represent the expression locus in class II, while either MTL2 or MTL3 may represent the expression locus in class III isolates. Class I isolates, on the other hand, carried identical MTLa1 ORFs at both the MTL1 and MTL2 loci. Therefore, in class I isolates, either MTL1 or MTL2 may represent the expression locus.
FIG. 7.
Northern analysis of the expression of MTLα1 and MTLa1 in 12 strains of C. glabrata. Approximately 20 μg of total cellular RNA was applied to each lane. Duplicate Northern blots were probed with either the 300-bp PCR fragment (primers CGAR1 and A1RACE1) containing the MTL1a1 ORF or the 550-bp PCR fragment (primers CGPIF1 and CGPIR2) containing the MTL1α1 ORF. To assess loading, ethidium bromide-stained 28S rRNA patterns are included. The approximate molecular sizes of transcripts are shown to the right of the hybridization patterns. The class and MTL1 expression pattern of each strain are noted.
Transcription of MTL genes during phenotypic switching.
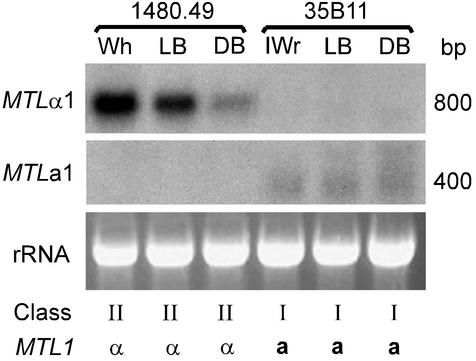
Since the differential expression of MATa and MATα genes confers cell type specificity in S. cerevisiae (2, 18, 23) and since white-opaque switching in C. albicans is intimately involved in the mating process (33, 34, 37), we compared expression of MTLα1 and MTLa1 among the phenotypes of the reversible high-frequency core switching system of C. glabrata (26, 27). Most strains of C. glabrata switch reversibly and at high frequency between the following four core phenotypes, graded in color on agar containing CuSO4: very dark brown, dark brown (DB), light brown (LB), and white (Wh) (26, 27). C. glabrata also switches reversibly between core phenotypes and an irregular wrinkle (IWr) phenotype composed primarily of pseudohyphae (27). Cells of C. glabrata strain 1480.49, an α-expressor of class II exhibiting the LB phenotype on agar containing CuSO4, were clonally plated, and Wh, LB, and DB colonies were analyzed. MTLα1 was expressed by cells with the tested core phenotypes in a graded fashion that correlated with color gradation (Wh > LB > DB) (Fig. 8). None of cells with the switch phenotypes expressed MTLa1 (Fig. 8). Cells of C. glabrata strain 35B11, an a-expressor exhibiting the LB phenotype on agar containing CuSO4, were clonally plated, and LB, DB, and IWr colonies were analyzed. Neither LB, DB, nor IWr cells expressed MTLα1, but all three expressed MTLa1 at similar low levels (Fig. 8). These results demonstrate that while general MTL expression is dictated by the genotype of the MTL1 or MTL2 locus, the core switching system influences the level of MTLα1 expression in a graded fashion.
FIG. 8.
Northern analysis of the expression of MTLα1 and MTLa1 in the switch phenotypes of an MTLα-expressing strain (1480.49) and an MTLa-expressing strain (35B11). Cells of the two strains were plated on agar containing 1 mM CuSO4, which discriminates between the switch phenotype in the core switching system of C. glabrata (26, 27). Cells from Wh, LB, and DB colonies of strain 1480.49 and from IWr, LB, and DB colonies of strain 35B11 were picked and replated before they were analyzed. Duplicate blots were probed with either the 550-bp PCR fragment (primers CGPIF1 and CGPIR1) containing the MTL1α1 ORF or the 300-bp PCR fragment (primers CGAR1 and A1RACE1) containing the MTL1a1 ORF. To assess loading, ethidium bromide-stained 28S rRNA patterns are included. The class and MTL1 expression pattern of each strain are noted.
DISCUSSION
Although C. glabrata represents the second most prevalent Candida species involved in human disease (15, 17, 32, 38, 41), research into its basic biology has been minimal compared to that for C. albicans, primarily because it has been assumed that information gathered for C. albicans would be transferable to other Candida species. However, genetic comparisons of the major Candida species have revealed that C. glabrata is far more related to S. cerevisiae than it is to C. albicans (3, 42, 49). Here we provide evidence for the first time that C. glabrata possesses three mating type-like loci with configurations similar to that of S. cerevisiae. Expression of mating type genes in C. glabrata may be restricted to a single mating type-like locus that can contain either a or α genes, suggesting that a cassette system similar to the one basic to S. cerevisiae mating may exist in C. glabrata (18).
Configuration of the three MTL loci.
C. glabrata possesses three independent mating type-like loci (MTL1, MTL2, and MTL3), each containing pairs of mating type genes. All three have been characterized. In our test strain 7549, both MTL1 and MTL2 were demonstrated to contain an MTLa1 ORF and an MTLa2 ORF on complementary Crick and Watson DNA strands. Although in-frame AUG codons were not evident in either MTLa1 or MTLa2, one out-of-frame AUG codon was present 39 and 15 nucleotides upstream of an isoleucine or glutamine codon in MTLa1 and MTLa2, respectively. It is not clear whether the hybridizable MTLa1 transcript identified in Northern blots or the 5′-RACE product represents the translatable mRNA. Interestingly, MATa1, the homolog of MTLa1, has no known function in haploid a cells of S. cerevisiae (32).
MTLa1 and MTLa2 were separated by an intergenic promoter region. This configuration is similar to that of the three mating type loci in S. cerevisiae (19, 25, 47), suggesting that regulation may also be similar. However, key regulatory sequences in the intergenic promoter of S. cerevisiae MATa1 and MATa2 were not shared with the comparable promoter of C. glabrata, suggesting differences in regulation. Characterization of the MTL1 locus in two additional strains revealed that in these strains the MTLα1 ORF and the MTLα2 ORF resided in the MTL1 locus on cDNA strands in a configuration similar to that of MTLa1 and MTLa2. Again, while the MTLα1-MTLα2 configuration included an intergenic promoter, like the one separating and regulating MATα1 and MATα2 in S. cerevisiae, the identified regulatory sequences differed. The intergenic promoter region of C. glabrata contained Rap1p, Mcm1p, and Rme1p binding sites (http://cgsigma.cshl.org/jian/), which are not present in the comparable S. cerevisiae promoter, and lacked an a1-α2 binding site present in the S. cerevisiae promoter (24). The latter observation suggests that in a cells of C. glabrata, transcription of MTLα1 may not be repressed by an MTLa1p-MTLα2p heterodimer as in a cells in S. cerevisiae. Instead, another transacting factor such as Rme1p, a repressor of meiotic gene expression in S. cerevisiae (16, 17), may substitute in the repression of MTLα1 transcription in a strains of C. glabrata. Alternatively, Rme1p, which can act as either an activator or a repressor (5, 10, 11), depending on the cis-acting element, may function as an activator of MTLα1 expression in α-type strains. Functional characterization of the MTL promoters will be the first step in revealing the unique mechanisms of regulation in C. glabrata.
Genetic models of the three MTL loci in class I, II, and III isolates.
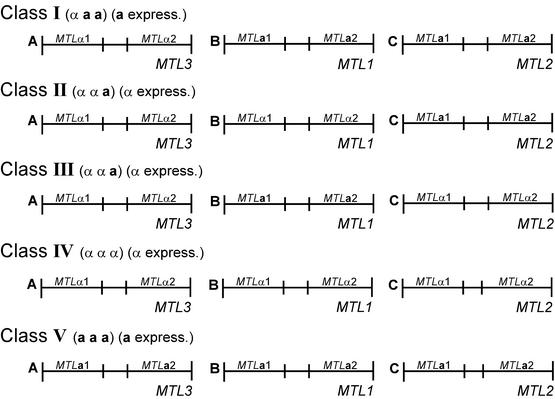
Using a combination of Southern analysis, PCR analysis, and sequencing, we were able to type each of the three MTL loci as a or α for a number of C. glabrata strains based on the presence of MTLa1-MTLa2 or MTLα1-MTLα2 pairs, which could be discriminated unambiguously at each of the three loci. Our results first demonstrate that the great majority of strains are either aαα or aaα. Only one of the 38 isolates tested, 1480.47, deviated from the two patterns, exhibiting an ααα genotype. Based on these studies, the 38 tested strains (97%) separated into four classes based on genotype and the size of the XbaI fragment harboring MTL1 or MTL2 (i.e., fragments B or C) (Fig. 9; Table 4). Class I strains were αaa and contained the combination of MTLa1 and MTLa2 at the MTL1 and MTL2 loci in bands B and C, respectively (Fig. 9). Class I strains also harbored the combination MTLα1-MTLα2 at the MTL3 locus in band A (Fig. 9). Class II strains were ααa and contained the combinations of MTL1α1 and MTL1α2 at the MTL1 locus in band B, MTL2a1 and MTL2a2 at the MTL2 locus in band C, and MTL3α1 and MTL3α2 at the MTL3 locus in band A (Fig. 9). Class III strains were also ααa but contained MTL2α1 and MTL2α2 at the MTL2 locus in band C rather than in band B (Fig. 9). Class III strains also harbored the combination MTL3α1-MTL3α2 at the MTL3 locus in band A, like the other two classes, and MTL2a1 and MTL2a2 in band B (Fig. 9). Based on the MTL genotypes in fragment B or C and the MTL flanking sequence, it is likely that the class IV isolate 1480.47 was derived from either a class II or class III isolate. Since our collection included only a limited number of strains, there is the possibility that additional configurations, such as aaa, exist (Fig. 9).
FIG. 9.
A model of the MTL loci in the four classes of C. glabrata strains. The three MTL loci were distinguished as XbaI fragments A, B, and C in Southern analyses (see Fig. 5). The genotypes of the loci in the different classes were interpreted from the combined results of Southern, PCR, and sequencing data. The strains are distinguished as αaa (class I), ααa (classes II and III), or ααα (class IV). A hypothesized class V is aaa. The expression patterns are distinguished as either a expression or α expression, as determined by Northern analysis.
Expression patterns: suggestion of a cassette system like that in S. cerevisiae.
Northern analysis revealed that C. glabrata, like S. cerevisiae, could be categorized based on mating type gene expression. Strains expressed either MTLa1 or MTLα1, never both, just as haploid S. cerevisiae expresses MATa1 or MATα1 but never both. In the case of S. cerevisiae, genes in HMR and HML loci are silent, while genes in the MAT locus are expressed (18). In C. glabrata, the expression pattern suggested a similar scenario. While class II and IV isolates expressed MTLα1, class I strains expressed MTLa1. The former classes of isolates possessed two MTLα loci, while the latter class possessed two MTLa loci. Northern analysis demonstrated a direct correlation between the expression of MTLa1 or MTLα1 and the presence of two corresponding ORFs at two MTLa and two MTLα loci, respectively. However, our data do not distinguish which of the two similar loci in each case is the expression locus.
If only one of the two loci in C. glabrata is the expression locus and a correlate to the MAT locus of S. cerevisiae and the other two MTL loci are correlates to the silent loci HMR and HML, then the three C. glabrata MTL loci may also function in a mobile cassette system similar to that of S. cerevisiae, in which copies of the silent MTL loci recombine with the MTL expression locus to switch mating type. Experiments to test this hypothesis are now in progress.
Expression patterns and phenotypic switching.
Our results indicate that MTL expression correlates with the genotype of the MTL1 or MTL2 locus. The α-expression strain 1480.49 expressed MTLα1 exclusively in all three tested phenotypes in the core switching system (Wh, LB, and DB), while the a-expressing strain 35B11 expressed MTLa1 exclusively in both core switch phenotypes (LB and DB) and the IWr phenotype. However, in the α-expressing strain, the levels of MTLα1 transcript differed between the three core switch phenotypes according to the hierarchy Wh > LB > DB, suggesting that MTLα1 expression is modulated in a graded fashion by the core switching system, just like transcription of the methalothionine gene MTII, for pigmentation and phloxine B staining (26, 27). Interestingly, the intergenic promoter that controls transcription of MTLα1 contains an Mcm1p binding site, which has been implicated in the regulation of phase-specific gene expression during white-opaque switching in C. albicans (31). The relationship between mating and switching in C. glabrata, therefore, deserves further investigation.
Acknowledgments
This research was supported by grants AI 2392 and DE014219.
We thank Claude Pujol, Shawn Lockhart, and Rui Zhao for helpful discussions.
REFERENCES
- 1.Astrom, S. U., A. Kegel, J. O. Sjostrand, and J. Rine. 2000. Kluyveromyces lactis Sir2p regulates cation sensitivity and maintains a specialized chromatin structure at the cryptic alpha-locus. Genetics 156:81-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Astell, C. R., L. Ahlstrom-Jonasson, M. Smith, K. Tatchell, K. A. Nasmyth, and B. D. Hall. 1981. The sequence of the DNAs coding for the mating-type loci of Saccharomyces cerevisiae. Cell 27:15-23. [DOI] [PubMed] [Google Scholar]
- 3.Barns, S. M., D. J. Lane, M. L. Sogin, C. Bibeau, and W. G. Weisburg. 1991. Evolutionary relationships among pathogenic Candida species and relatives. J. Bacteriol. 173:2250-2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blaschke-Hellmessen, R. 1996. Fluconazole and itraconazole susceptibility testing with clinical yeast isolates and algae of the genus Prototheca by means of the Etest. Mycoses 39(Suppl. 2):39-43. [DOI] [PubMed] [Google Scholar]
- 5.Blumental-Perry, A., W. Li, G. Simchen, and A. P. Mitchell. 2002. Repression and activation domains of RME1p structurally overlap, but differ in genetic requirements. Mol. Biol. Cell 13:1709-1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carninci, P., Y. Shibata, N. Hayatsu, Y. Sugahara, K. Shibata, M. Itoh, H. Konno, Y. Okazaki, M. Muramatsu, and Y. Hayashizaki. 2000. Normalization and subtraction of cap-trapper-selected cDNAs to prepare full-length cDNA libraries for rapid discovery of new genes. Genome Res. 10:1617-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Church, G. M., and W. Gilbert. 1984. Genomic sequencing. Proc. Natl. Acad. Sci. USA 81:1991-1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Claret, F. X., M. Hibi, S. Dhut, T. Toda, and M. Karin. 1996. A new group of conserved coactivators that increase the specificity of AP-1 transcription factors. Nature 383:453-457. [DOI] [PubMed] [Google Scholar]
- 9.Conner, B. J., A. A. Reyes, C. Morin, K. Itakura, R. L. Teplitz, and R. B. Wallace. 1983. Detection of sickle cell beta S-globin allele by hybridization with synthetic oligonucleotides. Proc. Natl. Acad. Sci. USA 80:278-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Covitz, P. A., I. Herskowitz, and A. P. Mitchell. 1991. The yeast RME1 gene encodes a putative zinc finger protein that is directly repressed by a1-alpha 2. Genes Dev. 5:1982-1999. [DOI] [PubMed] [Google Scholar]
- 11.Covitz, P. A., and A. P. Mitchell. 1993. Repression by the yeast meiotic inhibitor RME1. Genes Dev. 7:1598-1608. [DOI] [PubMed] [Google Scholar]
- 12.Csank, C., and K. Haynes. 2000. Candida glabrata displays pseudohyphal growth. FEMS Microbiol. Lett. 189:115-120. [DOI] [PubMed] [Google Scholar]
- 13.Eberstadt, M., B. Huang, Z. Chen, R. P. Meadows, S. C. Ng, L. Zheng, M. J. Lenardo, and S. W. Fesik. 1998. NMR structure and mutagenesis of the FADD (Mort1) death-effector domain. Nature 392:941-945. [DOI] [PubMed] [Google Scholar]
- 14.Eschrich, D., M. Buchhaupt, P. Kotter, and K. D. Entian. 2002. Nep1p (Emg1p), a novel protein conserved in eukaryotes and archaea, is involved in ribosome biogenesis. Curr. Genet. 40:326-338. [DOI] [PubMed] [Google Scholar]
- 15.Fidel, P. L. Jr., J. A. Vazquez, and J. D. Sobel. 1999. Candida glabrata: review of epidemiology, pathogenesis, and clinical disease with comparison to C. albicans. Clin. Microbiol. Rev. 1:80-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fortun, J., A. Lopez-San Roman, J. J. Velasco, A. Sanchez-Sousa, E. de Vicente, J. Nuno, C. Quereda, R. Barcena, G. Monge, A. Candela, A. Honrubia, and A. Guerrero. 1997. Selection of Candida glabrata strains with reduced susceptibility to azoles in four liver transplant patients with invasive candidiasis. Eur. J. Clin. Microbiol. Infect. Dis. 16:314-318. [DOI] [PubMed] [Google Scholar]
- 17.Hazen, K. C. 1995. New and emerging yeast pathogens. Clin. Microbiol. Rev. 4:462-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herskowitz, I., J. Rine, and J. N. Strathern. 1992. Mating-type determination and mating-type interconversion in Saccharomyces cerevisiae, p. 583-656. In E. W. Jones, J. R. Pringle, and J. R. Broach (ed.), The molecular and cellular biology of the yeast Saccharomyces. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 19.Hicks, J., J. N. Strathern, and A. J. Klar. 1979. Transposable mating type genes in Saccharomyces cerevisiae. Nature 282:478-483. [DOI] [PubMed] [Google Scholar]
- 20.Hitchcock, C. A., G. W. Pye, P. F. Troke, E. M. Johnson, and D. W. Warnock. 1993. Fluconazole resistance in Candida glabrata. Antimicrob. Agents Chemother. 37:1962-1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hull, C. M., and A. D. Johnson. 1999. Identification of a mating type-like locus in the asexual pathogenic yeast Candida albicans. Science 285:1271-1275. [DOI] [PubMed] [Google Scholar]
- 22.Hull, C. M., R. M. Raisner, and A. D. Johnson. 2000. Evidence for mating of the “asexual” yeast Candida albicans in a mammalian host. Science 289:307-310. [DOI] [PubMed] [Google Scholar]
- 23.Jacquet, M., J. M. Buhler, F. Iborra, M. C. Francingues-Gaillard, and C. Soustelle. 1991. The MAT locus revisited within a 9.8 kb fragment of chromosome III containing BUD5 and two new open reading frames. Yeast 7:881-888. [DOI] [PubMed] [Google Scholar]
- 24.Johnson, A. D. 1995. Molecular mechanisms of cell-type determination in budding yeast. Curr. Opin. Genet. Dev. 5:552-558. [DOI] [PubMed] [Google Scholar]
- 25.Klar, A. J., J. McIndoo, J. B. Hicks, and J. N. Strathern. 1980. Precise mapping of the homothallism genes HML and HMR in Saccharomyces cerevisiae. Genetics 96:315-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lachke, S. A., T. Srikantha, L. K. Tsai, K. Daniels, and D. R. Soll. 2000. Phenotypic switching in Candida glabrata involves phase-specific regulation of the metallothionein gene MT-II and the newly discovered hemolysin gene HLP. Infect. Immun. 68:884-895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lachke, S. A., S. Joly, K. Daniels, and D. R. Soll. 2002. Phenotypic switching and filamentation in Candida glabrata. Microbiology 148:2661-2674. [DOI] [PubMed] [Google Scholar]
- 28.Landsman, D., O. W. McBride, N. Soares, M. P. Crippa, T. Srikantha, and M. Bustin. 1989. Chromosomal protein HMG-14. Identification, characterization, and chromosome localization of a functional gene from the large human multigene family. J. Biol. Chem. 264:3421-3427. [PubMed] [Google Scholar]
- 29.Li, T., M. R. Stark, A. D. Johnson, and C. Wolberger, C. 1995. Crystal structure of the MATa1/MAT alpha 2 homeodomain heterodimer bound to DNA. Science 270:262-269. [DOI] [PubMed] [Google Scholar]
- 30.Lockhart, S. R., S. Joly, C. A. Pujol, J. D. Sobel, M. A. Pfaller, and D. R. Soll. 1997. Development and verification of fingerprinting probes for Candida glabrata. Microbiology 143:3733-3746. [DOI] [PubMed] [Google Scholar]
- 31.Lockhart, S. R., M. Nguyen, T. Srikantha, and D. R. Soll. 1998. A MADS box protein consensus binding site is necessary and sufficient for activation of the opaque-phase-specific gene OP4 of Candida albicans. J. Bacteriol. 180:6607-6616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lockhart, S. R., S. Joly, K. Vargas, J. Swails-Wenger, L. Enger, and D. R. Soll. 1999. Natural defenses against Candida colonization breakdown in the oral cavities of the elderly. J. Dent. Res. 78:857-868. [DOI] [PubMed] [Google Scholar]
- 33.Lockhart, S. R., C. A. Pujol, K. Daniels, M. G. Miller, A. D. Johnson, M. A. Pfaller, and D. R. Soll. 2002. In Candida albicans, white-opaque switchers are homozygous for mating type. Genetics 162:737-745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lockhart, S. R., K. Daniels, R. Zhao, D. Wessels, and D. R. Soll. 2003. The cell biology of mating in Candida albicans. Eukaryot. Cell 2:49-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Magee, B. B., and P. T. Magee. 2000. Induction of mating in Candida albicans by construction of MTLa and MTLalpha strains. Science 289:310-313. [DOI] [PubMed] [Google Scholar]
- 36.Marichal, P., H. Vanden Bossche, F. C. Odds, G. Nobels, D. W. Warnock, V. Timmerman, C. Van Broeckhoven, S. Fay, and P. Mose-Larsen. 1997. Molecular biological characterization of an azole-resistant Candida glabrata isolate. Antimicrob. Agents Chemother. 41:2229-2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miller, M. G., and A. D. Johnson. 2002. White-opaque switching in Candida albicans is controlled by mating-type locus homeodomain proteins and allows efficient mating. Cell 110:293-302. [DOI] [PubMed] [Google Scholar]
- 38.Odds, F. C. 1988. Candida and candidiasis. Bailliere. Tendall, London, United Kingdom.
- 39.Odds, F. C., M. G. Rinaldi, C. R. Cooper, Jr., A. Fothergill, L. Pasarell, and M. R. McGinniss. 1997. Candida and Torulopsis: a blinded evaluation of use of pseudohypha formation as basis for identification of medically important yeasts. J. Clin. Microbiol. 35:313-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pfaller, M. A. 1996. Nosocomial candidiasis: emerging species, reservoirs, and modes of transmission. Clin. Infect. Dis. 22(Suppl. 2):S89-S94. [DOI] [PubMed]
- 41.Pfaller, M. A., R. N. Jones, S. A. Messer, M. B. Edmond, and R. P. Wenzel. 1998. National surveillance of nosocomial blood stream infection due to Candida albicans: frequency of occurrence and antifungal susceptibility in the SCOPE Program. Diagn. Microbiol. Infect. Dis. 31:327-332. [DOI] [PubMed] [Google Scholar]
- 42.Santos, M. A., T. Ueda, K. Watanabe, and M. F. Tuite. 1997. The non-standard genetic code of Candida spp.: an evolving genetic code or a novel mechanism for adaptation? Mol. Microbiol. 26:423-431. [DOI] [PubMed] [Google Scholar]
- 43.Sobel, J. D. 1996. Candida vulvovaginitis. Semin. Dermatol. 15:17-28. [DOI] [PubMed] [Google Scholar]
- 44.Spinillo, A., E. Capuzzo, R. Gulminetti, P. Marone, L. Colonna, and G. Piazzi. 1997. Prevalence of and risk factors for fungal vaginitis caused by non-albicans species. Am. J. Obstet. Gynecol. 176:138-141. [DOI] [PubMed] [Google Scholar]
- 45.Srikantha, T., L. K. Tsai, K. Daniels, and D. R. Soll. 2000. EFG1 null mutants of Candida albicans switch but cannot express the complete phenotype of white-phase budding cells. J. Bacteriol. 182:1580-1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Srikantha, T., L. Tsai, K. Daniels, A. J. Klar, and D. R. Soll. 2001. The histone deacetylase genes HDA1 and RPD3 play distinct roles in regulation of high-frequency phenotypic switching in Candida albicans. J. Bacteriol. 183:4614-4625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Strathern, J. N., A. J. Klar, J. B. Hicks, J. A. Abraham, J. M. Ivy, K. A. Nasmyth, and C. McGill. 1982. Homothallic switching of yeast mating type cassettes is initiated by a double-stranded cut in the MAT locus. Cell 31:183-192. [DOI] [PubMed] [Google Scholar]
- 48.Wong, S., G. Butler, and K. H. Wolfe. 2002. Gene order evolution and paleopolyploidy in hemiascomycete yeasts. Proc. Natl. Acad. Sci. USA 99:9272-9277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Worley, K. C., B. A. Wiese, and R. F. Smith. 1995. BEAUTY: an enhanced BLAST-based search tool that integrates multiple biological information resources into sequence similarity search results. Genome Res. 5:173-184. [DOI] [PubMed] [Google Scholar]